Transcranial Magnetic Stimulation in the Treatment of Depression
Abstract
OBJECTIVE: Transcranial magnetic stimulation (TMS) is a noninvasive and easily tolerated method of altering cortical physiology. The authors evaluate evidence from the last decade supporting a possible role for TMS in the treatment of depression and explore clinical and technical considerations that might bear on treatment success. METHOD: The authors review English-language controlled studies of nonconvulsive TMS therapy for depression that appeared in the MEDLINE database through early 2002, as well as one study that was in press in 2002 and was published in 2003. In addition, the authors discuss studies that have examined technical, methodological, and clinical treatment parameters of TMS. RESULTS: Most data support an antidepressant effect of high-frequency repetitive TMS administered to the left prefrontal cortex. The absence of psychosis, younger age, and certain brain physiologic markers might predict treatment success. Technical parameters possibly affecting treatment success include intensity and duration of treatment, but these suggestions require systematic testing. CONCLUSIONS: TMS shows promise as a novel antidepressant treatment. Systematic and large-scale studies are needed to identify patient populations most likely to benefit and treatment parameters most likely to produce success. In addition to its potential clinical role, TMS promises to provide insights into the pathophysiology of depression through research designs in which the ability of TMS to alter brain activity is coupled with functional neuroimaging.
In 1831, Michael Faraday discovered that electrical currents can be converted into magnetic fields and vice versa. His principle of mutual induction is the basis of transcranial magnetic stimulation (TMS). In TMS, a bank of capacitors is rapidly discharged into an electric coil to produce a magnetic field pulse (Figure 1). When the coil is placed near the head of a human or animal, the magnetic field penetrates the brain and induces an electric field in the underlying region of the cerebral cortex (4) (Figure 2). An electrical field of sufficient intensity will depolarize cortical neurons, generating action potentials. These then propagate to exert their biological effects. For example, TMS over the left motor cortex causes action potentials that propagate through the corticospinal tract, causing twitches in contralateral skeletal muscles.
This new technology has been used to affect underlying brain tissue at several levels. First, TMS can alter regional activity within the cortex. Positron emission tomography (PET) has revealed changes in cortical metabolism and dose-dependent changes in regional cortical blood flow in response to TMS (5–7). Such changes are also observed at sites distant from the magnetic stimulus, showing that the effects of TMS propagate to other parts of the brain (6, 7). The connections demonstrated in this manner correspond to known neural pathways in nonhuman primates, suggesting that the propagation of TMS effects occurs by means of existing neural networks (8). These distant changes are functionally significant. Wassermann et al. (9) found that TMS to one primary motor cortex reduces the response of the contralateral motor cortex to magnetic stimuli. Similarly, TMS to one brain region can alter neurotransmitter release elsewhere. For example, TMS to the left prefrontal cortex has been shown to increase release of dopamine in the ipsilateral caudate nucleus (10). Last, TMS might directly alter gene expression patterns. A study of TMS-induced activation of c-fos expression in the thalamic paraventricular nucleus of rats found that the expression does not depend on the direction of magnetic stimulus in vivo or on the integrity of neural circuitry in brain slices (11). These findings suggest that magnetic stimulation might alter gene expression directly by a mechanism not dependent on the generation of action potentials.
Why This Review?
The initial observation that TMS can improve depressive symptoms was made serendipitously during a study testing whether TMS might be useful in treating Parkinson’s disease (12, 13). Since then, evidence suggesting that TMS might have antidepressant efficacy has accumulated. These findings have aroused growing interest in what potentially might become a novel treatment modality with rapid onset of action and a more favorable side effect profile than ECT or pharmacotherapy. However, it remains unclear how significant and durable the effects of TMS are. Recent work has implicated several variables, including brain physiology and duration of treatment, as influencing treatment success. In this review, we examine the current state of evidence concerning the use of TMS to treat depression. We review the clinical data and assess which types of patients are most likely to benefit and which technical parameters might bear on treatment success.
Clinical Results
Several early case reports (14) and reports of single pulses or very slow trains of TMS applied to multiple sites (15–17) or at the vertex (18) produced encouraging initial results. In the wake of these studies, more rigorous studies were performed in which sequences of repetitive transcranial magnetic stimulation (rTMS) were applied to one brain region. The studies vary in how they were controlled and how TMS was delivered.
Uncontrolled Studies
High-frequency rTMS
Three studies tested the effects of high-frequency (3–20 Hz) rTMS to the left prefrontal cortex in depressed patients but did not include depressed comparison subjects. All three studies produced positive results. George et al. (19) observed a significant decrease in Hamilton Depression Rating Scale scores (20) in a group of six inpatients with medication-resistant depression, of whom two patients responded robustly. In an open study of patients with Hamilton depression scale scores >20, Figiel et al. (21) observed a decline in scores of more than 60% in 21 of 50 patients after five daily treatments. Similarly, Triggs et al. (22) observed a decrease in Hamilton depression scale scores of ≥50% in five of 10 unmedicated depressed patients after 10 treatments.
Low-frequency rTMS
Initial studies of low-frequency (≤1 Hz) rTMS applied to the right prefrontal cortex were similarly encouraging. Feinsod et al. (23) treated 14 patients, of whom six experienced a >50% decrease in the Hamilton depression scale score after 10 treatments. Menkes et al. (24) treated eight outpatients and found significant decreases in their mean Hamilton depression scale and Beck Depression Inventory (25) scores.
Summary
Several initial studies of fast rTMS to the left prefrontal cortex and of slow rTMS to the right prefrontal cortex showed improvement in depressive symptoms.
