Altered Prefrontal Dopaminergic Function in Chronic Recreational Ketamine Users
Abstract
OBJECTIVE: Ketamine is a noncompetitive antagonist at the glutamatergic N-methyl-d-aspartate (NMDA) receptor that is used in human and animal medicine as an injectable anesthetic. The illegal use of ketamine as a recreational drug is rapidly growing. Very little is currently known about the consequences of repeated ketamine exposure in the human brain. Animal studies indicate that the prefrontal dopaminergic system is particularly vulnerable to the toxic effects of repeated administration of NMDA antagonists. In this study, dopamine D1 receptor availability was assessed by using positron emission tomography and the selective D1 receptor radioligand [11C]NNC 112 in a group of 14 recreational chronic ketamine users and matched healthy subjects. METHOD: History of ketamine abuse was confirmed in subjects by hair analysis. [11C]NNC 112 binding potential was measured with kinetic analysis using the arterial input function. RESULTS: Dorsolateral prefrontal cortex D1 receptor availability was significantly up-regulated in chronic ketamine users ([11C]NNC 112 binding potential: mean=1.68 ml/g, SD=0.40) relative to comparison subjects (mean=1.35 ml/g, SD=0.35). No significant differences were noted in other cortical, limbic, or striatal regions. In the chronic ketamine user group, dorsolateral prefrontal cortex [11C]NNC 112 binding potential up-regulation was significantly correlated with the number of vials of ketamine (with a vial representing approximately 200–300 mg of ketamine) used per week. CONCLUSIONS: Chronic ketamine users exhibited a regionally selective up-regulation of D1 receptor availability in the dorsolateral prefrontal cortex, a phenomenon observed following chronic dopamine depletion in animal studies. These data suggest that the repeated use of ketamine for recreational purposes affects prefrontal dopaminergic transmission, a system critically involved in working memory and executive function.
Ketamine, a noncompetitive antagonist at the glutamatergic N-methyl-d-aspartate (NMDA) receptor, is currently used in human and animal medicine as an injectable anesthetic (1, 2). Ketamine is also a controlled substance, illegally used as a recreational drug (“Special K,” “Vitamin K”). The recreational use of ketamine is prevalent at dance events (3, 4). While no epidemiological study has specifically addressed the scope and growth rate of the nonmedical use of ketamine, the rates of emergency room visits nationwide for use of ketamine grew 20-fold between 1994 and 1999 (5).
At subanesthetic doses, ketamine induces a state of dissociation (including distortion of space, time, and body image) and feelings ranging from euphoria to detachment, an experience described by users as a mind or spiritual exploration (6–8). While the cognitive deficits observed during acute administration of ketamine are well documented (7, 9, 10), very little is known of the long-term effects of repeated ketamine administration in the human brain. High incidence of psychiatric symptoms, such as recurrent hallucinations and psychotic episodes, have been described in subjects abusing phencyclidine (PCP), a more potent noncompetitive NMDA antagonist (11). In chronic ketamine abusers, limited studies suggest the persistence of neurocognitive deficits up to 3 days after use, but these studies are compromised by the polysubstance use in these samples (12, 13).
The animal literature suggests that repeated exposure to noncompetitive NMDA antagonists leads to sustained impairment of performance in numerous cognitive domains, such as working memory tasks (reviewed by Jentsch and Roth [14]). These deficits induced by NMDA antagonists have been linked to reduced function of the prefrontal dopaminergic system, which plays a critical role in sustaining working memory and executive functions (15–19). Monkeys chronically treated with the noncompetitive antagonist MK-801 showed decreased performance on working memory tasks and decreased prefrontal dopamine levels measured with microdialysis (20). Sustained decrease in prefrontal dopamine leads to an up-regulation of prefrontal dopamine D1 receptors, the main dopaminergic receptor in the cortex (21). A positron emission tomography (PET) study showed increased binding of the selective dopamine D1 receptor radiotracer [11C]NNC 112 in the prefrontal cortex of monkeys chronically exposed to MK-801 (20). Thus, in experimental animals, chronic exposure to NMDA antagonists has led to deficits in presynaptic dopaminergic function in the prefrontal cortex, which are associated with a compensatory up-regulation of postsynaptic dopamine D1 receptors.
