Dysfunctional Attitudes and 5-HT2 Receptors During Depression and Self-Harm
Abstract
OBJECTIVE: Dysfunctional attitudes are negatively biased assumptions and beliefs regarding oneself, the world, and the future. In healthy subjects, increasing serotonin (5-HT) agonism with a single dose of d-fenfluramine lowered dysfunctional attitudes. To investigate whether the converse, a low level of 5-HT agonism, could account for the higher levels of dysfunctional attitudes observed in patients with major depression or with self-injurious behavior, cortex 5-HT2 receptor binding potential and dysfunctional attitudes were measured in patients with major depressive disorder, patients with a history of self-injurious behavior, and healthy comparison subjects (5-HT2 receptor density increases during 5-HT depletion). METHOD: Twenty-nine healthy subjects were recruited to evaluate the effect of d-fenfluramine or of clonidine (control condition) on dysfunctional attitudes. Dysfunctional attitudes were assessed with the Dysfunctional Attitude Scale 1 hour before and 1 hour after drug administration. In a second experiment, dysfunctional attitudes and 5-HT2 binding potential were measured in 22 patients with a major depressive episode secondary to major depressive disorder, 18 patients with a history of self-injurious behavior occurring outside of a depressive episode, and another 29 age-matched healthy subjects. Cortex 5-HT2 binding potential was measured with [18F]setoperone positron emission tomography. RESULTS: In the first experiment, dysfunctional attitudes decreased after administration of d-fenfluramine. In the second experiment, in the depressed group, dysfunctional attitudes were positively associated with cortex 5-HT2 binding potential, especially in Brodmann’s area 9 (after adjustment for age). Depressed subjects with extremely dysfunctional attitudes had higher 5-HT2 binding potential, compared to healthy subjects, particularly in Brodmann’s area 9. CONCLUSIONS: Low levels of 5-HT agonism in the brain cortex may explain the severely pessimistic, dysfunctional attitudes associated with major depression.
In major depressive disorder, major depressive episodes may include symptoms of sad mood, anhedonia, sleep/wake disturbances, weight change, and cognitive changes. Many of the cognitive changes reflect an information processing bias in which the importance of negatively valent (pessimistic) information is exaggerated and the importance of positively valent (optimistic) information is minimized (1). These cognitive changes may influence dysfunctional attitudes. Dysfunctional attitudes are negatively biased views of oneself, the world, and the future (1). Modest levels of dysfunctional attitudes are healthy. The level of dysfunctional attitudes usually increases beyond the healthy range during major depressive episodes. With successful antidepressant treatment or cognitive behavior therapy, dysfunctional attitudes can normalize within a healthy range (2, 3). The level of dysfunctional attitudes has also been shown to be higher than normal in patients with self-harm behavior and personality disorder (4).
The relationship between dysfunctional attitudes and neurochemical pathology in patients with major depressive episodes and/or chronic self-harm behavior is unclear. However, available evidence suggests that a subpopulation of patients with major depression and/or chronic self-harm behavior have low levels of serotonin (5-HT) stimulation of 5-HT2 receptors. Investigations using animal models have reported that a chronic lack of stimulation by 5-HT produces an up-regulation of 5-HT2 receptors in the cortex (5, 6). Other studies have reported higher than normal levels of 5-HT2 receptor density in Brodmann’s area 9 in the prefrontal cortex in suicide victims as well as in suicide victims with major depressive episodes (7–13). Other indirect measures suggest that the level of 5-HT in the brain is low during major depressive episodes and/or suicidal states. For example, when brain 5-HT levels are low, cerebrospinal fluid concentration of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) is low (14), and low cerebrospinal fluid 5-HIAA concentration has been found in suicidal subjects as well as in subjects with major depressive episodes (15, 16). Given these findings, it appears that low 5-HT stimulation of 5-HT2 receptors in the prefrontal cortex occurs in some patients with major depressive episodes and/or chronic self-harm. Since a higher level of dysfunctional attitudes is an important common symptom in these illnesses, low 5-HT stimulation of 5-HT2 receptors in the prefrontal cortex may be related to higher levels of dysfunctional attitudes.
In this study, the phrase “5-HT agonism” is used to refer to a process that includes 5-HT release by neurons, the binding of 5-HT to a receptor, a conformational change in the receptor when bound to 5-HT, and subsequent intracellular changes consequent to the conformational change in the receptor. Multiple intracellular effects (second messenger cascades, down-regulation of receptors, etc.) may also be involved. In this study we measured the 5-HT2 receptor binding potential that is proportional to 5-HT2 receptor density. Increased 5-HT release after administration of monoamine oxidase inhibitors has been shown to be associated with a down-regulation of 5-HT2 receptors (17, 18), and decreased 5-HT release after administration of reserpine (5) or a tryptophan hydroxylase inhibitor (6) has been associated with increased 5-HT2 receptor density.
This study had two main purposes. The first was to examine the relationship between 5-HT agonism and dysfunctional attitudes in healthy subjects by assessing the change in dysfunctional attitudes after a single dose of d-fenfluramine. d-Fenfluramine selectively induces the release of serotonin from neurons (19, 20). Dysfunctional attitudes are measurable with the Dysfunctional Attitude Scale (21–23). We hypothesized that a higher level of 5-HT agonism would lower the level of dysfunctional attitudes, i.e., shift them toward optimism.
The second purpose was to assess whether low 5-HT agonism in the prefrontal cortex is related to the higher levels of dysfunctional attitudes found in patients with major depressive episodes secondary to major depressive disorder as well as in patients with chronic self-harm behavior. We hypothesized that 5-HT agonism would be lower than normal in the prefrontal cortex in some patients with major depressive episodes and in some patients with chronic self-harm behavior, resulting in greater dysfunctional attitudes and up-regulated 5-HT2 receptors. We specifically hypothesized that higher levels of 5-HT2 receptor binding potential in the prefrontal cortex would be associated with higher levels of dysfunctional attitudes in these two groups. 5-HT2 receptor binding potential was measured by using [18F]setoperone positron emission tomography (PET) (24). The binding potential is proportional to receptor density and affinity.
Method
Subjects and procedures
All procedures were approved by the University of Toronto Human Subjects Review Committee.