Sham-Controlled Studies
Several sham-controlled studies of depression treated with TMS have been published (3, 26–30, 32–35) (Table 1). In order to blind patients to whether they were part of the treatment or control group, several groups conducted trials in which comparison subjects received sham TMS. In sham TMS, the coil is positioned such that less of the magnetic stimulus penetrates the brain. The validity of sham controls and the question of whether they are truly inactive have been debated in the literature and will be addressed in detail later in this section.
Studies with concomitant pharmacotherapy
Most sham-controlled studies of high-frequency rTMS to the left prefrontal cortex have produced positive results. Pascual-Leone et al. (26) conducted a crossover study of patients with relapsing unipolar psychotic depression that was refractory to pharmacotherapy. In this study, rTMS applied to the left prefrontal cortex was compared to rTMS applied to other locations as well as to sham TMS. Patients were given five daily treatments and then evaluated over the next 4 weeks. During the first 2 weeks, rTMS to the left prefrontal cortex produced significantly better results than both rTMS to other locations and sham TMS. However, the benefit became insignificant by the 3rd week. George et al. (27) conducted a sham-controlled crossover study involving 12 outpatients with unipolar depression or bipolar II depression. In this study, a course of 10 daily rTMS treatments was associated with a significantly larger decrease in the mean Hamilton depression scale score, compared with sham rTMS. Eschweiler et al. (28) conducted a crossover study comparing rTMS administered over 5 days with sham rTMS. Four of 12 patients responded to the real rTMS phase of the study with a decrease in their Hamilton depression scale score of ≥30%, while only one of 10 patients responded to the sham rTMS. However, by 2 weeks there was no significant difference between the groups. A study by García-Toro et al. (29) included patients with major depression for whom two courses of pharmacotherapy had failed. The patients were randomly assigned to either rTMS or sham treatment. Although the treatment group had a significantly greater drop in their mean Hamilton depression scale score than the comparison subjects, only five of the 17 patients in the treatment group experienced remission, which was defined as a ≥50% decrease in the Hamilton depression scale score. In a later open phase the authors found better results with increased treatment duration and stimulus intensity. In a crossover study conducted by Loo et al. (30), 18 patients experiencing major depressive episodes were assigned for the first 2 weeks to either sham or rTMS groups. In contrast to other researchers, Loo et al. did not find a significant difference between rTMS and sham treatment. However, both the sham and the rTMS groups experienced significant reductions in scores on two depression rating scales, suggesting that the sham treatment in this study might have been active (see the later discussion). Similarly, in a study of patients beginning antidepressant therapy with sertraline, García-Toro et al. (32) did not find any added benefit of rTMS over sham treatment.
Authors’ critique of studies with concomitant pharmacotherapy
The design of the study by Pascual-Leone et al. (26) elegantly demonstrated that, of the anatomical sites tested, the left prefrontal cortex was associated with the most effective high-frequency rTMS results. However, the applicability of the findings to other groups of psychotically depressed patients is problematic. Most patients in the study by Pascual-Leone et al. were maintained in an ambulatory setting, and some were without other treatment, suggesting a milder illness than is often seen in psychotic depression. Eschweiler’s group (28) also used a crossover design and a short treatment course. The transient nature of the benefit patients experienced in both studies might be related to the short course of treatment. The study of Eschweiler et al. (28) especially and that of George et al. (27) are subject to Sackeim’s criticism (40) that success has been defined too weakly, as a modest decrease in rating scale score rather than as remission. Of the two studies that showed no added benefit from rTMS, García-Toro’s add-on study (29), which found that concomitant rTMS failed to augment newly initiated sertraline treatment, is more convincing because the kind of sham used in the study was less likely to be active (see later discussion).
Studies without concomitant pharmacotherapy
Sham-controlled studies have examined the effects of high-frequency rTMS to the left prefrontal cortex as a stand-alone treatment for depression in patients who were not receiving antidepressant medications. A study by Berman et al. (33) included 20 patients with a major depressive episode that was resistant to pharmacotherapy. Among the 10 patients who received 10 rTMS treatments over 2 weeks, one patient responded fully and three patients partly responded, compared with no responders in the sham group. Similarly, George et al. (34) treated nonpsychotic depressed outpatients with rTMS at 5 Hz, rTMS at 20 Hz, or sham rTMS. A decrease in Hamilton depression scale score of ≥50% was observed in nine of 20 (45%) rTMS patients. (The 5-Hz group did twice as well as the 20-Hz group, although the difference was not statistically significant.) None of the sham-treated patients responded.
Authors’ critique of studies without concomitant pharmacotherapy
Studies of rTMS involving patients who are not treated with concomitant pharmacotherapy are especially important for determining whether rTMS is an adequate stand-alone therapy. These two similarly designed studies produced very different degrees of treatment success. Three differences between them might account for the discrepancy and suggest parameters that merit further investigation. First, Berman et al. (33) recruited only patients for whom at least one drug trial had failed. Pharmacological treatment resistance might bear on responsiveness to rTMS. Second, Berman et al. used an 80% motor threshold (MT) stimulus, compared with a 100% MT stimulus in the study by George et al. (34). Last, some of the patients in the latter study received 5-Hz rTMS rather than 20-Hz rTMS. Perhaps the intensity and/or frequency of stimulation are relevant parameters.
Studies of slow rTMS
Low-frequency (≤1 Hz) rTMS has also been examined in a few sham-controlled studies. Padberg et al. (35) conducted a three-armed study of 18 patients comparing the effects of high-frequency, low-frequency, and sham rTMS to the left prefrontal cortex on pharmacotherapy-resistant major depression. They found a significant decrease in the mean Hamilton depression scale score only in the low-frequency (0.3 Hz) group after 5 days of daily treatment. Klein et al. (3) reported a double-blind, sham-controlled study of inpatients with medication-resistant major depression. Seventeen of 35 patients (49%) treated with low-frequency rTMS to the right prefrontal cortex experienced a ≥50% decrease in scores on a depression rating scale after 10 treatments. These results were significantly better than those for the sham group, in which only eight of 32 patients (25%) responded.