It is currently unknown if such a phenomenon (decreased prefrontal dopamine function and up-regulation of D1 receptors) is also present in humans chronically exposed to NMDA antagonists. Here, we studied the impact of repeated ketamine exposure on dorsolateral prefrontal cortex D1 receptor binding potential using PET and [11C]NNC 112 in a group of 14 recreational chronic ketamine users and 14 healthy comparison subjects matched for age, gender, race, socioeconomic status of the family of origin, and nicotine smoking.
In addition to examining potential toxic effect of repeated ketamine exposure, this study was also relevant to the pathophysiology of schizophrenia. In schizophrenia patients, we previously observed a regionally selective up-regulation of [11C]NNC 112 binding potential in the dorsolateral prefrontal cortex (22). A deficit in NMDA transmission has been implicated as a fundamental aspect of the pathophysiology of this illness (23–25). Therefore, we speculated that in schizophrenia, a chronic deficit in NMDA transmission might lead to a decrease in prefrontal dopamine function and D1 receptor up-regulation. To document that D1 receptor up-regulation might result from chronic NMDA antagonist exposure in humans would reinforce the biological plausibility of this model.
Method
Subjects
The study was approved by the institutional review boards of the New York State Psychiatric Institute and Columbia University Medical Center. Chronic ketamine users were recruited through flyers distributed by volunteers of Dance Safe.org (promoters of a harm reduction model within the drug abuse community); through discussions with rave/party organizers and closed web groups; and by word of mouth. Study criteria for chronic ketamine users were 1) age between 18 and 50 years; 2) history of at least 2 years of ketamine use, with an average use of one vial per week or more over the last 3 months (a vial contains 200 mg–300 mg of ketamine); 3) history of psychotic symptoms during acute ketamine intoxication; 4) ability to provide 3 cm of hair, and history of ketamine use confirmed by hair analysis (average hair ketamine concentration higher than 10 ng/ml per month in the last 3 months); 5) absence of DSM-IV axis I diagnosis other than ketamine or cannabis abuse or dependence; 6) absence of psychotropic medication for at least 30 days preceding study entry; 7) absence of concomitant or past severe medical conditions; and 8) absence of pregnancy. The healthy comparison subjects had no past or present neurological or psychiatric illnesses including substance abuse. Chronic ketamine users were admitted to an inpatient research unit 3 days before the PET scan to ensure they were drug-free at the time of the scan (the half-life of ketamine is 20 minutes). Healthy comparison subjects underwent the scan as outpatients.
Hair Analysis
Hair samples were collected, and the scalp end was taped to a card so that the laboratory could section the hair and give a chronological estimate of drug intake. The hair was then sectioned into 1–2 cm sections (sufficient to achieve >10 mg weight), weighed, and rinsed with distilled water, methanol, and methylene chloride and then allowed to air dry. Deuterated ketamine and PCP were added as internal standards, and the hair digested in 1M Na2S at 90°C for 10 minutes. Drug-free hair samples were spiked with pure compounds to form a 7-point standard curve encompassing the expected range and processed exactly as samples. The digest was then cooled in ice water and extracted with 1.5% iso-amyl alcohol in heptane and the organic phase back extracted into 0.1 M HCl. This aqueous phase was adjusted to pH 9.5 and extracted into 50 μl 15% iso-amyl alcohol in toluene. A 2-μl aliquot was injected into a GC/MS operated in the EI mode and fitted with a 15 m, 0.25 mm i.d. Trx-5-Amine capillary column (Restek Corp.,Bellefonte, Pa.). Simultaneous ion monitoring of the compounds of interest and their respective isotopomers enabled quantitation using the classical isotope dilution method. Standard curves were linear throughout the range with r2>0.99+. Interassay coefficient of variation was <8% for both analyses.
Imaging Protocol
[11C]NNC 112 was prepared by N-methylation of the desmethyl precursor (+)-5-(7-benzofuranyl)-8-chloro-7-hydroxy-3-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine using [11C]methyl triflate as previously described (26).
PET imaging sessions were conducted with the ECAT EXACT HR+ camera as previously described (27). Following a 10-minute transmission scan, [11C]NNC 112 was injected intravenously over a 45-second period, and emission data were collected in the three-dimensional mode for 90 minutes.
