In This Issue
Is the FDA Warning About Antidepressants Wrong?
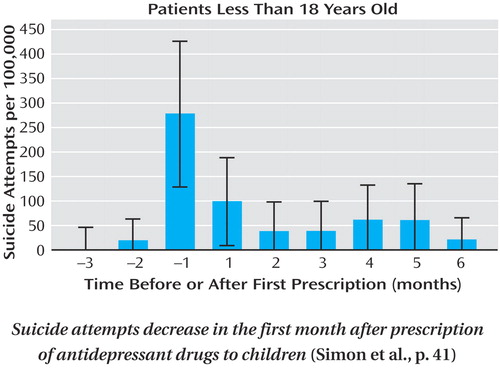
A 2004 advisory by the Food and Drug Administration (FDA) warns that suicidal behavior may emerge after antidepressant treatment is begun. A 10-year population-based study by Simon et al. (p. 41) indicates just the opposite. Among 65,103 patients in a large health plan who filled prescriptions for antidepressants during 1992–2003, the number of suicide attempts fell dramatically after antidepressant treatment began and then declined more slowly. Completed suicides were few and did not vary over the first 6 months of treatment. Adolescents had more suicidal behavior than adults, but attempts decreased by over 60% in the first month of treatment (see figure). The FDA advisory names 10 newer antidepressants, but in this study the decrease in suicide attempts after treatment began was actually greater with newer agents than with older antidepressants. Reconsideration of the FDA warning thus seems warranted, as it discourages use of an effective treatment.
DHEA Helps Mild Depression in HIV/AIDS
Minor forms of depression are common among patients with HIV/AIDS, but mildly depressed patients may not want to take antidepressants, and interactions with antiretroviral medications are a concern. Dehydroepiandrosterone (DHEA) seems to be an effective, safe, and familiar option. It is classified as a nutritional supplement and is viewed by many patients as an “alternative” treatment. Rabkin et al. (p. 59) report a placebo-controlled trial of DHEA for 145 patients with HIV/AIDS and either subthreshold depression or dysthymia (chronic depressed mood). Only 12 patients dropped out of the 8-week study. Among the completers, the rates of response were 62% for DHEA and 33% for placebo; side effects did not differ between groups. Confirmation of DHEA’s efficacy and safety could mean greater well-being and functioning for patients with HIV/AIDS.
Pitfalls in Treating Suicidal Patients
Hendin et al. (p. 67) offer specific recommendations for the treatment of seriously suicidal patients in psychotherapy. From 36 cases discussed with the therapists of patients who committed suicide while receiving open—ended psychotherapy and medication, several common themes emerged: poor communication between care providers, control of therapy by the patient or relatives, avoidance of sexuality issues, ineffective or coercive actions resulting from the therapist’s anxiety about suicide, not recognizing the meaning of the patient’s communications, and undertreatment of symptoms, including substance abuse. Therapeutic exploration of a patient’s demands, unstated conflicts, or medication noncompliance may go further in preventing suicide than does the therapist’s passive acceptance.
A Landmark Depression Study
The first report of the NIMH collaborative study of the treatment of depression, STAR*D (Sequenced Treatment Alternatives to Relieve Depression) (Trivedi et al., p. 28), is accompanied by an editorial from Thomas Insel, Director of NIMH (p. 5). This real-world outcome study finds that only 30% of chronically depressed patients achieve remission during initial treatment with the selective serotonin reuptake inhibitor (SSRI) citalopram. The serotonin transporter, which mediates the clinical effects of SSRIs, is explored in two PET imaging studies that examine its brain expression in relationship to genetic background and disease state (Parsey et al., p. 48 and p. 52). An editorial by Sibille and Lewis (p. 8) reviews the biology of the transporter, which is also featured in Images in Neuroscience (p. 12).
Predicting SSRI Nonresponse
The selective serotonin reuptake inhibitor (SSRI) antidepressants do not work for all depressed people. Slowed motor activity and verbal processing in a subgroup of depressed patients suggest abnormalities in dopamine, rather than serotonin, and this subgroup appears less likely to respond to SSRIs. Now there may be a practical clinical tool to spot SSRI nonresponders before they undergo weeks of ineffective treatment and become discouraged. The FAS test-listing as many words as possible in 1 minute that begin with “F,” with “A,” and with “S”-reveals impaired verbal fluency. Taylor et al. (p. 73) gave the test to patients before a trial of fluoxetine, an SSRI. Of patients scoring below the norm, only 40% responded to fluoxetine, compared to 100% of those scoring above the norm. All of the nonresponders scored below the norm. If it is established that non-SSRI antidepressants work better for this subgroup, this 5-minute test could bring help to many patients faster, before they abandon treatment.