Sustained Benefit of Supportive Intervention for Depressive Symptoms in Caregivers of Patients With Alzheimer’s Disease
Abstract
OBJECTIVE: The long-term effect of counseling and support on symptoms of depression was examined in spouse-caregivers of patients with Alzheimer’s disease. METHOD: The participants were 406 spouse-caregivers of Alzheimer’s disease patients who lived at home at baseline. The caregivers were randomly assigned to either a group receiving enhanced counseling and support treatment or a group receiving usual care (control group). Caregivers in the enhanced treatment group were provided with six sessions of individual and family counseling, agreed to join support groups 4 months after enrollment, and received ongoing ad hoc counseling. The Geriatric Depression Scale was administered at baseline and at regular follow-up intervals for as long as the caregiver participated in the study. RESULTS: After baseline differences were controlled for, caregivers in the enhanced treatment group had significantly fewer depressive symptoms after the intervention than did the control subjects. These effects were sustained for 3.1 years after baseline, similar across gender and patient severity level, and sustained after nursing home placement or death of the patient. CONCLUSIONS: Counseling and support lead to sustained benefits in reducing depressive symptoms in spouse-caregivers of Alzheimer’s disease patients and should be widely available to provide effective, evidence-based intervention for family caregivers.
Family members, often at great personal cost, provide much of the care for older adults with Alzheimer’s disease and other dementias in the community (1). Family caregivers of relatives with Alzheimer’s disease are at high risk for psychological distress, with rates of clinical depression and depressive symptoms far in excess of those for age-matched comparison subjects (2). This risk persists over the many years of caregiving (3) and even after caregiving ends with the death of the care recipient (4).
Carefully designed psychosocial interventions have been shown to be effective in reducing caregiver depressive symptoms (5, 6). Little is known about the long-term impact of caregiver interventions in reducing depressive symptoms. Caregiver intervention studies rarely follow participants for longer than a year or after potentially stressful transitions in caregiving, such as the nursing home placement or death of the care recipient. The New York University Spouse-Caregiver Intervention Study provided an ideal context in which to study the long-term impact of caregiver intervention on depressive symptoms. Over 9.5 years, 406 spouse-caregivers, enrolled in two successive cohorts, were randomly assigned to either enhanced counseling and support intervention or to usual care, which served as a control condition. The project is unique in that it has followed caregivers for a long period of time, with little attrition. Results from the first 206 subjects enrolled in the project have been reported previously (6, 7) and indicate that the intervention had an increasingly stronger effect on depressive symptoms in the first year after enrollment (6). Analyses of the entire study group of 406 caregivers and of the long-term effects of the intervention on depressive symptoms beyond the first year have not heretofore been reported.
Because the intervention was designed to improve caregiving skills, mobilize the support of naturally existing family networks, and provide the opportunity for counseling as needed over the entire course of caregiving, we hypothesized that the intervention would yield sustained benefits in reducing depressive symptoms, regardless of gender or level of dementia severity, not only while the family member continued to provide care at home but also after potentially stressful events such as nursing home placement and death of the patient. A secondary hypothesis was that the demonstrated effectiveness of the caregiver intervention in comparison to usual care for symptoms of depression 1 year after enrollment for the first cohort would be replicated in the second cohort, even though educational material and community supports have become increasingly available for caregivers since the study began. Finally, we were interested in exploring whether the intervention was of similar effectiveness for caregiving husbands and wives and for caregivers of patients at all levels of dementia severity.
Method
Subjects
Each study subject was the spouse of a patient with a clinical diagnosis of Alzheimer’s disease and had the primary responsibility for the patient’s care. All patients were living at home with their spouses at baseline. In each family, the patient or the caregiver had to have at least one other relative living in the New York City metropolitan area.
Subjects were recruited through the New York University Alzheimer’s Disease Center, the local chapters of the Alzheimer’s Association, media announcements, and referrals from physicians, social workers, lawyers, Alzheimer’s disease day care centers, and social service agencies. The institutional review board of the New York University School of Medicine reviewed and approved this project. Written consent to participate in the project was obtained from each caregiver, as well as from any other participating family members.
The total study group consisted of 406 caregivers. The study had two enrollment phases, resulting in two cohorts of subjects. In the first phase, 206 subjects were recruited over a 3.5-year period beginning in August 1987. In the second phase, an additional 200 subjects were recruited over a 5.5-year period beginning in June 1991.