Input function measurement
Following radiotracer injection, arterial samples were collected every 10 seconds with an automated sampling system for the first 2 minutes and manually thereafter at longer intervals (for a total of 30 samples per experiment). Six samples (collected at 2, 8, 16, 30, 50, and 70 minutes) were further processed by high-pressure liquid chromatography to measure the fraction of plasma activity representing unmetabolized parent compound. The measured input function values (Ca(t), μCi/ml) were analyzed as previously described and used as input to the kinetic analysis of the regional brain uptake (27). The clearance of the parent compound (liters/hour) was calculated as the ratio of the injected dose to the area under the curve of the input function. The determination of the plasma-free fraction (f1) was calculated as the ratio of ultrafiltrate to total activity concentrations as previously described (27).
MRI acquisition and segmentation procedures
MRIs were acquired on a GE 1.5-T Signa Advantage system. Steps for MRI segmentation performed within MEDx (Sensor Systems, Inc., Sterling, Va.), with original subroutines implemented in MATLAB (The Math Works, Inc., Natick, Mass.), included correction for field inhomogeneities, fitting of the voxel distribution to a combination of 3 Gaussian, voxel classification, and post filtering (27).
Image analysis
Image analysis was performed blind to subject diagnosis with MEDx (Sensor Systems, Inc., Sterling, Va.). Correction for head movement and coregistration of the PET data to the MR were done with the aid of automated image registration (27).
Derivation of receptor parameters
Derivation of [11C]NNC 112 regional distribution volumes (VT, ml of plasma/g of tissue) was performed with kinetic analysis using the arterial input function as previously described (22). A one-tissue compartment was used in the cerebellum, and a two-tissue compartment in other regions. The primary outcome measure was the binding potential (binding potential, ml/g), derived as the difference in VT between the region of interest and the cerebellum, a region with negligible levels of D1 receptors. The relationship of [11C]NNC 112 binding potential and D1 receptor parameters can be calculated as binding potential=f1Bmax/KD, where f1 (unitless) is the free fraction of [11C]NNC 112 in plasma, Bmax (nanomoles/g of tissue) is the regional concentration of D1 receptors, and KD (nanomoles/ml of water) is the affinity of [11C]NNC 112 for D1 receptors. Another outcome measure frequently used in PET neuroreceptor imaging is the equilibrium specific-to-nonspecific partition coefficient, denoted here V3′′. The relationship of V3′′ and D1 receptor parameters can be calculated as binding potential=Bmax/V2KD, where V2 (ml/g) is the nonspecific volume of distribution. V3′′ was calculated as binding potential/cerebellum VT.
Analyses
Region of interest
Kinetic analysis was performed first on region of interest time-activity curves. Regions of interest included the dorsolateral prefrontal cortex, medial prefrontal cortex, orbitofrontal cortex, parietal cortex, temporal cortex, occipital cortex, anterior cingulate cortex, amygdala, entorhinal cortex, hippocampus, parahippocampal gyrus, associative striatum, sensorimotor striatum, and ventral striatum. Anatomical criteria and methods used to delineate the cortical regions of interest and striatal regions of interest can be found in Abi-Dargham et al. (22) and Martinez et al. (28), respectively.
Voxelwise
In a secondary analysis, kinetic analysis was performed on each voxel to derive VT. Binding potential was computed at each voxel as VT(voxel)–VT(cerebellum), where VT(voxel) was computed using a two-tissue compartment model and VT(cerebellum) was computed as the mean across cerebellum voxels of VT computed with a one-tissue compartment model. All data were fitted with a basis function approach (29). Binding potential maps were then normalized to the MNI T1 template in SPM 99 (30) and compared across groups.
Statistical
For the region of interest analysis, between-group comparisons were performed using two-tailed unpaired t tests. For the voxelwise analysis a one-tailed two-group t test for binding potential of chronic ketamine users versus healthy subjects was computed in SPM 99. A probability value of 0.05 was selected as significance level for both analyses. On the basis of the aforementioned animal data, the primary hypothesis of this study was that dorsolateral prefrontal cortex D1 receptor availability would be altered in chronic ketamine users. Therefore, no correction for multiple comparison testing was applied to this region (31). Other regions were analyzed in an exploratory fashion, to investigate the regional specificity of potential findings in the dorsolateral prefrontal cortex.
Neurocognitive Assessment
A neurocognitive battery was administered the day before the scan to both groups of subjects. The primary neurocognitive test used in this study was the N-back test (32). The N-back paradigm engages white matter by requiring subjects to maintain information about previous stimuli as well as manipulate this information (i.e., to make a comparison with the current stimuli). The N-back was selected as the primary neurocognitive test because a previous study in patients with schizophrenia found a significant relationship between up-regulated D1 receptors in the dorsolateral prefrontal cortex (measured with [11C]NNC 112 using a method similar to the one used in this study) and poor performance on the N-back (22).