Experiment 1 examined the effect of d-fenfluramine or clonidine on dysfunctional attitudes in healthy subjects. Twenty-nine healthy subjects age 18–40 years (mean age=27 years, SD=5) were recruited. Each subject provided written consent after the procedures had been fully explained. All healthy subjects were screened with the nonpatient version of the Structured Clinical Interview for DSM-IV (SCID) (25) to rule out axis I disorders (either current or in remission), current suicidal ideation, history of self-harm behavior, and history of psychiatric illness. Clonidine was used in the control condition because its side effect profile is similar to that of d-fenfluramine after a single dose (26). Clonidine is an α2 receptor agonist (27). Twenty-eight subjects completed the protocol. Fifteen subjects received 0.3 mg/kg of intravenous d-fenfluramine, and 13 subjects received 1.4 μg/kg of intravenous clonidine (control group). These doses are typical for agonist challenge studies examining behavioral and neuroendocrine effects (28–31). Assessment measures were done 1 hour before and 1 hour after the agonist challenge. To assess dysfunctional attitudes, form A and form B of the Dysfunctional Attitude Scale were administered in a counterbalanced design between subjects. Visual analogue scales of mood, anxiety, and energy levels were also completed.
Experiment 2 examined the levels of 5-HT2 receptors and dysfunctional attitudes in patients with depression and in patients with self-harm behavior. Twenty-two subjects with a major depressive episode secondary to major depressive disorder (mean age=31 years, SD=6), 18 patients with a history of self-injurious behavior occurring outside of a depressive episode (mean age=31 years, SD=7), and another 29 age-matched healthy subjects (mean age=31 years, SD=7) received an [18F]setoperone PET scan. Subjects were between 18 and 44 years of age. Twenty-two healthy subjects were age-matched within 2 years to the depressed patients, and 18 healthy subjects were age-matched within 2 years to the patients with histories of self-injurious behavior. Two patients with self-harm behavior who received a [18F]setoperone PET scan did not complete the rest of the study. All subjects were physically healthy and free of psychotropic drug use for more than 4 weeks plus five half-lives of any medication. All healthy subjects were screened by using the nonpatient version of the SCID (25) to rule out axis I disorders (either current or in remission), current suicidal ideation, history of self-harm behavior, and history of psychiatric illness. For each subject, written consent was obtained after the procedures had been fully explained.
The diagnosis of major depressive episode secondary to major depressive disorder was based on the patient edition of the SCID (32) and a consultation by a psychiatrist. The recruiting methods for patients with major depressive disorder have been described previously (33). Subjects with major depressive disorder were eligible for enrollment on the basis of a minimum severity of depression indicated by a cutoff score of 17 on the 17-item Hamilton Depression Rating Scale. Patients with major depressive episodes with psychotic symptoms, bipolar disorder (type I or II), or comorbid axis I psychiatric diagnoses (either current or in remission) were excluded, as were subjects with a history of alcohol or drug abuse or dependence. Patients with major depressive episodes were screened to rule out self-harm and suicidality outside of their episodes of depression. Sixteen of the 22 depressed patients had never received a trial of antidepressant treatment. No depressed patient had received antidepressant treatment within the past 3 months.
The patients with self-harm behavior had a history of chronic suicidal ideation and a history of potentially lethal self-harm behavior. Table 1 lists the behaviors associated with the most severe (lifetime) incident of self-harm reported by each subject. The patients in this group also had current suicidal ideation. All had a diagnosis of borderline personality disorder, which was confirmed by the SCID for axis II disorders (34) and a consultation by a psychiatrist. Patients in this group were also interviewed with the SCID for axis I disorders (32) to exclude patients with bipolar disorder. Given the high comorbidity of major depressive disorder and other axis I disorders in patients with borderline personality disorder, patients with cormorbid major depressive disorder or other axis I illnesses were not excluded, provided they had a clear history of serious self-harm behavior outside of the episode of the axis I illness.
Urine drug screening was completed for all patients with major depressive episode and a history of any drug use as well as for all patients with self-harm behavior. All patients received routine blood tests (measures of thyroid function and electrolytes and a complete blood cell count) to rule out medical causes of disturbed mood.
[18F]Setoperone PET measurement of 5-HT2 binding potential was done with [18F]setoperone prepared by [18F]fluoride substitution on the nitro-derivative precursor of setoperone (35). [18F]Setoperone was of high radiochemical purity (>99%) and high specific activity (mean=48 GBq/mmol, SD=32, at the time of injection). Imaging was based on the approach described by Blin et al. (36). Subjects received an intravenous bolus injection of 185 MBq of [18F]setoperone. PET images were obtained by using a GEMS 2048-15B camera (Scanditronix, Uppsala, Sweden) (x, y, z voxel dimensions=2, 2, and 6.5 mm, respectively). Images were obtained in five 1-minute frames, followed by 17 5-minute frames. The images were corrected for attenuation by using a 68Ge transmission scan and reconstructed by filtered back projection (Hanning filter, 5 mm full width at half maximum).
The 5-HT2 binding potential in the cortex was measured both in regions and in each cortex voxel by using the ratio model during the pseudo-equilibrium period of 65 to 90 minutes after injection of [18F]setoperone (37, 38). For the region-of-interest analyses, a reference magnetic resonance imaging scan (GE Sigma 1.5-T scanner [General Electric, Milwaukee], spin-echo sequence proton density weighted image; x, y, z voxel dimensions=0.78, 0.78, 3 mm, respectively) was obtained for each subject. Regions of interest were found by using a semiautomated method (33, 39) and verified by visual assessment with reference to a coregistered magnetic resonance imaging scan. These methods have been described in more detail previously (33).
The regions of interest were within the middle frontal gyrus (Brodmann’s area 9), lateral orbitofrontal cortex, posterior medial temporal gyrus, and rostral anterior cingulate. For the voxel-by-voxel analysis, images composed of binding potential values were transformed and deformed into a common brain shape by using statistical parametric mapping and ligand-specific templates (40–43). Then the images were spatially smoothed with a Gaussian filter (12 mm full width at half maximum).
Statistical Analyses
In experiment 1, the analysis was designed to determine the effect of the drug on dysfunctional attitudes, after controlling for any differences in the order in which the versions of the Dysfunctional Attitude Scale were administered. For this analysis, the variables were change, order, and drug type. Change was the change in the score on the Dysfunctional Attitude Scale from before to after administration of the drug. Order referred to whether version A or version B of the Dysfunctional Attitude Scale was administered first. Drug type was either d-fenfluramine or clonidine. Analysis of variance (ANOVA) was used to examine the effect of drug type and order on change in dysfunctional attitudes. Drug type was the factor of primary interest.
In a previous analysis, 27 healthy subjects were assessed in a similar paradigm but without a pharmacological challenge (administration of version A of the Dysfunctional Attitude Scale, followed an hour later by version B, or vice versa) (data not reported). The mean difference (change) in the score, after removing order effects, was –1 (SD=14). In an analysis comparing 13 subjects receiving one type of pharmacological challenge and 15 subjects receiving another type (data not reported), a mean change of 14 in the score on the Dysfunctional Attitude Scale would be detected with a power of 75% (assuming an alpha of 0.05 and a similar standard deviation in each population).