Study Design
After a comprehensive baseline assessment, study subjects were randomly assigned by lottery to one of two groups—a treatment group that received enhanced counseling and support or a control group that received the usual care offered family members of patients at the New York University Alzheimer’s Disease Center. Participants were free to seek additional assistance and support elsewhere at any time throughout the study.
All caregivers were interviewed every 4 months during the first year and every 6 months thereafter, by telephone or in person, with the comprehensive battery of structured questionnaires first administered at baseline.
All caregivers were followed until 2 years after the death of the patient or until they refused or were no longer able to participate in the study. The analysis for this report is confined to the first 5 years after enrollment, the time period for which we have follow-up interviews for the most recently enrolled subjects. Thus, the analyses include the results of up to 12 interviews: intake, every 4 months for the first year, and every 6 months for years 2 to 5. Of the original 406 subjects, we assessed 380 (93.6%) at 1 year, 328 (80.8%) at 3 years, and 223 (54.9%) at 5 years of follow-up.
Treatment
The enhanced counseling and support treatment was delivered by counselors with advanced degrees in social work or allied professions and has been described in detail in a recent publication (8). The first component consisted of two individual and four family counseling sessions that included relatives suggested by the caregiver but never included the patient. The content of these sessions was determined by the needs of each caregiver (e.g., learning techniques for management of troublesome patient behavior, promoting communication among family members). Counselors also provided education about Alzheimer’s disease and community resources.
The second component of the intervention was participation in a support group, beginning after the first follow-up interview. Caregivers in the group receiving enhanced treatment agreed at baseline that they would join support groups that met weekly and provided a venue for continuous emotional support and education. The third component of the treatment was ad hoc counseling—the continuous availability of counselors to caregivers and families to help them deal with crises and with the changing nature and severity of their relatives’ symptoms over the course of the disease. The emergence of new psychiatric and behavioral problems of patients, which are generally more stressful than the need for assistance with activities of daily living or physical limitations (2), often precipitated ad hoc calls from caregivers.Ad hoc counseling made it possible for caregivers and families to determine the amount of contact they had with the counselors beyond the scheduled structured sessions. Each caregiver in the enhanced treatment group was offered all of the treatment components. Caregivers in the usual care group received the services provided to all families of patients at the New York University Alzheimer’s Disease Center, which included information about resources and advice upon request, but they did not have formal counseling sessions and their family members did not have contact with the counselors. They were free to participate in the same support groups and ad hoc counseling used by caregivers in the enhanced treatment group if they so chose. Because the same highly skilled and trained counselors at the New York University Alzheimer’s Disease Center were available to participants in both the intervention and control groups, caregivers in the control group undoubtedly received more information and support than is generally available in typical medical and community settings.
One counselor was assigned to each caregiver because we felt that counseling and support would be most effective if each caregiver had an ongoing relationship with someone who was familiar with his or her situation. Anecdotal evidence suggests that the follow-up assessments, conducted by the same counselors, were viewed as helpful by the caregivers receiving usual care, as well as by those receiving enhanced treatment.
The study had a low dropout rate; in the first 5 years after enrolling, 28 (6.9%) of the caregivers dropped out, of whom 13 (3.2%) were caring for patients at home at the time of refusal. An additional 55 caregivers dropped out because they became too ill to participate (N=20, 4.9%), entered nursing homes (N=4, 1.0%), or died (N=31, 7.6%). These retention rates are a major strength of the study and suggest that caregivers in both groups valued the contacts and assistance they received through the project.
Measures Used in the Analysis
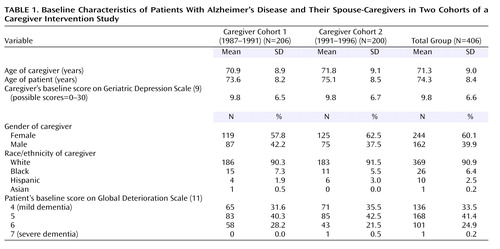
Caregiver depression was assessed at baseline and at every follow-up assessment with the Geriatric Depression Scale (9), a 30-item self-report questionnaire with a yes/no format that was specially developed for use with the elderly; possible scores range from 0 to 30 (alpha=0.94). A cutoff score of 11 yields a sensitivity of 84% and a specificity of 95% (10). At baseline, 42.9% of the caregivers in this study (52.0% of the women and 29.0% of the men) had scores above the cutoff, indicative of possible clinical depression.