In addition to the N-back task, subjects were evaluated using a battery of neurocognitive tests developed for the schizophrenia trial of the National Institute of Mental Health-sponsored Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project. The CATIE battery comprises a wide variety of tasks aiming to characterize subjects across multiple domains of cognitive functioning. The battery has been described in fuller detail by Keefe et al. (33).
Results
Following a telephone screening interview, 54 potential chronic ketamine users were evaluated at the research site. Thirty-three potential participants were excluded from the study because of medical conditions (N=4), psychiatric comorbidity (N=3), abuse of other drugs (N=12), or use of ketamine below study criteria (N=14). Among the 21 subjects who met study criteria, 14 agreed to participate in the study after explanation of the nature and the risks of the study. All recruited subjects completed the study.
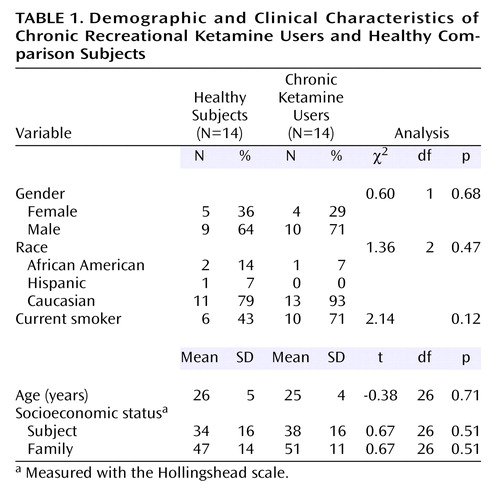
The two study groups are further described in Table 1. The groups were predominantly composed of young Caucasian males. Chronic ketamine users had used ketamine for a mean of 4.1 previous years (SD=2.4, range=11 months to 2 years). Age did not correlate with duration of use (r2=0.01, df=12, p=0.68). Over the preceding 3 months, the mean ketamine consumption was 2.8 vials per week (SD=1.9, range=1–7). Twelve subjects reported inhalation of ketamine as their usual mode of administration, and two subjects reported intramuscular injection. Two of the 14 chronic ketamine users also met criteria for cannabis dependence. Mean hair ketamine concentration was 32 ng/mg of hair (SD=21, range=9–70).
Groups were matched for familial socioeconomic status. No group difference was found in the socioeconomic status of the subjects. Among the chronic ketamine users, six were employed full-time, three were employed part-time, two were students, and three were unemployed. The difference between socioeconomic status of subjects and their family of origin was similar in the chronic ketamine users (mean=–10 points [SD=22] on the Hollingshead scale) and healthy subjects (mean=–13 points [SD=19]). Hence, the frequent use of ketamine did not result in a significantly lower socioeconomic status in chronic ketamine users than in healthy subjects from similar socioeconomic backgrounds.
Scan Parameters
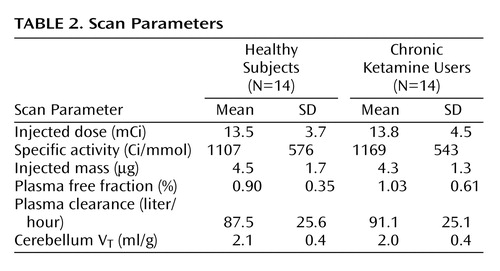
Critical PET scan parameters are listed in Table 2. [11C]NNC 112 injected dose, specific activity at time of injection, and injected mass did not differ between the groups. No significant between-group differences were observed in the clearance rate of [11C]NNC 112 from the plasma compartment, in [11C]NNC 112 plasma free fraction, or in [11C]NNC 112 cerebellum distribution volume.
Regional Volumes
No significant between-group differences were found in dorsolateral prefrontal cortex volumes (healthy subjects: mean=49.9 cm3 [SD=16.1]; chronic ketamine users: mean=47.9 cm3 [SD=10.0]) nor in volumes of the other regions. These data indicate that the use of ketamine in the subjects included in the study did not result in detectable changes in regional brain volumes.
