A Functional Magnetic Resonance Imaging Study of Auditory Mismatch in Schizophrenia
Abstract
OBJECTIVE: Previous research has noted functional and structural temporal lobe abnormalities in schizophrenia that relate to symptoms such as auditory hallucinations and thought disorder. The goal of the study was to determine whether the functional abnormalities are present in schizophrenia at early stages of auditory processing. METHOD: Functional magnetic resonance imaging activity was examined during the presentation of the mismatch stimuli, which are deviant tones embedded in a series of standard tones. The mismatch stimuli are used to elicit the mismatch negativity, an early auditory event-related potential. Ten patients with schizophrenia and 10 comparison subjects were presented the mismatch stimuli condition and a control condition in which only one tone was presented repeatedly. RESULTS: The superior temporal gyrus showed the most prevalent and consistent activation. The superior temporal gyrus showed less activation in the schizophrenic subjects than in the comparison subjects only during the mismatch stimuli condition. CONCLUSIONS: This result is consistent with those of mismatch negativity event-related potential studies and suggests that early auditory processing is abnormal in chronic schizophrenia.
The mismatch negativity is a negative-going brain potential elicited between 150 and 250 msec after the presentation of a deviant stimulus within a repetitive pattern (1) and is usually measured by subtracting the level of activity elicited by the standard stimuli from the level of activity elicited by the deviant stimuli. Naatanen and Gaillard (2) proposed that the mismatch negativity was evoked pre-attentively and that it indexed an echoic memory comparison of a stimulus to the neural trace of previous stimuli (3, 4). Thus, the mismatch negativity is the earliest identified cortical event-related potential that is elicited by deviant, infrequent auditory stimuli and that occurs independently of whether or not the stimuli are consciously attended (5).
Our laboratory recently used high-density event-related potential recording during a mismatch negativity paradigm and found an event-related potential scalp topography abnormality in schizophrenic subjects that was localized to the parietotemporal junction (6) and that was slightly more pronounced on the left. Mismatch negativity amplitude also correlated with several measures of psychopathology in that study, including the severity of auditory hallucinations. In two studies, no mismatch negativity event-related potential abnormalities were found in patients with schizophrenia at their first hospitalization, indicating a functional abnormality that may develop with disease chronicity (7, 8).
The current study used functional magnetic resonance imaging (fMRI) to examine brain activity during presentation of the mismatch stimuli to schizophrenic and comparison subjects. Several fMRI studies involving schizophrenic subjects (9) have found abnormal activity in the temporal lobe (as well as other regions) (10–12). The mismatch stimuli and tone repetition conditions were chosen for the fMRI studies because the related event-related potential response can occur without attention and does not require a response from the subject, thus minimizing effects due to attentional, motivational, performance, or motor differences that might exist between schizophrenic and comparison subjects. This study used the same parameters that were used in the previous event-related potential study from our laboratory (6) and used a block design similar to one used in a combined event-related potential fMRI study of mismatch negativity (13, 14). We predicted that the fMRI results would confirm the lower neural signal previously demonstrated by event-related potential mismatch negativity studies in schizophrenia. The results should also provide spatial source information that event-related potential measures cannot.
Method
Subjects
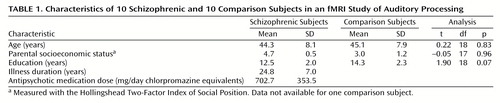
Ten patients with chronic schizophrenia who received medications and 10 comparison subjects were included in the study; all were right-handed men. The schizophrenic subjects were recruited from a pool of subjects that had previously received MRI scans for morphometric studies conducted by the Brockton Veterans Affairs Medical Center. The comparison subjects were recruited through newspaper advertisements. The patients’ diagnoses were made according to the DSM-III-R criteria with information obtained from chart reviews and from assessment with the Structured Clinical Interview for DSM-III-R (15, 16). Parental socioeconomic status was measured with the Hollingshead Two-Factor Index of Social Position. The comparison subjects were required to be 20–55 years of age with no history of electroconvulsive shock treatment, neurologic illness, or steroid use and no lifetime history of DSM-III-R drug or alcohol addiction or abuse within the last 5 years. They were also excluded if they had a history of psychiatric illness in themselves or in their first-degree relatives. The subject groups did not significantly differ (by a two-tailed t test) in age, education, or parental socioeconomic status. Table 1 summarizes the subjects’ characteristics, including doses of antipsychotic medications received by the patients, in mean mg/day of chlorpromazine equivalents. Written informed consent for the procedures was obtained for all subjects before MRI scanning.