The severity of the patient’s dementia was determined at baseline and at each follow-up interview by using the Global Deterioration Scale (11) (alpha=0.83), a semistructured rating of patient functioning by the interviewer based on information provided by the caregiver. Patients with dementia have scores ranging from 4 to 7 on this scale, with 4 representing mild dementia and 7 representing severe dementia.
Statistical Methods
Changes in depression over the first year of the clinical trial were examined by using an intent-to-treat analysis, with the last value carried forward for the 26 participants (6.4%) who did not provide complete data through the 1-year follow-up assessment. In addition, mixed-model growth curve analyses were conducted by using SAS Proc Mixed (12) to examine the longitudinal trajectories of depression. These growth curve models offer important advantages over more traditional repeated-measures analyses, especially in handling missing data (13). Growth curves were fit for each individual subject on the basis of the number of data this person provided, allowing subjects who discontinued or completed the study before the 5-year assessment to be included in the longitudinal analyses without imputation of data for the missing observations. Imputing the last observed value and carrying it forward was considered acceptable for the relatively few missing data in the first year, but it would have led to considerable bias if applied for the increasing number of missing data through 5 years after randomization.
Variability in the actual time of the assessments was explicitly included in the growth curve models by analyzing time as a random effect. Individual growth curve parameters were modeled as a function of group (enhanced treatment versus usual care) and other predictors of interest. Restricted maximum likelihood estimation was used, and an unstructured covariance structure was specified. The depression scores obtained after treatment onset (i.e., 4-month follow-up, 8-month follow-up, etc.) were analyzed as repeated observations of the dependent variable, with “mean-centered” baseline depression scores serving as a covariate. These “mean-centered” scores consisted of deviation scores; the mean baseline depression score across all caregivers (9.64) was subtracted from each individual caregiver’s baseline depression score. Other predictor variables included gender (female versus male), group (enhanced treatment versus usual care), the amount of time from baseline to when each follow-up depression score was obtained, the status of the patient at the time of the interview (living at home, in a nursing home, or dead), the interaction of group and time, and the interaction of baseline depression and time. The status of the patient was analyzed as a time-dependent covariate, whereas baseline caregiver depression, gender, and group were analyzed as time-invariant covariates.
Linear, quadratic, and logarithmic growth models were examined for two different time periods. First, we analyzed changes over the first year of the study only (4-month, 8-month, and 12-month follow-ups); the group main effect and group-by-time interaction effect from these analyses addressed hypotheses similar to those tested by the intent-to-treat analyses. Second, we analyzed effects from the end of year 1 to the end of year 5 (12-month, 18-month, 24-month,.… 60-month follow-ups).
Additional growth curve analyses were conducted to determine whether depression varied as a function of cohort (enrollment phase 1 versus enrollment phase 2, time-invariant) or patient Global Deterioration Scale score (time-dependent). For all growth curve models, the Akaike information criterion (14) was used to evaluate overall model fit and to select the best-fitting longitudinal change pattern (i.e., linear, quadratic, or logarithmic).
Results
The 203 caregivers assigned to enhanced counseling and support treatment had a mean age of 71.5 years (range=40–93) and consisted of 111 wives and 92 husbands. Caregivers in the usual care control group had a mean age of 71.1 years (range=47–95), and this group comprised 133 wives and 70 husbands. Table 1 shows demographic and clinical characteristics of the caregivers and patients at baseline. Even though the caregivers were randomly assigned to the intervention and control groups, the difference in the gender composition of the two groups was statistically significant (χ2=4.65, df=1, p=0.03). Baseline depression scores were also significantly lower for the caregivers receiving enhanced treatment (mean=8.9, SD=5.7) than for the caregivers in the control group (mean=10.6, SD=7.2) (F=6.59, df=1, 404, p=0.01), mostly because wives (of whom there were more in the control group) had higher baseline depression scores (mean=11.1, SD=6.7) on average than husbands (mean=7.7, SD=5.7) (F=28.93, df=1, 404, p<0.0001). These baseline differences indicated that baseline depressive symptoms and gender should be included as covariates in the longitudinal models.