D1 Receptor Measurement
Region of interest analysis
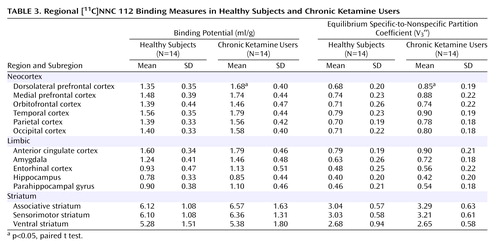
As seen in Figure 1, dorsolateral prefrontal cortex [11C]NNC 112 binding potential was significantly higher in chronic ketamine users compared with the healthy subjects. Similar results were obtained when V3′′ was used as the outcome measure (healthy subjects: mean=0.68 ml/g, SD=0.20; chronic ketamine users: mean=0.85 ml/g, SD=0.22) (t=2.37,df=26, p<0.03). The two chronic ketamine users with highest dorsolateral prefrontal cortex [11C]NNC 112 values were the subjects who reported predominant intramuscular administration. The group difference in dorsolateral prefrontal cortex [11C]NNC 112 binding potential remained significant after exclusion of the two subjects with concomitant cannabis dependence (p=0.048). [11C]NNC 112 binding potential and V3′′ values in other regions are listed in Table 3. No significant between-group differences were observed in [11C]NNC 112 binding potential and V3′′ in other regions examined. In the chronic ketamine users, dorsolateral prefrontal cortex [11C]NNC 112 binding potential positively correlated with the number of vials of ketamine used per week (Figure 2). Dorsolateral prefrontal cortex [11C]NNC 112 binding potential was not associated with the duration of ketamine use (r2<0.01, p=0.77) nor with the average hair ketamine concentration (r2<0.01, p=0.87).
Statistical parametric mapping analysis
Group comparison of [11C]NNC 112 binding data on a voxel basis (results shown in Figure 3) revealed various clusters of increased [11C]NNC 112 in chronic ketamine users relative to comparison subjects. The main area was localized within the dorsolateral prefrontal cortex, confirming the finding of the region of interest analysis. No clusters of decreased [11C]NNC 112 in chronic ketamine users relative to comparison subjects were identified.
Hair Analysis
Mean hair ketamine concentration in the chronic ketamine users was 32 ng/mg of hair (SD=21, range=9–70). Mean hair concentration was not correlated with the average number of vials reported per week (r2=0.07, p=0.33). This lack of correlation was not unexpected because of possible errors in the reported frequency of use and because of several factors such as hair pigmentation and frequency of hair washing, which affect the relationship between dose exposure and hair concentration.
Neurocognitive Assessment
No significant performance differences were found between the groups on tests involving working memory (semantic, visuospatial, and auditory), executive functions, attention, reaction time, verbal learning and memory, verbal fluency, motor function, and intellectual functioning. Chronic ketamine users performed better than healthy subjects on a social cognition task. In the chronic ketamine users, no significant relationships were found between dorsolateral prefrontal cortex [11C]NNC 112 binding potential and performance on the N-back tests (1-back: r2=0.19, p=0.15; 2-back: r2=0.05, p=0.50; 3-back: r2=0.19, p=0.14).
Discussion
In this study, we investigated D1 receptor availability in a group of subjects who regularly used ketamine for recreational or mind-exploring purposes. These subjects had a strong and exclusive interest in ketamine (with the exception of two subjects who also abused cannabis). The regular use of ketamine in this cohort did not result in a detectable downward shift in socioeconomic status. As per inclusion criteria, these subjects were free of medical, neurological, and psychiatric conditions while not under the influence of ketamine. In contrast to the ketamine users studied previously, who were polydrug abusers (12, 13), subjects included in this cohort did not exhibit neurocognitive alterations on a battery of tests administered after 2 days of monitored abstinence. The clinical read and volumetric analysis of the MRI was unremarkable.
In contrast to cognitive performances and regional brain volumes, which were similar in healthy subjects and chronic ketamine users, the PET scan detected an increase in [11C]NNC 112 binding potential in chronic ketamine users that reached significance only in the dorsolateral prefrontal cortex in both the region-of-interest and voxel-based analyses. The quantitative method used in this study is not sensitive to possible alterations in regional cerebral blood flow that could be associated with ketamine use (34–36). In addition, no between-group differences were observed in [11C]NNC 112 plasma-free fraction, in [11C]NNC 112 nonspecific binding (cerebellum VT), or in dorsolateral prefrontal cortex volumes. Under these conditions, increased [11C]NNC 112 binding potential and V3′′ clearly indicated increased availability of D1 receptors. This increased availability could be due to increased receptor density or affinity. The imaging methods used in this study do not discriminate between these mechanisms.