MR Image Acquisition Parameters
All images were acquired at the Brigham and Women’s Hospital with a 1.5-T GE Horizon system (General Electric Medical Systems, Milwaukee). Low-resolution anatomical images were acquired after initial sagittal localizer scans. Twenty-one 7-mm thick contiguous coronal oblique spoiled-gradient-recalled images were taken by using the same slice thickness and in the same location and plane as the functional images. The protocol used the following parameters: TE=5 msec, TR=35 msec, one repetition, nutation angle=45°, field of view=24 cm, matrix=256 × 192. Note that low-resolution refers to the slice thickness, not the in-plane resolution of the images. The images were acquired perpendicular to the line of the superior temporal sulcus and covered the majority of the brain for most subjects.
The functional images were acquired in a continuous manner, with 102 whole brain acquisitions (the first two acquisitions are discarded, and the remaining 100 are used in analyses) taken in 306 seconds. Each set of 102 images is referred to as a functional experiment. The functional images were acquired by using the Epibold pulse sequence (General Electric Medical Systems, Milwaukee). This gradient-echo echo-planar sequence was used to acquire 21 contiguous 7-mm coronal slices of the whole brain with the following parameters: TE=40 msec, TR=3 seconds, field of view=24 cm, image resolution=64 × 64, in-plane voxel edge=3.75 mm. The functional images were reconstructed off-line.
High-resolution spoiled-gradient-recalled anatomical images with 124 1.5-mm thick coronal slices were acquired in a separate session and were used for anatomical localization and three-dimensional visualization of activation. The acquisition consisted of the following parameters: TE=5 msec, TR=35 msec, field of view=24 cm, acquisition matrix=256 × 256, voxel dimensions=0.975 mm × 0.975 mm × 1.5 mm.
The high-resolution spoiled-gradient-recalled images were then registered into the lattice of the low-resolution spoiled-gradient-recalled images by using the Mutual Information algorithm that was implemented within an image viewing/editing program (3D Slicer) developed at our site (17).
Procedures for an fMRI Session
General procedures
Before being placed into the MRI device, subjects received the following instructions: “Several images will be taken of your brain; each acquisition will last approximately 5 minutes. It is extremely important not to move while the magnet is acquiring images. I will tell you, through the headphones, when we are about to take images, and then you will hear the magnet sounds as the images are taken. You may also hear tones. Please just ignore them.”
Subjects were placed in the MRI device with the head held within an air-filled Vac-Fix cushion (S&S X-Ray Products, Brooklyn, N.Y.) that was inflated to conform to head shape to secure the head against movement. Tape was then placed across the forehead and the chin. A personal computer equipped with a digital input/output board (model AT-MIO-16E-10, National Instruments, Austin, Tex.) was used to control sound presentation. A signal indicating the application of radio frequency pulses by the functional pulse sequence was also fed into the computer and was used to trigger the sound presentation programs. This input/output hardware was used in conjunction with stimulus control programs written with the Labview software package (National Instruments, Austin, Tex.).
The sound tasks were produced and stored on the computer with the Sound Forge sound editor (Sonic Foundry, Inc., Madison, Wis.). During an fMRI session, the sound tasks were presented through sound-insulated earphones (Silent Scan, Avotec, Jensen Beach, Fla.) that were connected to the computer audio output. The dB level of the tones as heard from the earphones was checked before each session and was approximately 80 dB for each session.
Tasks during functional imaging
Each fMRI experiment contained 30-second epochs (10 scans) of rest alternated with 30-second epochs of tones (either control or mismatch stimuli) that were repeated five times during the continuous acquisition. Tones of 1,000 Hz and 1,200 Hz were presented. All tones were of a 100-msec duration with equally spaced delays between tones of 300 msec, resulting in a presentation rate of three tones per second. Both the 1,000-Hz tone and the 1,200-Hz tone contained a 10-msec rise at the beginning of the tone and 10-msec fall at the end of the tone. During the mismatch stimuli condition, a 1,000-Hz tone was presented as the frequent tone (probability=0.95) and a 1,200-Hz tone as the infrequent tone (probability=0.05); the task was presented for 30 seconds in each epoch. Presentation of the mismatch stimuli condition alternated with 30-second periods of silence. During the control condition, only the dominant or frequent tone (1,000 Hz) was presented for 30 seconds in each epoch, alternated by 30-second periods of silence.