Effects of Intervention Over First Year
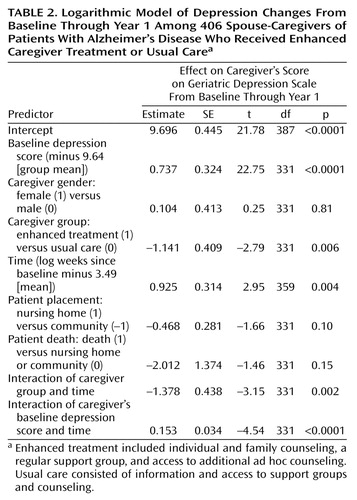
In the first year after baseline, there was a gradual decrease in symptoms of depression among caregivers in the group receiving enhanced treatment and an increase among the group receiving usual care. At the 1-year follow-up, the difference in the change on the Geriatric Depression Scale score between the enhanced treatment group (mean=–1.1, SD=5.0) and the usual care group (mean=0.3, SD=6.0) was statistically significant (F=6.40, df=1, 404, p=0.02) according to the intent-to-treat approach. For the growth curve analyses examining changes over the first year after randomization, the logarithmic growth curve model was found to provide better fit than either the linear or quadratic model, as indicated by the lowest Akaike information criterion score (logarithmic=5985, linear=6002, quadratic=6016). This means that better fit was obtained when the rate of change in depression was allowed to gradually decrease over time (the logarithmic model) than when this rate of change was constrained to be constant over time (the linear model). Table 2 presents the results for the logarithmic growth model during the first year after baseline for participants in both intervention groups from both cohorts. The predictor labeled “time” was obtained by calculating the natural logarithm of the number of weeks from baseline to an assessment and “centering” this value by subtracting its mean of 3.49.
The significant predictors of depressive symptoms over the first year after randomization were the baseline depression score, intervention group, time since baseline, the group-by-time interaction effect, and the baseline-by-time interaction effect. The predicted effects for group, time, and the group-by-time interaction are depicted in Figure 1. The model-predicted values indicate that depression scores decreased during the first year for caregivers in the enhanced treatment group but increased slightly during the first year for those in the control group. As in the intent-to-treat analyses, the treatment and control groups were significantly different on covariate-adjusted depression scores at 1 year (p=0.0005) but not at 4 months (p=0.58) after randomization. In contrast to the intent-to-treat analyses, the covariate-adjusted growth curve models also revealed significant group differences at 8 months (p=0.004). The analysis also revealed that 39.9% of the caregivers in the group receiving enhanced treatment were above the threshold for clinically significant depression at baseline, while only 29.8% of these caregivers exceeded the cutoff after 1 year of intervention. The corresponding percentages for the caregivers in the control group were 45.8% and 45.1% for baseline and 1 year, respectively.
We investigated whether the previously published findings on the first cohort 12 months after randomization (6) could be replicated by using mixed-model growth curve analyses. Traditional regression analyses of data from the first cohort had shown a steadily increasing difference between the enhanced treatment and usual care caregivers in the change from baseline in the number of depressive symptoms. In the present study, we investigated the replicability of this longitudinal pattern by fitting growth models for the first year for the two cohorts separately. Consistent effects were found across both cohorts, with the group-by-time interaction effect from the logarithmic model significant for both cohort 1 (t=–2.38, df=167, p=0.02) and cohort 2 (t=–2.13, df=161, p=0.04).
Sustainability of Intervention Effects in Years 1–5
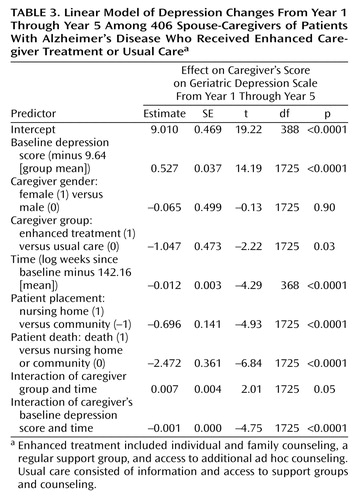
For the growth curve analyses examining long-term effects from the end of year 1 through year 5 after baseline, a linear growth model was found to provide better fit than either a quadratic or logarithmic model (Akaike information criterion: linear=13,848, quadratic=13,867, logarithmic=13,877). This suggests that the rate of change in depression was fairly constant across time for both groups. Table 3 presents the results for the linear growth model for the two cohorts combined. Significant effects were found for all predictors except gender. The baseline-by-time interaction effect reflects the finding that depression scores were less correlated with baseline depression scores as the time from baseline increased. Figure 2 shows the significant group main effect, time main effect, and group-by-time interaction effect from this linear model. The caregivers receiving enhanced treatment averaged 1.05 depression points lower than the caregivers receiving usual care over this time period. While the difference between groups decreased in magnitude as time went on, post hoc analysis of the least squares means indicated that, after baseline differences were controlled for, caregivers in the enhanced treatment group had significantly lower depression scores (p<0.05) than caregivers in the control group through 161 weeks (3.1 years) after enrollment.