The significance of this finding did not survive correction for multiple comparisons, either in region-of-interest or in statistical parametric analyses. Since animal data indicated that the dorsolateral prefrontal cortex dopamine projections were especially vulnerable to repeated NMDA antagonist administration, this study was primarily designed to look at this region, and under these conditions, no multiple comparison correction is required to ascertain the statistical significance of the findings. Regarding statistical parametric analysis, it has been argued by Friston and colleagues (31) that the use of “corrected” p values in statistical parametric analysis is unnecessary and inappropriately conservative when the target region of interest is predicted in advance.
It cannot be ascertained if this increased D1 receptor availability in the dorsolateral prefrontal cortex of chronic ketamine users is associated with a vulnerability to develop ketamine abuse or is a consequence of repeated ketamine exposure. The positive correlation between average number of ketamine vials used per week and dorsolateral prefrontal cortex [11C]NNC 112 binding potential does not, per se, indicate that this alteration is a result of toxic effects of ketamine. However, the fact that a similar increase in dorsolateral prefrontal cortex [11C]NNC 112 binding potential has been observed in nonhuman primates chronically treated with the NMDA antagonist MK-801 strongly supports the hypothesis that elevated [11C]NNC 112 binding potential is a consequence of repeated ketamine exposure. In rodents, glutamatergic projections from the prefrontal cortex to the ventral tegmental area make direct synaptic contacts onto dopaminergic cells that project back to the cortex (37). This circuit provides an anatomical substrate by which prefrontal dopamine projections might be more vulnerable than other dopamine projections to repeated alterations of glutamatergic transmission induced by ketamine.
The normality of the cognitive performances in the chronic ketamine users was an unanticipated result of this study. From the aforementioned animal studies, it is reasonable to postulate that the D1 receptor up-regulation observed in the present study might be secondary to a drug-induced deficit in prefrontal dopamine function. Given the absence of detectable neurocognitive impairment observed in these subjects, it is tempting to speculate that, at this stage in the condition, the up-regulation of D1 receptors might be relatively efficient at compensating for a deficit in prefrontal dopamine function. However, this up-regulation might be an early sign of system dysregulation. Because of the design of the study, subjects with potentially more severe consequences of NMDA antagonist exposure were less likely to participate, since alcoholism, polysubstance abuse, and comorbid psychopathology tend to develop with the progression of the addiction and were exclusion criteria.
It is very important to stress that the recruitment criteria (absence of psychiatric comorbidity and other substance abuse) used in this study resulted in a sample of “high-functioning” recreational ketamine users, and that this group might not be representative of the majority of ketamine abusers. Therefore, the absence of cognitive deficits in chronic ketamine users enrolled in this study does not indicate that the recreational use of ketamine is safe for cognitive functions. In fact, this study shows that, even in the absence of cognitive deficits, repeated ketamine exposure is associated with signs of disruptions of a critical component of cognition, the prefrontal dopamine system.
The findings of the present study also have implications for the pathophysiology of schizophrenia. Schizophrenia, a severe and chronic mental illness, is believed to be associated with an imbalance in dopamine transmission, characterized by a persistent deficit in prefrontal cortical dopamine function involving D1 receptors (contributing to the cognitive impairment) and an intermittent excess of subcortical dopamine function involving D2 receptors (contributing to the emergence of psychotic states) (38, 39). In addition, several lines of evidence suggest that a deficit in NMDA transmission might be a fundamental aspect of the pathophysiology of this illness (23–25). Schizophrenia is associated with a regionally selective up-regulation of [11C]NNC 112 binding potential in the dorsolateral prefrontal cortex (22), an observation that might reflect a sustained deficit in presynaptic dopamine function. The fact that chronic ketamine users and patients with schizophrenia exhibit the same endophenotypic trait (up-regulated D1 receptor expression in the dorsolateral prefrontal cortex) supports the hypothesis that in schizophrenia, this alteration might be secondary to NMDA dysfunction (40).