In experiment 2, the main analysis, using ANOVA, examined the effect of dysfunctional attitudes on regional 5-HT2 binding potential, after controlling for the effect of age in each patient group. Separate analyses were done for each brain region. The analysis was also done for each voxel in the brain. ANOVA was also used to consider whether the subgroup of patients defined by high levels of dysfunctional attitudes (those with Dysfunctional Attitude Scale scores above the median in the patient group) had a different level of 5-HT2 binding potential than the healthy subjects, after controlling for the effect of age.
For the first 12 healthy subjects scanned, mean cortex 5-HT2 binding potential in the regions chosen was approximately 2.0 (SD=0.4), after age was controlled and binding potential was adjusted to the typical value for 30-year-olds. The power to detect a 25% difference between two groups of nine subjects was 85% (assuming an alpha significance of 0.05 and a similar standard deviation in each population sampled).
Results
In experiment 1, the decrease in dysfunctional attitudes after d-fenfluramine was significantly greater than changes observed after clonidine (ANOVA examining effects of drug type and order of administration of the attitude scale versions on change in dysfunctional attitudes—effect of drug type: F=17.3, df=1, 25, p<0.001; effect of order [version A versus version B]: F=15.8, df=1, 25, p=0.001). After the order effect was controlled, the mean decrease in the Dysfunctional Attitude Scale score was 14 points (SD=11) after d-fenfluramine and 0 points (SD=10) after clonidine. The order effect was controlled by determining the mean change in dysfunctional attitude scores for the subjects who received version A then B of the Dysfunctional Attitude Scale and for the subjects who received version B then A. The difference between the two mean changes in score was determined, and the change in score for each subject was increased or reduced by half the difference between the mean changes in score for the two order groups so that there was no difference between the scores for the two order groups after the transformation. Figure 1 illustrates the changes in Dysfunctional Attitude Scale scores after d-fenfluramine or clonidine administration.
No significant changes in the visual analogue scales for mood, anxiety, and energy levels were found (ANOVA examining effects of order of administration of attitude scale versions and drug type—effect of drug type on mood change: F<0.1, df=1, 25, p>0.9; effect of drug type on anxiety change: F<0.1, df=1, 25, p>0.90; effect of drug type on energy level change: F=1.6, df=1, 25, p=0.19). The effects of drug type alone (without considering order of administration of attitude scale versions) on changes in visual analogue scales were also nonsignificant.
In experiment 2, Dysfunctional Attitude Scale scores covaried strongly with 5-HT2 binding potential in all cortex brain regions in the patients with a major depressive episode (analysis of covariance [ANCOVA] with Dysfunctional Attitude Scale score and age as covariates—Dysfunctional Attitude Scale score effect: F=4.6 to 9.9, df=1, 19, p=0.04 to 0.005) (Figure 2). After the effects of age were controlled, the attitude scale scores were significantly associated with 5-HT2 binding potential throughout the entire cortex (Figure 3). No such associations were present in self-harming patients with chronic suicidal ideation (ANCOVA with Dysfunctional Attitude Scale score and age as covariates—Dysfunctional Attitude Scale score effect: F=0.5 to 1.5, df=1, 13, p=0.48 to 0.24). The mean Dysfunctional Attitude Scale scores in the major depressive episodes group and the self-injurious group were 162 (SD=32) and 164 (SD=52), respectively. Consistent with previous reports (7–13), 5-HT2 binding potential declined with age in the healthy, major depressive episodes, and self-harming groups, as shown in both regional analyses and voxel-based analyses (ANCOVA for regional analyses: F=31.4 to 52.7, df=1, 20, and F=22.2 to 50.1, df=1, 11, p≤0.002 for all analyses; voxel-based analyses: N=80265 to 85333 suprathreshold voxels, p [cluster size]<0.001).
The patients with major depressive episodes were divided into two groups on the basis of whether their Dysfunctional Attitude Scale score was above or below the median score of 166 for the entire group of depressed patients. The subgroup with high Dysfunctional Attitude Scale scores had significantly greater 5-HT2 binding potential in all brain regions than the age-matched healthy subjects (ANCOVA with group and age as covariates—effect of group [depressed with high Dysfunctional Attitude Scale score]: F=5.1 to 11.5, df=1, 19, p=0.04 to 0.003; effect of age: F=34.4 to 53.7, df=1, 19, p<0.001). In the regions analyzed, the mean 5-HT2 binding potential was 21% to 29% higher in the high Dysfunctional Attitude Scale score group than in the healthy subjects, with the greatest difference in the middle frontal gyrus (Brodmann’s area 9) bilaterally (29% higher) (Figure 4).
Within the major depressive episodes group, regional and voxel analyses showed no associations of 5-HT2 binding potential with score on the suicidal ideation subscale of the Hamilton Depression Rating Scale, overall severity of depressive symptoms (total Hamilton depression scale score), number of previous episodes, duration of depression, past antidepressant use, or any of the five orthogonal factors (44) within the Hamilton depression scale (mood depression, sleep disturbance, weight loss, somatization, agitation/anxiety).
In regional and voxel-by-voxel comparisons of 5-HT2 binding potential, no significant differences were found between the patients with serious self-harm behavior and the age-matched healthy patients, although 5-HT2 binding potential was a mean of 4% lower in the self-harm group (ANCOVA with age covariate—effect of self-harm: F<0.1 to 1.3, df=1, 33, p=0.98 to 0.27). Two subgroup analyses were done. Patients with a history of more severe self-harm behavior (i.e., stabbing self in chest or more severe behaviors [N=10]) were compared to age-matched healthy subjects, and no significant differences in 5-HT2 binding potential were found, although 5-HT2 binding potential was a mean of 10% lower in the severe self-harm group (ANCOVA with age covariate—effect of severe self-harm: F<0.1 to 2.8, df=1, 17, p=0.79 to 0.11). Patients with a lifetime history of more than five self-harm attempts (N=12) were compared to age-matched healthy subjects, and no significant differences in 5-HT2 binding potential were found, although 5-HT2 binding potential was a mean of 5% lower in the frequent self-harm group (ANCOVA with age covariate—effect of frequent self-harm: F<0.1 to 1.1, df=1, 21, p=0.99 to 0.29).
Discussion
This study had three main findings. The first was that dysfunctional attitudes decreased after administration of d-fenfluramine in healthy subjects. The second was that higher levels of dysfunctional (more pessimistic) attitudes during major depressive episodes were associated with higher 5-HT2 binding potential in the cortex. The third main finding was that patients with major depressive episodes and high levels of dysfunctional (pessimistic) attitudes had higher 5-HT2 binding potential in the cortex, compared to healthy subjects.