Authors’ critique of studies of slow rTMS
Low-frequency rTMS has been less well studied than higher-frequency treatment. In principle, the two types of treatment can be expected to have opposite physiological effects (see later discussion). In this light, the findings of the small study of Padberg et al. (35), which showed that left-sided rTMS at 0.3 Hz provided a greater, albeit mild, improvement than 10 Hz, are surprising. A larger number of subjects and a longer treatment course would provide more convincing evidence. The study by Klein et al. (3) examining the effects of rTMS to the right side, on the other hand, with its larger number of subjects and more rigorous definition of success, suggested that slow rTMS to the right prefrontal cortex might be effective and deserves more study. Theoretical considerations of asymmetric alterations of brain function in depression (41–44) also support further studies of this type.
Summary
Most, but not all, sham-controlled studies of fast rTMS and all sham-controlled studies of slow rTMS show rTMS to be superior to sham treatment.
ECT-Controlled Studies
Four studies have compared high-frequency rTMS to the left prefrontal cortex with ECT (36–39) (Table 1). These studies are particularly important because if rTMS, with its benign side effect profile, is as effective as ECT even for only a subset of patients, then it might provide a safer and more tolerable alternative. Our group has studied patients with major depression who were not taking antidepressant medication and who were randomly assigned to receive either rTMS for 20 treatment days or to receive ECT. We found that 16 (80%) of 20 patients responded to ECT but that only nine (45%) of 20 responded fully to rTMS. However, when the data were stratified by the presence or absence of psychotic features, a different picture emerged. For psychotic patients, ECT was clearly superior to rTMS, with 10 of 10 patients responding to ECT but only two of nine responding to rTMS. But among nonpsychotic patients, both treatments met with similar success rates, with six (60%) of 10 patients responding to ECT and seven (64%) of 11 responding to rTMS (36). In a follow-up study (37), only patients with nonpsychotic major depression for whom 4 weeks of antidepressant pharmacotherapy had failed were included. In the ECT group, 60% of patients responded, compared with 55% in the rTMS group (37). Pridmore et al. (38) randomly assigned 32 patients with treatment-resistant depression to either ECT or rTMS. The duration of rTMS treatment was tailored for each patient. Two assays were performed. In the first, patients were considered responders if they achieved a Hamilton depression scale score ≤8; 69% of patients from each group were responders by this criterion. However, in the second assay, only 43% of the rTMS patients achieved a Beck Depression Inventory (25) score of ≤15, compared with 75% of the ECT patients. A recent study by Janicak et al. (39) of severely depressed patients who were not taking antidepressant medications produced similar results. Using conditions designed to optimize both treatment arms and a rigorous definition of treatment response, they found similar remission rates for patients receiving rTMS (46%) and those receiving ECT (56%). Their study included too few psychotic patients to stratify the data.
A significant limitation of the studies reviewed in this section is the lack of long-term follow-up. They leave open the question of how durable the antidepressant effects of rTMS are over time. Our group recently addressed this issue. We followed a group of patients randomly assigned to receive ECT or rTMS at 3- and 6-month time points. We found no significant difference in the relapse rate between the two groups (45). If this promising result is replicated, rTMS might prove to provide a durable benefit similar to that of ECT.
Authors’ critique
We believe that our studies (36, 37) and that of Janicak et al. (39), in which rTMS was used for patients who were not taking antidepressant medication, should stand along with those of Berman’s (33) and George’s (34) groups described earlier in bolstering the case for rTMS as a stand-alone therapy. One feature that sets these studies and that of Pridmore et al. (38) apart is the long treatment course. Pridmore et al. observed visual analog scale scores over the first 15 treatment days and found a trend toward continuing improvement, providing evidence that prolonged courses of rTMS provide accumulating benefit. This trend was found even though the patients in their study had medication-resistant depression and represented a population that previous studies have found to be difficult to treat with rTMS. It is also noteworthy that the studies described in this section applied a rigorous standard of treatment success and still found that rTMS produced results comparable to ECT, at least for some patients.
Summary
Studies comparing long courses of high-frequency rTMS to ECT show comparable results in certain patient populations.
Patient Parameters
One of the striking differences between the published studies is the range of effectiveness observed. Some studies demonstrated significant antidepressant effects, while others showed only a mild effect or none at all. What factors account for the disparity of results? The published literature points to several clinical parameters that might affect treatment success.
Psychotic Features
Pascual-Leone et al. (26) and our group have studied patients with psychotic depression. Pascual-Leone et al. described improvement in scores on two depression rating scales after rTMS, although it is unclear whether the patients in their study were experiencing psychosis at the time they were treated. In contrast, our study (36) compared depressed patients with and without psychosis at the time of treatment. Our results suggested that the absence of psychosis might be a predictor of treatment success, even when success is defined by a rigorous standard. At this point it is not clear whether psychotic depression represents an illness that responds poorly to rTMS in general or whether different TMS parameters, possibly longer or more intense treatment, are required for patients with psychotic depression to respond.
Age
Figiel et al. (21) and Kozel et al. (46) both observed that older patients respond less well to rTMS. In older patients, onset of depression after age 65 was also associated with less response to treatment (21). Similarly, in a sham-controlled study of a brief, low-intensity course of fast rTMS in older patients, Manes et al. (47) found little response and no difference between the treatment and comparison groups. However, this result might be an artifact of insufficient treatment parameters. This conclusion is supported by Janicak et al. (39), who, in a trial of high-intensity and longer rTMS treatments, found a correlation between age and the number of treatments required to achieve a clinical response.