In conclusion, repeated exposure to ketamine in humans is associated with up-regulation of D1 receptors in the dorsolateral prefrontal cortex. Animal data suggest that this phenomenon could be related to deficits in prefrontal presynaptic dopamine function induced by intermittent and repeated NMDA blockade. Thus, repeated use of ketamine for recreational purposes might be associated with detrimental effects on brain neurotransmission. Given the critical role of this system in executive functions, more studies are needed to evaluate this neurotoxic effect of ketamine and the reversibility of these changes upon long-term abstinence.
 |
 |
 |
Received April 14, 2004; revision received Jan. 10, 2005; accepted Feb. 23, 2005. From the Departments of Psychiatry and Radiology, Columbia University College of Physicians and Surgeons, New York; and the Department of Psychiatry, Duke University, Durham, NC. Address correspondence and reprint requests to Dr. Narendran, New York State Psychiatric Institute, 1051 Riverside Dr., Box 31, New York, NY 10032; [email protected] (e-mail). Supported by a grant from the Lieber Center for Schizophrenia Research at Columbia and an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Abi-Dargham. The authors thank Carol Caton, Ph.D., and John Keilp, Ph.D., from Columbia University for their assistance in conducting the study.
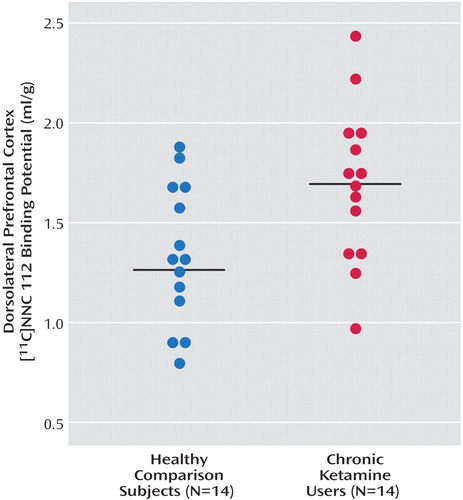
Figure 1. Distribution of [11C]NNC 112 Binding Potential in the Dorsolateral Prefrontal Cortex of Healthy Subjects and Chronic Ketamine Usersa
aChronic ketamine users displayed increased D1 receptor availability in the dorsolateral prefrontal cortex ([11C]NNC 112 binding potential: mean=1.68 ml/g, SD=0.40) relative to healthy subjects (mean=1.35 ml/g, SD=0.35) (t=2.34, df=26, p<0.03).
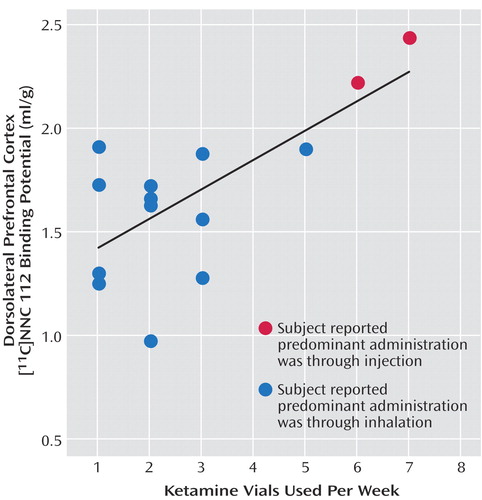
Figure 2. Correlation Between Dorsolateral Prefrontal Cortex Binding Potential and the Vials of Ketamine Used per Weeka
aOne vial equals approximately 200–300 mg of ketamine. The correlation was significant (r2=0.48, p=0.005).
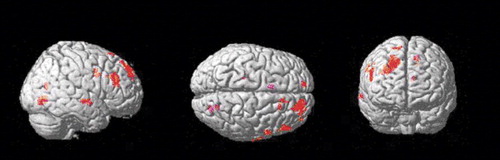
Figure 3. Regions of Significantly Greater [11C]NNC 112 Binding Potential in Chronic Ketamine Users (N=14) Relative to Healthy Comparison Subjects (N=14)a
aOne-tailed two-group test computed in SPM 99; the rendered t statistic is thresholded to uncorrected p<0.01. The primary brain region showing at this level is the right dorsolateral prefrontal cortex.