The first finding indicates that increasing 5-HT agonism can lower dysfunctional attitudes. d-Fenfluramine causes an increase in extracellular 5-HT concentration by inducing the neuronal release of 5-HT (19, 20). The optimistic shift in dysfunctional attitudes after d-fenfluramine administration demonstrates a role for 5-HT-releasing neurons as modulators of dysfunctional attitudes in humans.
This role of 5-HT-releasing neurons as modulators of dysfunctional attitudes may explain the second finding of an association between dysfunctional attitudes and cortex 5-HT2 binding potential during major depressive episodes. The converse of the first finding is that lower levels of 5-HT agonism may be related to higher levels of dysfunctional attitudes. Low 5-HT agonism has been shown to up-regulate 5-HT2 receptors (5, 6). Thus, low 5-HT agonism during major depressive episodes can account for both an increase in dysfunctional attitudes (toward pessimism) and an increase in 5-HT2 binding potential: the lower the 5-HT agonism, the greater the increase in both 5-HT2 binding potential and dysfunctional attitudes. This would create an association between 5-HT2 binding potential and dysfunctional attitudes, as observed.
This interpretation of low 5-HT agonism resulting in both higher levels of dysfunctional attitudes and higher 5-HT2 binding potential also explains the third finding. Patients with a major depressive episode and greater severity of dysfunctional attitudes have very low 5-HT agonism, and 5-HT2 binding potential in this subgroup is distinguishably higher than that in healthy subjects.
The third finding reflects a key difference in the approach taken in the current study, compared to previous imaging investigations of major depressive episodes and 5-HT2 binding potential. Previous studies tested the hypothesis that all patients with major depressive episodes have an abnormal 5-HT2 binding potential (45–48). The main drawback of this method is that the diagnosis of major depressive episodes is based on a symptom cluster and individual symptoms of major depressive episodes are not always present. The current study tested the hypothesis that patients with more severe symptoms, as indicated by higher scores on the Dysfunctional Attitude Scale, would show an abnormally high 5-HT2 binding potential in the prefrontal cortex. Another important aspect of the current study design is that all of the patients were drug-free for at least 1 month and five half-lives of any previous medication. We are aware of only one large study that reported lower 5-HT2 binding potential during major depressive episodes, and this study selected patients who had recently been treated with antidepressants that increase 5-HT concentrations (48). To our knowledge, the number of patients with major depressive episodes in the current study is larger than in any previous study in this area. In addition, this study examined two different groups of patients—those with major depressive episodes and those with self-harm behaviors—with respect to a common symptom of dysfunctional attitudes.
The present study suggests a new interpretation for the results of postmortem investigations of suicide victims that have reported higher 5-HT2 receptor density in Brodmann’s area 9 (7–13). These findings may represent patients with major depressive disorder and major depressive episodes who have high levels of dysfunctional attitudes. In the present study, the subgroup of patients with major depressive episodes and high levels of dysfunctional attitudes had a higher 5-HT2 binding potential in the cortex (including Brodmann’s area 9), compared to healthy subjects. The presence of major depressive disorder is the most likely explanation of the postmortem findings, as suggested by previous findings that more than 50% of suicide victims have major depressive episodes secondary to major depressive disorder (49, 50). In addition, two studies of drug-free suicide victims with major depressive episodes secondary to major depressive disorder found higher 5-HT2 receptor density in Brodmann’s area 9 (11, 12). Higher levels of dysfunctional attitudes during major depressive episodes may be linked to suicide, as suggested by findings that scores on the Beck Hopelessness Scale are predictive of eventual suicide (51, 52). Also, in studies with large numbers of patients with major depressive episodes, Beck Hopelessness Scale scores were consistently correlated with Dysfunctional Attitude Scale scores (3, 53–55). To our knowledge, no study has explicitly addressed whether dysfunctional attitudes are predictive of eventual death by suicide during major depressive episodes.
This new interpretation is incompatible with the reports of two postmortem studies that did not find higher 5-HT2 receptor density in the temporal cortex of suicide victims (9, 13). However, this discrepancy could reflect differences in the sensitivity of imaging and postmortem techniques in detecting change in some cortex regions.
Cortex 5-HT2 binding potential appears to be unrelated to the higher levels of dysfunctional attitudes observed in the patients with chronic self-harm behavior. It is not surprising that the etiology of higher levels of dysfunctional attitudes in self-harming patients with borderline personality disorder would be different from that in patients with major depressive episodes secondary to major depressive disorder. For example, psychological factors can influence dysfunctional attitudes, and the psychological intervention of cognitive behavior therapy has been shown to reduce dysfunctional attitudes in patients with major depressive episodes (2, 3). If psychological factors are extreme, they could introduce sufficient variance to obscure a relationship between dysfunctional attitudes and serotonin measures. Self-harming patients with borderline personality disorder often report extremely abnormal experiences associated with a long history of relationships with very negative outcomes, including early childhood abuse and/or a lifetime of disturbing short-term relationships (56, 57).
The lack of a relationship between dysfunctional attitudes and 5-HT2 binding potential in patients with recurrent self-harm behavior should not be interpreted as ruling out other 5-HT abnormalities that do not influence 5-HT2 binding potential (i.e., 5-HT lesions [58] or 5-HT abnormalities in locations separated from 5-HT2-containing pyramidal cell neurons). It has been reported that a-[11C]methyltryptophan uptake in cortex is reduced in patients with borderline personality disorder (59).
In this study, age was a covariate of the cortex measure of 5-HT2 binding potential. 5-HT2 receptors are mostly contained in dendrites of pyramidal cell neurons, and the density of pyramidal cell neuron dendrites declines sharply with age over the second to the fourth decades (60, 61). By covarying the effects of age, we were able to distinguish this and other age-related effects from the effect of illness on cortex 5-HT2 binding potential.