Four fMRI experiments were conducted in one scanning session with each subject as follows: 1) presentation of the mismatch stimuli condition, 2) presentation of the mismatch stimuli condition with a different random mix of frequent and infrequent tones, 3) presentation of the control condition that used a 1,000-Hz tone, and 4) repeat of the control condition. The task order was counterbalanced between subjects.
Data Analysis
Any movement of a subject’s head was detected by viewing the images through a cine loop and by using a low-threshold t test analysis to detect activated regions that followed brain edges (18). Since each subject data set had two fMRI runs per experimental condition, each run was examined for movement and only those that were free of movement were used in the analyses. The data for two comparison subjects and one schizophrenic subject were excluded from further analyses because of movement. It is important to note that a recent study showed that head movement during fMRI procedures does not occur more frequently with schizophrenic patients than with normal subjects (19). Even with a stringent criterion of exclusion for head movement, only 15% of this study’s subjects (roughly equal numbers in the patient and comparison groups) had to be excluded from the study.
The fMRI data analyses were done with the Matlab software programs (Math-works, Inc., Natick, Mass.) that have been developed at our site. Pixels from the fMRI images were subjected to linear detrending and then analyzed by using cross-correlation with a box-car function that was delayed by 6 seconds (two acquisitions) (20). Pixels were considered to be significantly activated if they showed a correlation of 0.55 or above, which was still significant at p<0.0001 after being conservatively corrected for the number of correlations by using the number of voxels in the entire temporal lobe of one randomly chosen comparison subject. The data were analyzed by using several cross-correlation thresholds; it is important to note that the same pattern of significant results was obtained by using thresholds ranging from r=0.45 to the one presented here, r=0.55. Single activated pixels were excluded from the analyses.
We used a region-of-interest approach to classify the region to which active voxels belonged in each subject (21). The regions and methods used in this approach were based on previously published data from morphometric studies in our laboratory (22–24). The regions of interest were the superior temporal gyrus (the anterior and posterior portions were combined); the remaining temporal lobe (regions of the cortex of the temporal lobe that are not in the superior temporal gyrus); the temporal-parietal junction and the inferior parietal lobule; the inferior, middle, and superior frontal gyrus; and the cingulate gyrus.
The activated pixels for each subject were viewed in relationship to both the high- and low-resolution anatomical images within the 3D Slicer program (image editor and viewing program) developed in our laboratory (17, 25). The activated pixels were then reclassified with a preassigned pixel value according to the anatomical region to which they belonged. This was done by a neuroscientist (C.G.W.) who had previously classified structural MRI regions of interest according to our group’s criteria. The number of pixels for these regions were then counted by using a histogram program.
It was evident that the superior temporal gyrus was the only region that consistently showed activation. A subsequent subdivision of the superior temporal gyrus was done to further characterize this activation. Pixels (or voxels) on coronal slices that were in the superior temporal plane were classified as being from either the anterior, posterior, or middle portions of that region. These pixels roughly corresponded to those that were anterior to, or posterior to, or part of/adjacent to Heschl’s gyrus (the middle portion). It is noteworthy that the relatively large voxel size of the fMRI data did not allow for as precise a determination of location as can be found in structural volumetric studies. There were also a few subjects for whom pixels were found on the ventral gray matter of the superior temporal gyrus, and these were classified as ventral superior temporal gyrus activations. The anterior superior temporal gyrus plane designation included voxels found in any coronal slices anterior to those containing the transverse gyri or gyrus (Heschl’s gyrus). The middle superior temporal gyrus plane designation was given to voxels that were on any coronal slice that also contained a portion of the transverse gyrus. The posterior superior temporal gyrus plane designation was given to voxels that were on any coronal slice that was posterior to the last slice containing the transverse gyrus.
A repeated measures analysis of variance (ANOVA) was then performed by using the number of active pixels in the left and right superior temporal gyrus for each subject. The ANOVA used the factors group, task, and hemisphere; all significance tests were two-tailed. The percentages of total activation accounted for by the right and left anterior, middle, posterior, and ventral superior temporal gyrus subdivisions were also computed. Correlations were performed between activation measures and the z score of the relative volume of the left and right posterior superior temporal gyrus (roughly encompassing the middle and posterior subdivisions as described here; the regions of interest are defined in reference 23). These volumetric measures were obtained from ongoing volumetric projects in the laboratory.