1. Anis NA, Berry SC, Burton NR, Lodge D: The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 1983; 79:565–575Crossref, Medline, Google Scholar
2. Zukin SR, Zukin RS: Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci USA 1979; 76:5372–5376Crossref, Medline, Google Scholar
3. Mattison AM, Ross MW, Wolfson T, Franklin D: Circuit party attendance, club drug use, and unsafe sex in gay men. J Subst Abuse 2001; 13:119–126Crossref, Medline, Google Scholar
4. Ross MW, Mattison AM, Franklin DR Jr: Club drugs and sex on drugs are associated with different motivations for gay circuit party attendance in men. Subst Use Misuse 2003; 38:1173–1183Crossref, Medline, Google Scholar
5. Freese TE, Miotto K, Reback CJ: The effects and consequences of selected club drugs. J Subst Abuse Treat 2002; 23:151–156Crossref, Medline, Google Scholar
6. Hansen G, Jensen SB, Chandresh L, Hilden T: The psychotropic effect of ketamine. J Psychoactive Drugs 1988; 20:419–425Crossref, Medline, Google Scholar
7. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers M Jr, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
8. Jansen KL: A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drugs 2000; 32:419–433Crossref, Medline, Google Scholar
9. Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A: NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996; 14:301–307Crossref, Medline, Google Scholar
10. Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH: Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N–methyl-d–aspartate receptor antagonists. Arch Gen Psychiatry 2000; 57:270–276Crossref, Medline, Google Scholar
11. Allen RM, Young SJ: Phencyclidine-induced psychosis. Am J Psychiatry 1978; 135:1081–1084Link, Google Scholar
12. Curran HV, Morgan C: Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 2000; 95:575–590Crossref, Medline, Google Scholar
13. Curran HV, Monaghan L: In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 2001; 96:749–760Crossref, Medline, Google Scholar
14. Jentsch JD, Roth RH: The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999; 20:201–225Crossref, Medline, Google Scholar
15. Brozoski TJ, Brown RM, Rosvold HE, Goldman PS: Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 1979; 205:929–932Crossref, Medline, Google Scholar
16. Deutch AY, Tam SY, Freeman AS, Bowers MB Jr, Roth RH: Mesolimbic and mesocortical dopamine activation induced by phencyclidine: contrasting pattern to striatal response. Eur J Pharmacol 1987; 134:257–264Crossref, Medline, Google Scholar
17. Carlezon WA Jr, Wise RA: Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci 1996; 16:3112–3122Crossref, Medline, Google Scholar
18. Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N: Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res 1994; 633:337–342Crossref, Medline, Google Scholar
19. Verma A, Moghaddam B: NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 1996; 16:373–379Crossref, Medline, Google Scholar
20. Kakiuchi T, Nishiyama S, Sato K, Ohba H, Nakanishi S, Tsukada H: Effect of MK801 on dopamine parameters in the monkey brain. Neuroimage 2001; 16:110Google Scholar
21. Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A: Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology 2003; 28:1703–1711Crossref, Medline, Google Scholar
22. Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M: Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22:3708–3719Crossref, Medline, Google Scholar
23. Javitt DC, Zukin SR: Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301–1308Link, Google Scholar
24. Moghaddam B: Bringing order to the glutamate chaos in schizophrenia. Neuron 2003; 40:881–884Crossref, Medline, Google Scholar
25. Tsai G, Coyle JT: Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 2002; 42:165–179Crossref, Medline, Google Scholar
26. Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J, Sedvall G, Farde L: Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1–dopamine receptors. J Nucl Med 1998; 39:2061–2068Medline, Google Scholar
27. Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M: Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 2000; 20:225–243Crossref, Medline, Google Scholar
28. Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M: Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23:285–300Crossref, Medline, Google Scholar
29. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ: Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6:279–287Crossref, Medline, Google Scholar
30. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frakowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
31. Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD: Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 1996; 4(3, part 1):223–235Google Scholar
32. Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC: Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp 1994; 1:293–304Crossref, Medline, Google Scholar
33. Keefe RS, Mohs RC, Bilder RM, Harvey PD, Green MF, Meltzer HY, Gold JM, Sano M: Neurocognitive assessment in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project schizophrenia trial: development, methodology, and rationale. Schizophr Bull 2003; 29:45–55Crossref, Medline, Google Scholar
34. Lahti AC, Holcomb HH, Medoff DR, Tamminga CA: Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 1995; 6:869–872Crossref, Medline, Google Scholar
35. Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D: Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 1997; 154:805–811Link, Google Scholar
36. Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J: Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [F-18]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 1997; 7:9–24Crossref, Medline, Google Scholar
37. Carr DB, Sesack SR: Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 2000; 20:3864–3873Crossref, Medline, Google Scholar
38. Weinberger DR: Implications of the normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
39. Davis KL, Kahn RS, Ko G, Davidson M: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474–1486Link, Google Scholar
40. Laruelle M, Kegeles LS, Abi-Dargham A: Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann NY Acad Sci 2003; 1003:138–158Crossref, Medline, Google Scholar