In this paper we presented a brief overview of the relationship between 5-HT and the 5-HT2 receptor. However, the available information regarding the regulation of the 5-HT2 receptor is complicated. In reviewing this information, it is useful to differentiate two categories of relationship: 1) the relationship between 5-HT concentration and 5-HT2 receptor regulation and 2) the relationship between 5-HT2-binding medications and 5-HT2 receptor regulation. In this study, the first of these relationships is most relevant. Earlier studies have demonstrated that increased 5-HT after administration of selective and nonselective monoamine oxidase-A inhibitors is associated with a down-regulation of 5-HT2 receptors (17, 18). It has also been demonstrated that decreased 5-HT after administration of reserpine (5) or the tryptophan hydroxylase inhibitor p-chlorophenylalanine (6) is associated with increased 5-HT2 receptor density. These specific interventions appear to influence the presynaptic storage of 5-HT and consequently increase (monoamine oxidase inhibitor [62]) or decrease (p-chlorophenylalanine [63], reserpine [64]) extracellular 5-HT. The intact functioning of the synapse may be important for the relationship between 5-HT and 5-HT2 receptors because lesioning of the 5-HT-releasing neurons does not result in up-regulation of 5-HT2 receptors (58, 65). In summary, the relationship between 5-HT concentration and 5-HT2 receptor regulation is best demonstrated by manipulations of 5-HT that influence presynaptic 5-HT storage in functional neurons (and indirectly influence synaptic concentrations of 5-HT).
As for the relationship between 5-HT2-binding medications and 5-HT2 receptor regulation, antagonists are traditionally associated with up-regulation and agonists with down-regulation of postsynaptic, G-protein-coupled receptors (66). Several agonists for 5-HT2 receptors are associated with the traditional, expected, down-regulation of 5-HT2 receptors (66, 67). At least one antagonist (SR 46349B) is consistently associated with the traditional, expected, up-regulation of 5-HT2 receptors (68–70). However, some 5-HT2-binding medications with antagonist effects can also down-regulate 5-HT2 receptors (66). There is no reason to assume that medications cannot induce conformational changes in the receptor such that the traditional category of agonist and antagonist no longer applies. Some categories of medications have a mix of antagonist, agonist, and other interactions with receptors (partial agonists, inverse agonist, etc.). It has been proposed that 5-HT2 antagonists with down-regulation properties exert these properties by promoting internalization (traditionally associated with agonists) (66). The effects of medications on receptor regulation may provide information about how a receptor regulates. However, there is no reason to assume that the interaction between specific medications and a receptor is fully representative of the interaction between the endogenous neurotransmitter and the receptor.
This study had limitations typical of ligand PET and brain imaging studies in humans. 5-HT concentrations in the brain cannot be measured directly in humans; therefore, we used indirect measures and made interpretations about these measures. The binding potential reflects Bmax/Kd (density × affinity). Although we are unable to discern between these two parameters, it is likely that an increase in 5-HT2 binding potential reflects an increase in 5-HT2 Bmax. Postmortem studies of suicide victims report higher levels of 5-HT2 Bmax(7–13), and animal models of 5-HT depletion report higher levels of 5-HT2 Bmax(5, 6). Even if decreased 5-HT2 receptor stimulation resulted in an increase in affinity (decrease in Kd), binding potential would still be increased in the same direction, and this result would not confound our main interpretations.
We found higher 5-HT2 binding potential throughout the cortex. Our interpretation is that the higher 5-HT2 binding potential can be attributed to a lower level of 5-HT in the cortex with normally functioning 5-HT2 receptors. This interpretation need not apply to other 5-HT receptor abnormalities reported during depressive episodes. Lower levels of 5-HT1A binding potential have been found in most cortex regions during depression (71, 72). Decreased 5-HT transporter density within the prefrontal cortex was found in a large postmortem study of depressed subjects (73); however, this finding is not consistently reported (12, 73–76).
The conclusion that low 5-HT agonism is responsible for the association between higher levels of 5-HT2 binding potential and dysfunctional attitudes is the simplest explanation of the highly significant findings of the separate experiments of this study. Usually the simplest explanation (with the fewest assumptions) for multiple observations is the correct one; however, it is possible that more complicated explanations may account for the association between dysfunctional attitudes and 5-HT2 binding potential during depressive episodes. To resolve this issue, dysfunctional attitudes should be measured in future investigations of serotonin abnormalities during depressive episodes.
In summary, this study had several novel findings. Dysfunctional attitudes decreased after administration of d-fenfluramine, suggesting that neuronal release of 5-HT may modulate dysfunctional attitudes in healthy humans. Abnormal functioning of 5-HT modulation during major depressive episodes can explain the association between cortex 5-HT2 binding potential and dysfunctional attitudes: low 5-HT agonism may lead to higher levels both of 5-HT2 binding potential in the cortex and of dysfunctional attitudes. A subtype of major depressive episodes with higher levels of dysfunctional attitudes was identified, and subjects with this subtype had higher 5-HT2 binding potential in the cortex, compared to healthy subjects. These findings indicate an important role for abnormal serotonergic neuromodulation in the pathophysiology of dysfunctional attitudes during major depressive episodes. These findings have significant implications for future research on suicide in depression.
 |
Received Aug. 21, 2001; revisions received April 16 and Aug. 6, 2002; accepted Aug. 14, 2002. From the Vivian M. Rakoff PET Imaging Centre and the Mood and Anxiety Disorders Division, Clarke Division, and the Dialectical Behaviour Therapy Clinic, Centre for Addiction and Mental Health, Department of Psychiatry, University of Toronto; and St. Michael’s Hospital, University of Toronto, Toronto. Address reprint requests to Dr. Meyer, Vivian M. Rakoff PET Imaging Centre, Clarke Division, Centre for Addiction and Mental Health, Department of Psychiatry, University of Toronto, 250 College St., Toronto, ON, M5T 1R8, Canada; [email protected] (e-mail). Supported by the Canadian Institutes of Health Research, the National Alliance for Research on Schizophrenia and Depression, and a grant to Dr. Meyer from the Canadian Institutes of Health Research new investigator program. The authors thank Alex Kecojevic, Beata Eisfeld, Fiona Downey, Doug Hussey, Kevin Cheung, Armando Garcia, Li Jin, and Ruiping Guo for assistance with the study.
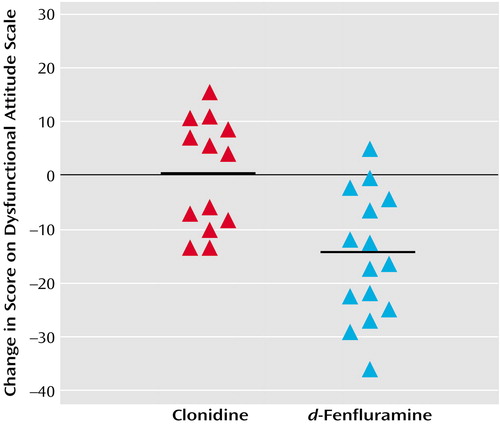
Figure 1. Change in Dysfunctional Attitude Scale Scores for 28 Healthy Subjects After a Single Dose of Intravenous Clonidine or d-Fenfluraminea
aThe change was significantly greater for subjects who received d-fenfluramine than for subjects who received clonidine, after removal of the effect of the order in which subjects were tested with the two versions (version A or B) of the Dysfunctional Attitude Scale (effect of drug type: F=17.3, df=1, 25, p<0.001; order effect [version A followed by version B versus version B followed by version A]: F=15.8, df=1, 25, p=0.001). The horizontal bars represent the mean change in dysfunctional attitudes for each group.