Previous Response to rTMS
Preliminary evidence suggests that clinical response to rTMS might itself be a predictor of the success of future treatment. In a small trial, we have shown that rTMS produces significant clinical improvement in patients with major depression who had previously responded to rTMS but had since relapsed (48). Conversely, two studies of rTMS patients who were later treated with ECT suggested that rTMS nonresponders might be less likely to respond to ECT (49, 50). Whether rTMS nonresponse defines a group of patients with treatment-resistant depression or whether rTMS treatment itself affects the success of subsequent therapies is not known.
Underlying Brain Physiology
Teneback et al. (51) used single photon emission computed tomography to compare regional brain activity in depressed patients before and after treatment with high-frequency rTMS. They found that baseline activity in the inferior frontal lobe was higher in patients who went on to respond to rTMS than in those who did not. Thus, activity in that region might be a predictor of success for high-frequency rTMS. Kimbrell et al. (52) used PET analysis to measure cerebral glucose metabolism in depressed patients undergoing TMS to the left prefrontal cortex. They found that hypometabolism in the cerebellum, both temporal lobes, and the occipital and anterior cingulate regions was associated with a positive response to 20-Hz treatment, but that hypermetabolism was associated with improvement with 1-Hz treatment.
Summary
Greater responsiveness to rTMS may be predicted by several patient factors, including the absence of psychosis, younger age, previous response to rTMS therapy, and certain brain physiologic markers.
Technical Parameters
At this time it is not known what technical parameters produce the most clinical benefit. As a result, studies differ from each other in a number of technical respects. Most investigators have used high-frequency rTMS directed to the left prefrontal cortex, but not all. In addition to differences in the frequency and location of stimulation, studies differ in pulse intensity and the duration of treatment (Table 2).
Pulse Frequency—Slow Versus Fast rTMS
The frequency at which the magnetic field oscillates during magnetic stimulation differs between studies. Although different studies have used various frequencies, two overall types have been used: low frequency (≤1 Hz) and high frequency (3–20 Hz). This distinction is important because the two types of stimulation have opposite effects on brain physiology.
Pascual-Leone et al. (53) showed that high-frequency (≥3 Hz) rTMS, when applied to the motor cortex, generates motor evoked potentials of progressively increasing amplitude. This increase in cortical excitability varies with rTMS frequency and intensity (53) and has been correlated with increased regional cerebral blood flow (54). Excitability can spread within the brain even to the extent of causing a generalized seizure (53). Conversely, low-frequency rTMS (≤1 Hz) has been shown to decrease both cortical excitability (55, 56) and regional blood flow (9, 54). This inhibition can propagate by means of neural pathways to other areas (9). These findings mean that rTMS frequency may be adjusted as desired to provoke either an increase or a decrease in cortical excitability and metabolism.
This frequency dependence might be clinically significant. Most studies have reported clinical success when applying excitatory stimuli to the left prefrontal cortex of depressed patients (19, 21, 22, 26–29, 33, 34, 36–39). A few have reported success when applying inhibitory stimuli to the opposite side (3, 23, 34). Speer et al. (54) and Kimbrell et al. (52) compared the effects of excitatory and inhibitory stimulation to the left prefrontal cortex with two interesting results. First, depressed patients who responded well to one frequency tended to have an opposite response to the other frequency. Second, the type of stimulation that led to clinical improvement varied with underlying brain physiology. Patients with global cerebral hypometabolism responded better to excitatory treatment, while hypermetabolism was associated with response to inhibitory TMS (52).
These results suggest several possibilities about the pathophysiology of depression. One is that there might be subpopulations of depressed patients with different underlying abnormalities of cerebral function, some with increased and others with decreased metabolism (52). Another is that TMS affects a lateralized element of mood control. Observations of decreased glucose metabolism (57) and excitability (58) and localized areas of reduced volume (44) in the left cortex as well as data from stroke victims (19, 59) have suggested a relative hypofunctioning of the left frontal lobe in depression (24, 41). If this were the case in many but not all patients, it would help explain why excitatory stimuli to the left side and inhibitory ones to the right have generally been successful treatment strategies. A reversed pattern of lateralization in a subset of patients could explain why some patients paradoxically do better with an inhibitory stimulus to the left prefrontal cortex and worse with an excitatory one (52, 54). The success of Grisaru’s group in treating mania with high-frequency rTMS to the right prefrontal cortex complements this model (60).
Course Duration, Pulse Intensity, and Quantity of Pulses
Systematic analysis of course duration, pulse intensity, and quantity of pulses has not been performed, and the current data do not allow each factor to be analyzed in isolation from other variables. However, differences in these parameters between studies point to their possible importance in treatment effectiveness. A crude analysis summarized in Figure 3 suggests that studies in which rTMS was given for 10 treatment days (27, 29, 32–34) had fewer treatment successes than studies in which more than 10 days of treatment were given (36–39). This result is consistent with the finding of Pridmore et al. (38), who, in following visual analog scale scores through the course of treatment, found progressive benefit even on later treatment days. A similar analysis suggested that studies in which more intense magnetic pulses (100%–110% of motor threshold) were given (34, 38, 39) had better results than those in which less intense pulses (80%–90% of motor threshold) were given (27, 29, 32, 33, 36, 37) (Figure 3). Finally studies in which more pulses per day (1200–1600 pulses per day) were given (29, 32, 34, 37, 38) had better results than those in which fewer pulses per day (800–1000 pulses per day) were given (27, 33, 39) (Figure 3). It should be noted that there was overlap in these variables between the studies that were analyzed and that the available data do not allow a true factor analysis.
Coil Placement
Most investigators target rTMS to the dorsolateral prefrontal cortex. To do this, most use magnetic stimuli to identify the motor cortex and then move the coil 5 cm rostrally. A study using MRI-based neuronavigation showed this method to be anatomically unreliable most of the time (61). Commonly used figure-eight coils are particularly sensitive to precise navigation, as the intensity of the magnetic field drops off sharply with the distance from the center of the field (1, 2) (Figure 1). Methods to accurately target TMS on the basis of mapping of brain anatomy by MRI have been described (62). It will be useful to test whether anatomical accuracy enhances clinical efficacy.