The proportion of subjects above the threshold for clinically significant depression remained higher in the control group throughout the 5 years of the analysis. Among caregivers in the enhanced treatment group, 29.8% exceeded this threshold after 1 year of intervention, 26.2% exceeded the threshold after 3 years, and 27.0% exceeded the threshold after 5 years. The corresponding rates for caregivers in the control group were 45.1%, 31.9%, and 30.0% for 1, 3, and 5 years after baseline, respectively.
Additional models were run to examine the effects of caregiver gender and of patient severity of dementia, nursing home placement, and death on depressive symptoms and treatment-related changes. The patient’s Global Deterioration Scale score was added to the growth models as a time-dependent categorical covariate with four levels. Significant main effects were found for dementia severity both for the first year (F=3.69, df=3, 327, p=0.02) and for years 1–5 (F=3.94, df=3, 1548, p=0.009), indicating that caregiver depression was higher when the patient’s dementia was more severe. However, neither severity of dementia nor caregiver gender had a significant interaction with treatment group in either the analysis for the first year or the analysis for years 1–5, indicating that the intervention was equally effective in reducing symptoms of depression across dementia severity level and gender. While the analyses showed that caregiver depressive symptoms decreased significantly after nursing home placement or death of the care recipient (Table 3), the interactions between treatment group and these patient outcomes were not significant, suggesting that the intervention continued to have an impact even after these highly stressful transitional events.
Discussion
The results indicate that the enhanced counseling and support intervention is an effective treatment for caregiver distress. Spouse-caregivers who received this intervention showed fewer depressive symptoms than participants receiving usual care at the 1-year follow-up, and sustained improvements were detectable more than 3 years after enrollment. The effects were replicated across cohort, caregiver gender, dementia severity level, and even nursing home placement or death of the patient.
These intervention effects were detected despite comparison with a control group that was likely to have received much more assistance than the typical care available in community and medical settings. In contrast to longitudinal studies following family caregivers of dementia patients without providing intervention, which have shown stable levels of depressive symptoms over time (3, 4), this study provided benefits to caregivers in the usual care control group. They, as well as caregivers in the enhanced treatment group, showed significant decreases in depression from years 1 through 5, suggesting benefit from study participation. Thus, our results may actually underestimate the full impact of the intervention.
Few caregiver intervention studies have demonstrated effects on symptoms of depression beyond 12 months of follow-up, and we are aware of none that has shown results beyond 18 months. In our study, while no group differences were evident at the first follow-up (4 months after baseline), there were increasing differences apparent at 8 and 12 months, suggesting that the benefit of treatment was fully realized only after the caregivers received all three components of the intervention. The sustained effects demonstrated by the intervention may be due to its flexibility and the opportunity to learn skills or develop psychosocial coping resources useful over the long course of providing care for a patient with Alzheimer’s disease.
Although the present project has attained a large number of subjects, long follow-up, and low rate of attrition that we believe are unique in the caregiving intervention literature, several limitations should be noted. The counselors who provided the enhanced treatment or usual care, and therefore were not blinded to treatment condition, conducted the follow-up interviews. However, the Geriatric Depression Scale consists of yes/no self-report questions, which are less subject to interviewer bias than interviewer rating measures. Previous research has shown that even when interviewers are blinded, 86% can accurately guess which participants have received active psychotherapy in a randomized trial, but only interviewer rating measures (not used as outcomes in our project) are typically affected by interviewer knowledge of group assignment (15). In studies of psychosocial intervention, it is not possible for the subjects themselves to be blinded to treatment condition. However, the subjects in this study completed the Geriatric Depression Scale, a symptom inventory, without reference to the treatment they received. Moreover, the subjects in the usual care group also received a considerable amount of support and counseling. Consequently, the lack of blinding in our study is unlikely to explain the observed differences in scores on the Geriatric Depression Scale. Another limitation is that we have studied the impact of this intervention only on spouses in an urban setting, and our subjects were predominantly Caucasian. Future studies should investigate whether similar interventions are as effective with more diverse groups of caregivers.