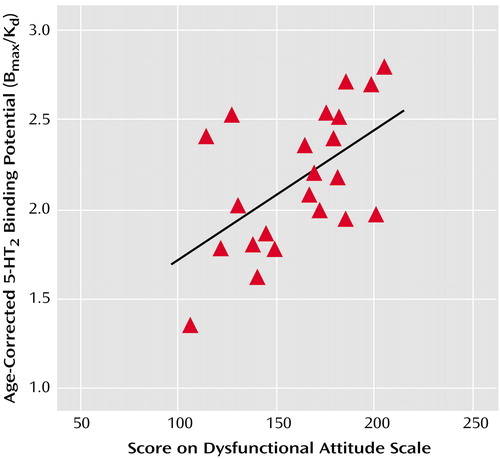
Figure 2. Correlation of Age-Corrected 5-HT2 Binding Potential in the Prefrontal Cortex With Scores on the Dysfunctional Attitude Scale for 22 Subjects With a Major Depressive Episode Secondary to Major Depressive Disordera
ar=0.56, p=0.009 for the correlation between age-corrected 5-HT2 binding potential in the bilateral prefrontal cortex (Brodmann’s area 9) and Dysfunctional Attitude Scale scores. Higher scores on the Dysfunctional Attitude Scale indicate higher levels of dysfunctional attitudes. Age-corrected 5-HT2 binding potential was calculated by linear regression analysis with the predictor variables of age and Dysfunctional Attitude Scale score. The slope of the line for the age predictor was used to normalize each subject’s 5-HT2 binding potential to that expected for a 30-year-old subject.
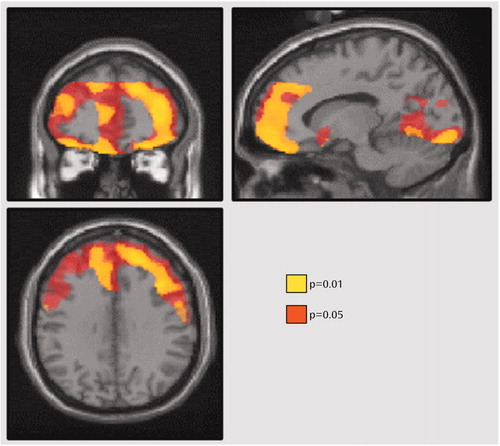
Figure 3. Brain Areas Where 5-HT2 Binding Potential Was Associated With Dysfunctional Attitudes in 22 Subjects With a Major Depressive Episode Secondary to Major Depressive Disordera
aStatistical probability maps are superimposed on a representative T1-weighted magnetic resonance imaging scan and displayed in three sections in standardized Montreal Neurological Space. Sections are presented at coordinates 13, 43, 33 mm in the x, y, and z axes relative to the anterior commissure (0, 0, 0). The p values represent the significance of the age-corrected effect of Dysfunctional Attitude Scale scores on 5-HT2 receptor binding potential (proportional to Bmax/Kd). Although the association appears to be somewhat stronger in the prefrontal cortex, a generalized cortical association is also suggested.
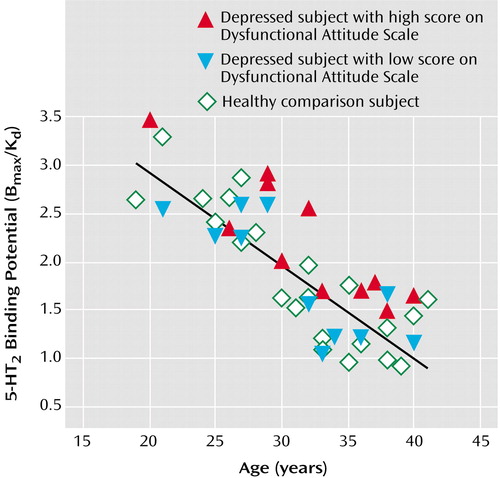
Figure 4. 5-HT2 Binding Potential in 22 Healthy Subjects and 22 Subjects With a Major Depressive Episode Secondary to Major Depressive Disorder and High or Low Scores on the Dysfunctional Attitude Scalea
a5-HT2 receptor binding potential in the bilateral middle frontal gyrus (Brodmann’s area 9) is plotted against age to show the relationship between depressed and healthy subjects. The 22 depressed patients were divided into two groups on the basis of whether their Dysfunctional Attitude Scale score was above or below the median score of 166 for the entire group of depressed patients. Patients with high scores had significantly higher age-corrected 5-HT2 receptor binding potential than healthy subjects (F=11.5, df=1, 19, p=0.003).