Coil-Brain Distance
Since magnetic fields weaken with distance, investigators have asked whether the coil-to-cortex distance is clinically relevant. Increased distance to the cortex raises the motor threshold in both depressed (46) and healthy (13) individuals. Also, the distance to the prefrontal cortex is greater than that to the motor cortex and tends to increase with age (46). Kozel et al. (46) did not observe a correlation between distance to the prefrontal cortex and clinical response. However, they did detect a maximum combined threshold of age and distance to the prefrontal cortex above which subjects did not respond to rTMS. The related parameter of frontal lobe volume has also been positively correlated with treatment response in older patients (47).
Sham TMS as a Control
During TMS, patients feel stimulation of scalp nerves and muscles and hear an acoustic artifact. An ideal sham control would simulate this subjective experience without any physiologic effect on the brain. The sham treatments in controlled studies involve discharging the coil at an angle to the head with only one edge in contact with the scalp as opposed to holding it tangential to the scalp as in real rTMS. After Loo et al. (30) published a study in which both real rTMS and a sham treatment had antidepressant efficacy, the question of which sham geometries were more likely to be active and which more closely approximated the ideal control condition received critical attention.
Loo et al. (63) examined variations of the common sham practice of holding a figure-eight coil with one edge touching the scalp at a 45° angle to the head. They found that sham variants that more closely simulated the experience of TMS also generated more motor evoked potentials, although less than real treatment. Lisanby et al. (64) measured the activity of 45° and 90° sham variants using both an assay of motor evoked potentials in human volunteers and direct voltage measurements in monkeys. They found that the 45° sham variant in which both wings of the coil were in contact with the scalp, such as was used by Loo et al. (30) in their clinical study, reduced the induced voltage in the brain by only 24%. However, a 45° sham with one wing touching, a 90° sham with one wing touching, and 90° sham with both wings touching all reduced the induced voltage by 67%–73%. Furthermore, neither 90° sham variant generated motor evoked potentials even at maximum stimulator output (64).
Among the studies reviewed here, five (3, 28, 29, 32, 35) used a 90° sham. Most other sham-controlled experiments (26, 27, 30, 34) used a 45° sham condition. Despite the problematic nature of the control conditions, Loo et al. (30) and García-Toro et al. (32) were the only groups to find no difference between sham and real TMS. A crude analysis that aggregated sham-controlled trials in which remission was rigorously defined (Hamilton depression scale score decrease of 50% or score ≤8) shows that active treatment is significantly more effective than sham. In these studies, 20 (29%) of 70 patients had remission of depression after rTMS treatment, while only four (7%) of 61 experienced remission with sham treatment, a significant difference (χ2=10.6, df=1, p≤0.001). This analysis, although potentially subject to a reporting bias, suggests that sham treatment is at worst only partly active and that results from sham-controlled trials are probably meaningful.
Summary
| 1. | High-frequency rTMS increases cortical excitability and metabolism, while low-frequency stimulation does the opposite. Current data support antidepressant effects of excitatory stimulation to the left prefrontal cortex and possibly inhibition of the right prefrontal cortex, although some patients respond paradoxically. | ||||
| 2. | Longer treatment courses and higher-intensity pulses may be more effective. | ||||
| 3. | Some studies may be complicated by active sham controls. | ||||
| 4. | Conclusions about the importance of anatomically accurate coil placement and the distance from the coil to the brain await further investigation. | ||||
Conclusions
Early studies demonstrated that short courses of rTMS produced modest benefit in the mean Hamilton depression scale scores of groups of patients, although significant remission of depression in individual patients was rare. However, refinements in TMS methods have led to improvement on these initial results. In the studies reviewed here that measured clinical remission of depressive symptoms, 41% of 139 patients treated with high-frequency rTMS to the left prefrontal cortex achieved either a 50% decrease in their Hamilton depression scale scores or a final score of ≤8. Recent studies have pointed to, but not yet proven, longer treatment courses, more magnetic pulses, and increased field intensity as likely contributors to treatment success, even when rTMS is the only antidepressant therapy, and have produced results with rTMS that are comparable to those of ECT. Initial work has also identified several potentially important clinical factors that might enhance treatment success, including the absence of psychosis, younger age, and previous TMS responsiveness. Interpretation of the data has been confounded by the possibility that sham TMS, which was intended to be a placebo, might be partially active. However, sham conditions that minimize physiologic effects have been described. In most studies, and when the aggregate data are tested, real rTMS is superior to sham controls.
In addition to developing TMS as a clinical tool, recent work has advanced the understanding of the physiologic effects of TMS and has provided clues to the pathophysiology of depression. Findings supporting the clinical efficacy of excitatory rTMS to the left prefrontal cortex and, although less well studied, inhibitory rTMS to the right prefrontal cortex have provided a functional correlate to data from imaging and lesion studies suggesting that lateralized alterations in brain activity might play a role in depressive symptoms. Furthermore, evidence linking regional brain activity to treatment responsiveness and the paradoxical response of some patients have allowed Kimbrell’s group (52) to identify two metabolically distinct populations that have different responses to excitatory and inhibitory treatment frequencies.
Given the encouraging results, it is time to ask what is necessary to transform this promising experimental treatment into part of our clinical armamentarium. We believe that there are two urgent priorities. The first is systematic investigation of the parameters of treatment. Treatment intensity, duration, and the number of magnetic pulses are all easily tested parameters that need to be analyzed separately. Neuronavigational techniques promise to allow more precise application of TMS. However, it is not known whether these techniques will improve clinical efficacy, although they would certainly increase the cost of TMS. Patient parameters, both clinical and physiologic, are even less well explored and deserve systematic investigation as well.