In summary, our results suggest that a short course of intensive counseling and readily available supportive maintenance can have long-lasting effects in reducing symptoms of depression among caregivers of dementia patients. Caregivers generally do not have access to such intense, individualized, multifaceted, and carefully planned interventions. In most clinical settings, caregivers may be referred to support groups and advised to use informative self-help materials, such as The 36-Hour Day(16). Our results, and those of previous studies (5), suggest that support and information alone are helpful but are not optimal interventions for caregivers.
The New York University Alzheimer’s Disease Center counseling and support intervention, if widely available, could have a major impact on health care costs, on the emotional distress associated with caregiving, and perhaps on factors related to depressive symptoms, including health, disability, and related health care utilization and costs (17). Since family caregiving affects about 25 million American families (1), providing effective interventions for caregivers should become a high priority. With the increasing emphasis on providing patients with evidence-based treatment, caregivers should have access to interventions that have demonstrated effectiveness.
 |
 |
 |
Received Nov. 25, 2002; revisions received May 6 and Sept. 15, 2003; accepted Sept. 18, 2003. From the Silberstein Aging and Dementia Research Center, New York University School of Medicine; the Department of Biostatistics, School of Public Health, University of Alabama at Birmingham; the Institute on Aging, San Francisco; and the School of Aging Studies, University of South Florida, Tampa. Address reprint requests to Dr. Mittelman, Silberstein Institute for Aging and Dementia, Department of Psychiatry, New York University School of Medicine, 550 First Ave., New York, NY 10016; [email protected] (e-mail). Supported by grants from NIMH (R01 MH-42216) and the National Institute on Aging (R01 AG-14634). Additional resources were provided by the New York University Alzheimer’s Disease Center (supported by grant P30 AG-08051 from the National Institute on Aging). The authors thank the New York University Counseling Team, especially Emma Shulman, C.S.W., and Gertrude Steinberg, M.A., for inspiring the New York University Spouse-Caregiver Intervention Study and Olivio Clay, who assisted with the data analysis.
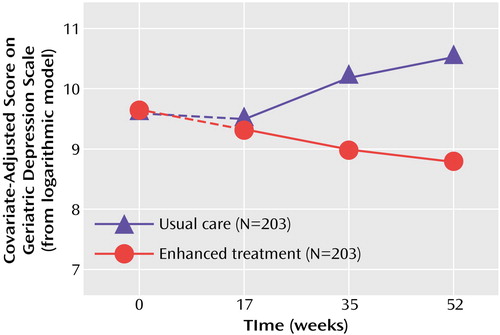
Figure 1. Covariate-Adjusted Depression Scores From Baseline Through Year 1 Among Spouse-Caregivers of Patients With Alzheimer’s Disease Who Received Enhanced Caregiver Treatment or Usual Carea
aEnhanced treatment included individual and family counseling, a regular support group, and access to additional ad hoc counseling. Usual care consisted of information and access to support groups and counseling. The dashed lines represent the baseline covariate adjustment, that is, the equating of groups at baseline, with the actual covariate-adjusted curves beginning at the 4-month assessment point.
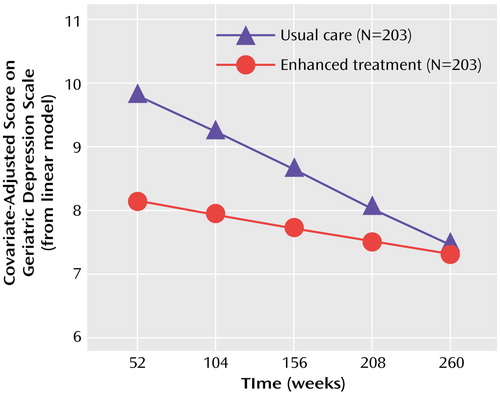
Figure 2. Covariate-Adjusted Depression Scores From Year 1 Through Year 5 Among Spouse-Caregivers of Patients With Alzheimer’s Disease Who Received Enhanced Caregiver Treatment or Usual Carea
aEnhanced treatment included individual and family counseling, a regular support group, and access to additional ad hoc counseling. Usual care consisted of information and access to support groups and counseling.