1. Beck A: Cognitive Therapy and the Emotional Disorders. New York, International Universities Press, 1976Google Scholar
2. Simons AD, Garfield SL, Murphy GE: The process of change in cognitive therapy and pharmacotherapy for depression: changes in mood and cognition. Arch Gen Psychiatry 1984; 41:45-51Crossref, Medline, Google Scholar
3. DeRubeis R, Hollon S, Grove W, Evans M, Garvey M, Tuason V: How does cognitive therapy work? cognitive change and symptom change in cognitive therapy and pharmacotherapy for depression. J Consult Clin Psychol 1990; 58:862-869Crossref, Medline, Google Scholar
4. O’Leary K, Cowdry R, Gardner D, Leibenluft E, Lucas P, de Jong-Meyer R: Dysfunctional attitudes in borderline personality disorder. J Personal Disord 1991; 5:233-242Crossref, Google Scholar
5. Stockmeier C, Kellar K: In vivo regulation of the serotonin-2 receptor in rat brain. Life Sci 1985; 38:117-127Crossref, Google Scholar
6. Roth B, McLean S, Zhu X, Chuang D: Characterization of two [3H]ketanserin recognition sites in rat striatum. J Neurochem 1987; 49:1833-1838Crossref, Medline, Google Scholar
7. Stanley M, Mann JJ: Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983; 1:214-216Crossref, Medline, Google Scholar
8. Mann JJ, Stanley M, McBride PA, McEwen BS: Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry 1986; 43:954-959Crossref, Medline, Google Scholar
9. Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ: Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 1990; 47:1038-1047Crossref, Medline, Google Scholar
10. Arora RC, Meltzer HY: Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry 1989; 146:730-736Link, Google Scholar
11. Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN: 5HT2 receptor changes in major depression. Biol Psychiatry 1990; 27:489-496Crossref, Medline, Google Scholar
12. Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M: 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res 1993; 614:37-44Crossref, Medline, Google Scholar
13. Arango V, Underwood M, Mann J: Alterations in monoamine receptors in the brain of suicide victims. J Clin Psychopharmacol 1992; 12:8S-12SCrossref, Medline, Google Scholar
14. Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ: Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology 1998; 19:26-35Crossref, Medline, Google Scholar
15. Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V: “Serotonin depression”—a biochemical subgroup within the affective disorders? Science 1976; 191:478-480Crossref, Medline, Google Scholar
16. Oreland L, Wiberg A, Asberg M, Traskman L, Sjostrand L, Thoren P, Bertilsson L, Tybring G: Platelet MAO activity and monoamine metabolites in cerebrospinal fluid in depressed and suicidal patients and in healthy controls. Psychiatry Res 1981; 4:21-29Crossref, Medline, Google Scholar
17. O’Regan D, Kwok RP, Yu PH, Bailey BA, Greenshaw AJ, Boulton AA: A behavioural and neurochemical analysis of chronic and selective monoamine oxidase inhibition. Psychopharmacology (Berl) 1987; 92:42-47Crossref, Medline, Google Scholar
18. Todd KG, McManus DJ, Baker GB: Chronic administration of the antidepressants phenelzine, desipramine, clomipramine, or maprotiline decreases binding to 5-hydroxytryptamine2A receptors without affecting benzodiazepine binding sites in rat brain. Cell Mol Neurobiol 1995; 15:361-370Crossref, Medline, Google Scholar
19. Puig de Parada M, Parada MA, Pothos E, Hoebel BG: d-Fenfluramine, but not d-norfenfluramine, uses calcium to increase extracellular serotonin. Life Sci 1995; 56:L415-L420Google Scholar
20. Rothman RB, Elmer GI, Shippenberg TS, Rea W, Baumann MH: Phentermine and fenfluramine: preclinical studies in animal models of cocaine addiction. Ann NY Acad Sci 1998; 844:59-74Crossref, Medline, Google Scholar
21. Weissman A: The Dysfunctional Attitude Scale: a validation study. Diss Abstr Int 1979; 40:1389B-1390BGoogle Scholar
22. Dobson K, Breiter H: Cognitive assessment of depression: reliability and validity of three measures. J Abnorm Psychol 1983; 92:107-109Crossref, Medline, Google Scholar
23. Oliver J, Baumgart E: The Dysfunctional Attitude Scale: psychometric properties and relation to depression in an unselected adult population. Cognit Ther Res 1985; 9:161-167Crossref, Google Scholar
24. Blin J, Pappata S, Kiyosawa M, Crouzel C, Baron J: [18F]Setoperone: a new high-affinity ligand for positron emission tomography study of the serotonin-2 receptors in baboon brain in vivo. Eur J Pharmacol 1988; 147:73-82Crossref, Medline, Google Scholar
25. First M, Spitzer R, Williams J, Gibbon M: Structured Clinical Interview for DSM-IV—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1995Google Scholar
26. Canadian Pharmacists Association: Compendium of Pharmaceuticals and Specialties. Toronto, Webcom Ltd, 2001Google Scholar
27. Seeman P: Receptor Tables, Vol 2: Drug Dissociation Constants for Neuroreceptors and Transporters. Toronto, Schizophrenia Research, 1993Google Scholar
28. Casanueva FF, Villanueva L, Penalva A, Cabezas-Cerrato J: Depending on the stimulus, central serotoninergic activation by fenfluramine blocks or does not alter growth hormone secretion in man. Neuroendocrinology 1984; 38:302-308Crossref, Medline, Google Scholar
29. Sulaiman WR, Johnson RH: Effect of fenfluramine on human growth hormone release. Br Med J 1973; 2:329-332Crossref, Medline, Google Scholar
30. Corn TH, Hale AS, Thompson C, Bridges PK, Checkley SA: A comparison of the growth hormone responses to clonidine and apomorphine in the same patients with endogenous depression. Br J Psychiatry 1984; 144:636-639Crossref, Medline, Google Scholar
31. Brown GM, Mazurek M, Allen D, Szechtman B, Cleghorn JM: Dose-response profiles of plasma growth hormone and vasopressin after clonidine challenge in man. Psychiatry Res 1990; 31:311-320Crossref, Medline, Google Scholar
32. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
33. Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH: The effect of paroxetine on 5-HT2A receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry 2001; 158:78-85Link, Google Scholar
34. Blais MA, Norman DK: A psychometric evaluation of the DSM-IV personality disorder criteria. J Personal Disord 1997; 11:168-176Crossref, Google Scholar
35. Maziere B, Crouzel C, Venet M, Stulzaft O, Sanz G, Ottaviani M, Sejourne C, Pascal O, Bisserbe J: Synthesis, affinity and specificity of 18F-setoperone, a potential ligand for in-vivo imaging of cortical serotonin receptors. Nucl Med Biol 1988; 15:463-468Google Scholar
36. Blin J, Sette G, Fiorelli M, Bletry O, Elghozi JL, Crouzel C, Baron JC: A method for the in vivo investigation of the serotonergic 5-HT2 receptors in the human cerebral cortex using positron emission tomography and 18F-labeled setoperone. J Neurochem 1990; 54:1744-1754Crossref, Medline, Google Scholar
37. Petit-Taboue MC, Landeau B, Osmont A, Tillet I, Barre L, Baron JC: Estimation of neocortical serotonin-2 receptor binding potential by single-dose fluorine-18-setoperone kinetic PET data analysis. J Nucl Med 1996; 37:95-104Medline, Google Scholar