Another priority is larger-scale studies whose outcome measure is clinical remission. Large, multicenter studies or smaller studies that are sufficiently similar to permit meta-analysis could prove (or disprove) the clinical efficacy of TMS. If data from such studies support the clinical value of TMS, it would then be possible to define a clinical role for TMS and to address the issues of whether TMS is most useful as an adjunct or stand-alone therapy, whether it is as effective as current first-line therapies, and whether maintenance TMS is beneficial.
In addition to contributing to the clinical development of TMS, future studies promise to help elucidate the neuroanatomical correlates of disease and recovery in this as yet poorly understood illness. The coupling of TMS with functional neuroimaging is already providing an opportunity for structure-function analysis of depression. Further structure-function studies might elucidate the neuroanatomical basis of depression, including the roles of specific neural pathways and of brain lateralization.
 |
 |
Received May 3, 2002; revision received Sept. 4, 2002; accepted Sept. 17, 2002. From the Psychiatry Division, Chaim Sheba Medical Center; and the Department of Psychiatry, Sackler Medical School, Tel Aviv University, Tel Aviv, Israel. Address reprint requests to Prof. Grunhaus, Psychiatry Department C, Chaim Sheba Medical Center, Tel Hashomer, Israel; [email protected] (e-mail). The authors thank Dana Polak for assistance with statistical analyses and Douglas G. Adler, M.D., and Elliot S. Gershon, M.D., for comments on the manuscript.
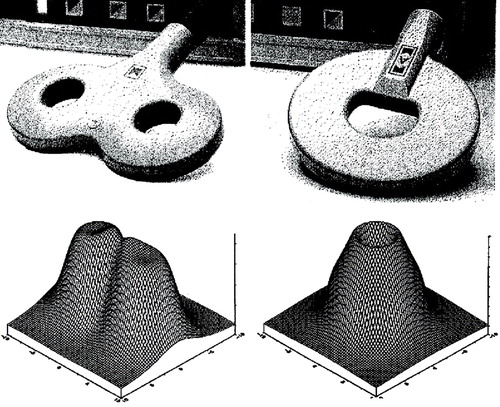
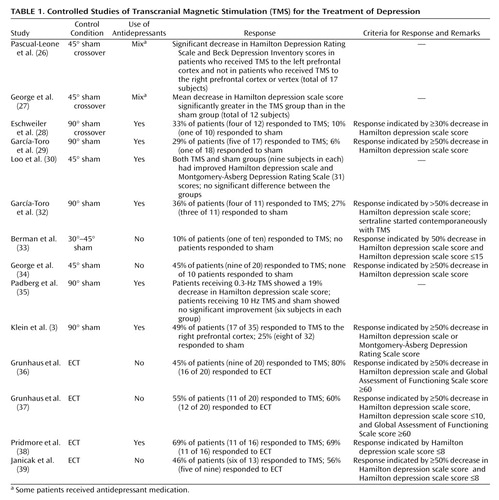
Figure 1. Two Types of Transcranial Magnetic Stimulation (TMS) Coils and Representations of the Magnetic Fields They Generatea
aOn the left is a figure-eight coil similar to those used in most clinical TMS studies. Note that the intensity of the magnetic field drops off sharply with the distance from the center of the field (1, 2). Circular coils, such as the one shown on the right, have been used in a few studies and generate a diffuse magnetic field over a relatively large area of cortex (3). (Figure reproduced with the permission of Magstim Company, Whitland, U.K.)
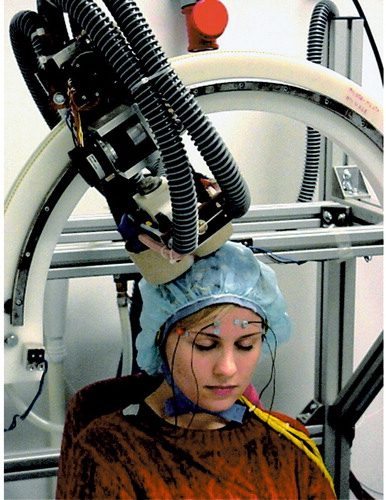
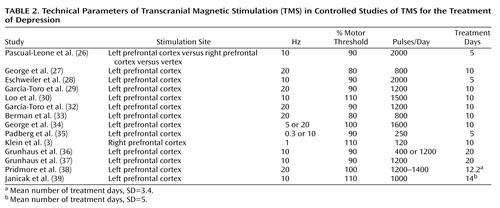
Figure 2. A Subject Undergoing Transcranial Magnetic Stimulation (TMS) a
aMost investigators perform TMS with the operator holding the coil flat over the target brain region. In this illustration, the coil is mounted and the electrodes are in place to allow continuous EEG monitoring. Mounted coils and head supports might play a role in future strategies for anatomically precise stimulation. (Photograph reproduced with the permission of Dubravko Kicic, BioMag Laboratory, Helsinki University Central Hospital, Finland.)
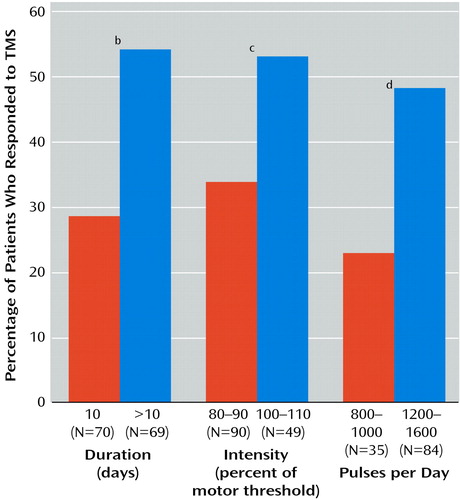
Figure 3. Number of Patients Who Responded to Transcranial Magnetic Stimulation (TMS) in Controlled Studies of TMS for the Treatment of Depression, by Technical Parameters of TMSa
aCumulative results of controlled studies of TMS targeting the left prefrontal cortex in which treatment response was rigorously defined (decrease in Hamilton Depression Rating Scale score of 50% or Hamilton Depression Rating Scale score ≤8) are represented.
bSignificant difference in response rate by duration of treatment (χ 2=9.0, df=1, p<0.01).
cSignificant difference in response rate by intensity of stimulation (χ 2=4.5, df=1, p<0.05).
dSignificant difference in response rate by number of pulses per day (χ2=6.2, df=1, p<0.05).