1. Ory MG, Yee JL, Tennstedt SL, Schulz R: The extent and impact of dementia care: unique challenges experienced by family caregivers, in Handbook on Dementia Caregiving: Evidence-Based Interventions for Family Caregivers. Edited by Schulz R. New York, Springer Publishing, 2000, pp 1–32Google Scholar
2. Schulz R, O’Brien AT, Bookwala J, Fleissner K: Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist 1995; 35:771–791Crossref, Medline, Google Scholar
3. Roth DL, Haley WE, Owen JE, Clay OJ, Goode KT: Latent growth models of the longitudinal effects of dementia caregiving: a comparison of African American and White family caregivers. Psychol Aging 2001; 16:427–436Crossref, Medline, Google Scholar
4. Robinson-Whelen S, Tada Y, MacCallum RC, McGuire L, Kiecolt-Glaser JK: Long-term caregiving: what happens when it ends? J Abnorm Psychol 2001; 110:573–584Crossref, Medline, Google Scholar
5. Sorensen S, Pinquart M, Duberstein P: How effective are interventions with caregivers? an updated meta-analysis. Gerontologist 2002; 42:356–372Crossref, Medline, Google Scholar
6. Mittelman MS, Ferris SH, Shulman E, Steinberg G, Ambinder A, Mackell J, Cohen J: A comprehensive support program: effect on depression in spouse-caregivers of AD patients. Gerontologist 1995; 35:792–802Crossref, Medline, Google Scholar
7. Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B: A family intervention to delay nursing home placement of patients with Alzheimer disease: a randomized controlled trial. JAMA 1996; 276:1725–1731Crossref, Medline, Google Scholar
8. Mittelman MS, Epstein C, Pierzchala A: Counseling the Alzheimer’s Caregiver: A Resource for Health Care Professionals. Chicago, AMA Press, 2002Google Scholar
9. Sheikh JI, Yesavage JA: Geriatric Depression Scale (GDS): recent evidence and development of a shorter version, in Clinical Gerontology: A Guide to Assessment and Intervention. Edited by Brink TL, Brink T. New York, Haworth Press, 1986, pp 165–173Google Scholar
10. Brink TL, Yesavage JA, Owen L, Heersema PH, Adey M, Rose TL: Screening tests for geriatric depression. Clin Gerontol 1982; 1:37–44Crossref, Google Scholar
11. Reisberg B, Ferris SH, De Leon MJ, Crook T: The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139Link, Google Scholar
12. Littell RC, Milliken GA, Stroup WW, Wolfinger RD: SAS System for Mixed Models. Cary, NC, SAS Institute, 1996Google Scholar
13. Francis DJ, Fletcher JM, Stuebing KK, Davidson KC, Thompson NM: Analysis of change: modeling individual growth. J Consult Clin Psychol 1991; 59:27–37Crossref, Medline, Google Scholar
14. Akaike H: A new look at statistical model identification. IEEE Transactions on Automatic Control 1974; 19:716–723Crossref, Google Scholar
15. Carroll KM, Rounsaville BJ, Nich C: Blind man’s bluff: effectiveness and significance of psychotherapy and pharmacotherapy blinding procedures in a clinical trial. J Consult Clin Psychol 1994; 62:276–280Crossref, Medline, Google Scholar
16. Mace NL, Rabins PV: The 36-Hour Day: A Family Guide to Caring for a Person With Alzheimer’s Disease, Related Dementing Illnesses, and Memory Loss in Later Life, 3rd ed. Baltimore, Johns Hopkins University Press, 1999Google Scholar
17. Unutzer J, Patrick DL, Simon G, Grembowski D, Walker E, Rutter C, Katon W: Depressive symptoms and the cost of health services in HMO patients aged 65 years and older: a 4-year prospective study. JAMA 1997; 277:1618–1623Crossref, Medline, Google Scholar