38. Fischman A, Bonab A, Babich J, Alpert N, Rauch S, Elmaleh D, Shoup T, Williams S, Rubin R: Positron emission tomographic analysis of central 5-hydroxytryptamine2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent ziprasidone. J Pharmacol Exp Ther 1996; 279:939-947Medline, Google Scholar
39. Rabiner E, Gunn R, Castro M, Sargent P, Cowen P, Koepp M, Meyer J, Bench C, Harrison P, Pazos A, Sharp T, Grasby P: Beta-blocker binding to human 5-HT1A receptors in vitro and in vivo: implications for antidepressant therapy. Neuropsychopharmacology 2000; 23:285-293Crossref, Medline, Google Scholar
40. Friston K, Ashburner J, Poline J, Frith C, Heather J, Frackowiak R: Spatial realignment and normalization of images. Hum Brain Mapp 1995; 2:165-169Crossref, Google Scholar
41. Friston K, Frith C, Liddle P, Frackowiak R: Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994; 1:214-220Google Scholar
42. Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189-210Crossref, Google Scholar
43. Meyer JH, Gunn RN, Myers R, Grasby PM: Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 1999; 9:545-553Crossref, Medline, Google Scholar
44. Rhoades H, Overall J: The Hamilton depression scale: factor scoring and profile classification. Psychopharmacol Bull 1983; 19:91-95Google Scholar
45. D’haenen H, Bossuyt A, Mertens J, Bossuyt-Piron C, Gijesmans M, Kaufman L: SPECT imaging of serotonin2 receptors in depression. Psychiatry Res Neuroimaging 1992; 45:227-237Crossref, Medline, Google Scholar
46. Meyer JH, Kapur S, Houle S, DaSilva J, Owczarek B, Brown GM, Wilson AA, Kennedy SH: Prefrontal cortex 5-HT2 receptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry 1999; 156:1029-1034Abstract, Google Scholar
47. Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben-Eliezer D, Lopresti B, DeKosky ST, Reynolds CF III: PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am J Psychiatry 1999; 156:1871-1878Abstract, Google Scholar
48. Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ: Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry 2000; 57:850-858Crossref, Medline, Google Scholar
49. Barraclough B, Bunch J, Nelson B, Sainsbury P: A hundred cases of suicide: clinical aspects. Br J Psychiatry 1974; 125:355-373Crossref, Medline, Google Scholar
50. Robins E, Murphy G, Wilkinson R, Gassner S, Kayes J: Some clinical considerations in the prevention of suicide based on a study of 134 successful suicides. Am J Public Health 1959; 49:888-899Crossref, Google Scholar
51. Beck AT, Steer RA, Kovacs M, Garrison B: Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985; 142:559-563Link, Google Scholar
52. Beck AT, Brown G, Steer RA: Prediction of eventual suicide in psychiatric inpatients by clinical ratings of hopelessness. J Consult Clin Psychol 1989; 57:309-310Crossref, Medline, Google Scholar
53. Norman WH, Miller IW, Dow MG: Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cognit Ther Res 1988; 12:39-51Crossref, Google Scholar
54. Bouvard M, Charles S, Guerin J, Aimard G, Cottraux J: [Study of Beck’s Hopelessness Scale: validation and factor analysis]. Encephale 1992; 18:237-240 (French)Medline, Google Scholar
55. Cannon B, Mulroy R, Otto MW, Rosenbaum JF, Fava M, Nierenberg AA: Dysfunctional attitudes and poor problem solving skills predict hopelessness in major depression. J Affect Disord 1999; 55:45-49Crossref, Medline, Google Scholar
56. van der Kolk BA, Hostetler A, Herron N, Fisler RE: Trauma and the development of borderline personality disorder. Psychiatr Clin North Am 1994; 17:715-730Crossref, Medline, Google Scholar
57. Figueroa E, Silk KR: Biological implications of childhood sexual abuse in borderline personality disorder. J Personal Disord 1997; 11:71-92Crossref, Google Scholar
58. Blackshear MA, Steranka LR, Sanders-Bush E: Multiple serotonin receptors: regional distribution and effect of Raphe lesions. Eur J Pharmacol 1981; 76:325-334Crossref, Medline, Google Scholar
59. Leyton M, Young SN, Benkelfat C: Relapse of depression after rapid depletion of tryptophan (letter). Lancet 1997; 349:1840-1841Crossref, Medline, Google Scholar
60. Jakab R, Goldman-Rakic P: 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA 1998; 95:735-740Crossref, Medline, Google Scholar
61. Jacobs B, Driscoll L, Schall M: Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol 1997; 386:661-680Crossref, Medline, Google Scholar
62. Romero L, Hervas I, Artigas F: The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci Lett 1996; 219:123-126Crossref, Medline, Google Scholar
63. Prinssen EP, Assie MB, Koek W, Kleven MS: Depletion of 5-HT disrupts prepulse inhibition in rats: dependence on the magnitude of depletion, and reversal by a 5-HT precursor. Neuropsychopharmacology 2002; 26:340-347Crossref, Medline, Google Scholar
64. Heslop KE, Curzon G: Depletion and repletion of cortical tissue and dialysate 5-HT after reserpine. Neuropharmacology 1994; 33:567-573Crossref, Medline, Google Scholar
65. Fischette C, Nock B, Renner K: Effects of 5,7-dihydroxytryptamine on serotonin1 and serotonin2 receptors throughout the rat central nervous system using quantitative autoradiography. Brain Res 1987; 421:263-279Crossref, Medline, Google Scholar
66. Gray JA, Roth BL: Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull 2001; 56:441-451Crossref, Medline, Google Scholar
67. Leysen J, Pauwels P: 5-HT2 receptors, roles and regulation. Ann NY Acad Sci 1990; 600:183-191Crossref, Medline, Google Scholar
68. Rinaldi-Carmona M, Congy C, Santucci V, Simiand J, Gautret B, Neliat G, Labeeuw B, Le Fur G, Soubrie P, Breliere JC: Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther 1992; 262:759-768Medline, Google Scholar
69. Rinaldi-Carmona M, Bouaboula M, Congy C, Oury-Donat F, Simiand J, Shire D, Casellas P, Soubrie P, Breliere JC, Le Fur G: Up-regulation of 5-HT2 receptors in the rat brain by repeated administration of SR 46349B, a selective 5-HT2 receptor antagonist. Eur J Pharmacol 1993; 246:73-80Crossref, Medline, Google Scholar
70. Chaouloff F, Kulikov A, Mormede P: Repeated DOI and SR 46349B treatments do not affect elevated plus-maze anxiety despite opposite effects on cortical 5-HT2A receptors. Eur J Pharmacol 1997; 334:25-29Crossref, Medline, Google Scholar
71. Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C: PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999; 46:1375-1387Crossref, Medline, Google Scholar
72. Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ: Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry 2000; 57:174-180Crossref, Medline, Google Scholar
73. Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V: A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000; 57:729-738Crossref, Medline, Google Scholar
74. Arora RC, Meltzer HY: Laterality and 3H-imipramine binding: studies in the frontal cortex of normal controls and suicide victims. Biol Psychiatry 1991; 29:1016-1022Crossref, Medline, Google Scholar
75. Lawrence KM, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW: Brain 5-HT uptake sites, labelled with [3H]paroxetine, in antidepressant-free depressed suicides. Brain Res 1990; 526:17-22Crossref, Medline, Google Scholar
76. Little KY, McLauglin DP, Ranc J, Gilmore J, Lopez JF, Watson SJ, Carroll FI, Butts JD: Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry 1997; 41:1156-1164Crossref, Medline, Google Scholar