1. Bohning DE: Introduction and overview of TMS physics, in Transcranial Magnetic Stimulation in Neuropsychiatry. Edited by George MS, Belmaker RH. Washington, DC, American Psychiatric Publishing, 2000, pp 13-44Google Scholar
2. Cohen LG, Roth BJ, Nilsson J, Nguyet D, Panizza M, Bandinelli S, Friauf W, Hallett M: Effects of coil design on delivery of focal magnetic stimulation: technical considerations. Electroencephalogr Clin Neurophysiol 1990; 75:350-357Crossref, Medline, Google Scholar
3. Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Ben-Shachar D, Feinsod M: Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry 1999; 56:315-320Crossref, Medline, Google Scholar
4. Barker AT, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex. Lancet 1985; 1:1106-1107Crossref, Medline, Google Scholar
5. Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC: Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol 1998; 79:1102-1107Crossref, Medline, Google Scholar
6. Wassermann EM, Kimbrell TA: Local and distant changes in cerebral glucose metabolism during repetitive transcranial magnetic stimulation (rTMS) (abstract). Neurology 1997; 48:A107-A108Google Scholar
7. Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P: Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 1997; 8:2787-2791Crossref, Medline, Google Scholar
8. Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC: Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 1997; 17:3178-3184Crossref, Medline, Google Scholar
9. Wassermann EM, Wedegaertner FR, Ziemann U, George MS, Chen R: Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett 1998; 250:141-144Crossref, Medline, Google Scholar
10. Strafella AP, Paus T, Barrett J, Dagher A: Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001; 21:RC157Google Scholar
11. Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, Rupp F: Repetitive transcranial magnetic stimulation activates specific regions in rat brain. Proc Natl Acad Sci USA 1998; 95:15635-15640Crossref, Medline, Google Scholar
12. Belmaker RH, Einat H, Levkovitz Y, Segal M, Grisaru N: TMS effects in animal models of depression and mania: implications of hippocampal electrophysiology, in Transcranial Magnetic Stimulation in Neuropsychiatry. Edited by George MS, Belmaker RH. Washington, DC, American Psychiatric Publishing, 2000, pp 99-114Google Scholar
13. McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, George MS: The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry 2001; 49:454-459Crossref, Medline, Google Scholar
14. Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller HJ: Application of transcranial magnetic stimulation in treatment of drug-resistant major depression: a report of two cases. Hum Psychopharmacol 1993; 8:361-365Crossref, Google Scholar
15. Geller V, Grisaru N, Abarbanel JM, Lemberg T, Belmaker RH: Slow magnetic stimulation of prefrontal cortex in depression and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:105-110Crossref, Medline, Google Scholar
16. Conca A, Swoboda E, König P, Koppi S, Beraus W, Künz A, Fritzsche H, Weiss P: Clinical impacts of single transcranial magnetic stimulation (sTMS) as an add-on therapy in severely depressed patients under SSRI treatment. Hum Psychopharmacol Clin Exp 2000; 15:429-438Crossref, Medline, Google Scholar
17. Conca A, Koppi S, Konig P, Swoboda E, Krecke N: Transcranial magnetic stimulation: a novel antidepressive strategy? Neuropsychobiology 1996; 34:204-207Crossref, Medline, Google Scholar
18. Kolbinger HM, Hoflich G, Hufnagel A, Moller H-J, Kasper S: Transcranial magnetic stimulation (TMS) in the treatment of major depression: a pilot study. Hum Psychopharmacol 1995; 10:305-310Crossref, Google Scholar
19. George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853-1856Crossref, Medline, Google Scholar
20. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
21. Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, Glover S: The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci 1998; 10:20-25Crossref, Medline, Google Scholar
22. Triggs WJ, McCoy KJ, Greer R, Rossi F, Bowers D, Kortenkamp S, Nadeau SE, Heilman KM, Goodman WK: Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry 1999; 45:1440-1446Crossref, Medline, Google Scholar
23. Feinsod M, Kreinin B, Chistyakov A, Klein E: Preliminary evidence for a beneficial effect of low-frequency, repetitive transcranial magnetic stimulation in patients with major depression and schizophrenia. Depress Anxiety 1998; 7:65-68Crossref, Medline, Google Scholar
24. Menkes DL, Bodnar P, Ballesteros RA, Swenson MR: Right frontal lobe slow frequency repetitive transcranial magnetic stimulation (SF r-TMS) is an effective treatment for depression: a case-control pilot study of safety and efficacy. J Neurol Neurosurg Psychiatry 1999; 67:113-115Crossref, Medline, Google Scholar
25. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561-571Crossref, Medline, Google Scholar
26. Pascual-Leone A, Rubio B, Pallardo F, Catala MD: Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233-237Crossref, Medline, Google Scholar
27. George MS, Wasserman EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM: Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752-1756Link, Google Scholar
28. Eschweiler GW, Wegerer C, Schlotter W, Spandl C, Stevens A, Bartels M, Buchkremer G: Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res 2000; 99:161-172Crossref, Medline, Google Scholar
29. García-Toro M, Mayol A, Arnillas H, Capllonch I, Ibarra O, Crespí M, Micó J, Lafau O, Lafuente L: Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J Affect Disord 2001; 64:271-275Crossref, Medline, Google Scholar
30. Loo C, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S: Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry 1999; 156:946-948Link, Google Scholar
31. Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382-389Crossref, Medline, Google Scholar
32. García-Toro M, Pascual-Leone A, Romera M, González A, Micó J, Ibarra O, Arnillas H, Capllonch I, Mayol A, Tormos JM: Prefrontal repetitive transcranial magnetic stimulation as add on treatment in depression. J Neurol Neurosurg Psychiatry 2001; 71:546-548Crossref, Medline, Google Scholar
33. Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, Charney DS, Boutros NN: A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000; 47:332-337Crossref, Medline, Google Scholar
34. George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li X, Arana GW, Risch SC, Ballenger JC: A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000; 48:962-970Crossref, Medline, Google Scholar
35. Padberg F, Zwanzger P, Thoma H, Kathmann N, Haag C, Greenberg BD, Hampel H, Moller HJ: Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res 1999; 88:163-171Crossref, Medline, Google Scholar
36. Grunhaus L, Dannon PN, Schreiber S, Dolberg OH, Amiaz R, Ziv R, Lefkifker E: Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000; 47:314-324Crossref, Medline, Google Scholar
37. Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN: A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003; 53:324-331Crossref, Medline, Google Scholar
38. Pridmore S, Bruno R, Turnier-Shea Y, Reid P, Rybak M: Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol 2000; 3:129-134Crossref, Medline, Google Scholar
39. Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J, Strong MJ, Sharma R, Rosen C, Viana M: Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002; 51:659-667Crossref, Medline, Google Scholar
40. Sackeim HA: Repetitive transcranial magnetic stimulation: what are the next steps? Biol Psychiatry 2000; 48:959-961Crossref, Medline, Google Scholar
41. George MS, Ketter TA, Post RM: Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59-72Crossref, Google Scholar
42. Soares JC, Mann JJ: The functional neuroanatomy of mood disorders. J Psychiatr Res 1997; 31:393-432Crossref, Medline, Google Scholar
43. Cummings JL: The neuroanatomy of depression. J Clin Psychiatry 1993; 55(Nov suppl):14-20Google Scholar
44. Drevets WC: Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813-829Crossref, Medline, Google Scholar
45. Dannon PN, Dolberg OT, Schreiber S, Grunhaus L: Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002; 51:687-690Crossref, Medline, Google Scholar
46. Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, Risch SC, George MS: How coil-cortex distance relates to age, motor threshold, and antidepressive response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000; 12:376-384Crossref, Medline, Google Scholar
47. Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S, Robinson RG: A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr 2001; 13:225-231Crossref, Medline, Google Scholar
48. Dannon PN, Schreiber S, Dolberg OT, Shemer L, Grunhaus L: Transcranial magnetic stimulation is effective in the treatment of relapse depression. Int J Psychiatry Clin Pract 2000; 4:223-226Crossref, Medline, Google Scholar
49. Eschweiler GW, Plewnia C, Batra A, Bartels M: Does clinical response to repetitive prefrontal transcranial magnetic stimulation (rTMS) predict response to electroconvulsive therapy (ECT) in cases of major depression? Can J Psychiatry 2000; 45:845-846Medline, Google Scholar
50. Dannon PN, Grunhaus L: Effect of electroconvulsive therapy in repetitive transcranial magnetic stimulation non-responder MDD patients: a preliminary study. Int J Neuropsychopharmacol 2001; 4:265-268Crossref, Medline, Google Scholar
51. Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings ME, Spicer KM, Risch SC, George MS: Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci 1999; 11:426-435Medline, Google Scholar
52. Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wasserman EM, Repella JD, Danielson AL, Willis MW, Benson BE, Speer AM, Osuch E, George MS, Post RM: Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry 1999; 46:1603-1613Crossref, Medline, Google Scholar
53. Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M: Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994; 117:847-858Crossref, Medline, Google Scholar
54. Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, Post RM: Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000; 48:1133-1141Crossref, Medline, Google Scholar
55. Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG: Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997; 48:1398-1403Crossref, Medline, Google Scholar
56. Wasserman EM, Grafman J, Berry C, Hollnagel C, Wild K, Clark K, Hallett M: Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr Clin Neurophysiol 1996; 101:412-417Crossref, Medline, Google Scholar
57. Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E: The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport 1998; 9:3253-3258Crossref, Medline, Google Scholar
58. Maeda F, Keenan JP, Pascual-Leone A: Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry 2000; 177:169-173Crossref, Medline, Google Scholar
59. Morris PL, Robinson RG, Raphael B, Hopwood MJ: Lesion location and poststroke depression. J Neuropsychiatry Clin Neurosci 1996; 8:399-403Crossref, Medline, Google Scholar
60. Grisaru N, Chudakov B, Yaroslavsky Y, Belmaker RH: Transcranial magnetic stimulation in mania: a controlled study. Am J Psychiatry 1998; 155:1608-1610Link, Google Scholar
61. Herwig U, Padberg F, Unger J, Spitzer M, Schönfeldt-Lecouna C: Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry 2001; 50:58-61Crossref, Medline, Google Scholar
62. Herwig U, Schonfeldt-Lecuona C, Wunderlich AP, von Tiesenhausen C, Thielscher A, Walter H, Spitzer M: The navigation of transcranial magnetic stimulation. Psychiatry Res 2001; 108:123-131Crossref, Medline, Google Scholar
63. Loo CK, Taylor JL, Gandevia SC, McDarmont BM, Mitchell PB, Sachdev PS: Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry 2000; 47:325-331Crossref, Medline, Google Scholar
64. Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA: Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry 2001; 49:460-463Crossref, Medline, Google Scholar