Results
The superior temporal gyrus was the most active region in all subjects and was the only region that was consistently activated across subjects. Significant effects of task (F=6.98, df=1, 18, p<0.02), side (F=6.14, df=1, 18, p=0.02), and the interaction of group and task (F=7.12, df=1, 18, p<0.02) were found. As illustrated in Figure 1, activation was generally larger during the mismatch stimuli condition than during the control condition, especially for comparison subjects. The mean activation for schizophrenic subjects was similar for all of the tasks. Individual follow-up t tests showed that comparison and schizophrenic subjects differed in the number of activated voxels in the superior temporal gyrus during the mismatch (t=2.52, df=18, p<0.03) but not during the control condition (t=–0.92, df=18, p=0.37). The mean activation for schizophrenic subjects during the control condition was slightly larger than the mean for comparison subjects, although this difference was not statistically significant. Figure 2 shows a slice through the superior temporal plane containing the active voxels for a comparison subject during the control and mismatch stimuli conditions.
Most of the superior temporal gyrus activation (70% bilaterally; 68% on the left and 71% on the right side) was located in the middle of the superior temporal plane (near Heschl’s gyrus) and in the posterior portion of this gyrus (21% bilaterally; 30% on the left and 13% on the right side). A few pixels were found in the anterior superior temporal gyrus only on the left (1% of the total left superior temporal gyrus activation). Also, in two subjects, one schizophrenic patient and one comparison subject, the right ventral gray matter of the superior temporal gyrus showed activation.
The z score for the absolute volume of the posterior superior temporal gyrus volume correlated with activation during the mismatch stimuli condition only in schizophrenic subjects and only on the right side (r=0.67, df=9, p<0.03), but this value was not significant after Bonferroni correction for the number of tests.
Discussion
The fMRI activation during the mismatch stimuli condition was predominately located in the middle (70% of the total superior temporal gyrus activation) and posterior portion of the superior plane of the superior temporal gyrus. The superior temporal plane contains auditory and verbal language processing regions, including the primary auditory cortex (Heschl’s gyrus), which receives input from both ears and contains several distinct tonotopically organized regions (26). The control condition, repeated presentation of one tone, also activated these regions but produced less overall activation (likely from habituation) than the mismatch stimuli condition, especially in comparison subjects (Figure 1). The activation during both tasks was generally larger in the left hemisphere for both groups of subjects. This is consistent with other fMRI reports showing that tones and white noise, respectively, activated the superior temporal gyrus and produced a more pronounced response in the left hemisphere (27, 28).
The schizophrenic subjects showed significantly less activation in both the left and right superior temporal gyrus during the mismatch stimuli condition than did the comparison subjects. The two groups did not differ significantly in terms of activation during the control condition. The schizophrenic subjects did show a greater mean activation to presentation of the repeated tones than the comparison subjects, but this difference was not statistically significant. Further studies of stimulus repetition will be needed to determine whether schizophrenic subjects have abnormal habituation to tones. Exploratory correlations showed a positive correlation between right superior temporal gyrus activity during the mismatch negativity condition and the relative volume of the middle/posterior right superior temporal gyrus in schizophrenic subjects only.
The finding of decreased activation of the superior temporal gyrus in schizophrenic subjects during the mismatch stimuli condition is consistent with the findings of a number of studies. Using a high-density 64-channel event-related potential recording montage, investigators in our laboratory found a reduced mismatch negativity potential in subjects with chronic schizophrenia that was present bilaterally but more pronounced in the left hemisphere (6). This result replicated those found previously in both medicated and unmedicated schizophrenic subjects (29–33). The current study did not find a lateralized abnormality, which, although found previously in our laboratory, has not been found in all studies.
Because of differences in temporal resolution between fMRI and event-related potential, the superior temporal gyrus activation abnormality that was found cannot be definitively identified as the source of mismatch negativity event-related potential abnormalities in schizophrenia, although it is compatible with that interpretation. In a combined event-related potential and fMRI study of the mismatch negativity task that used inverse source analyses to localize the mismatch negativity signal, the superior temporal gyrus was found to be the most dominant contributor to the event-related potential signal within the 100–160-msec time window (13, 14). Magnetoencephalographic event-related potential source analysis (34–36) and depth recordings in humans (37, 38) and animals (39, 40) also suggest that mismatch negativity is generated in the primary auditory cortex and adjacent regions of the superior temporal gyrus.
Additional evidence of superior temporal gyrus abnormalities in schizophrenic subjects comes from fMRI and positron emission tomography (PET) studies that have used auditory stimulation. A recent study found that fMRI activity in Heschl’s gyrus was significantly increased during auditory hallucinations, providing a strong link between superior temporal gyrus activity and schizophrenic symptoms (41). Another fMRI study found decreased activation in schizophrenic subjects in the superior temporal gyrus (and some frontal regions) during both a tone working-memory task and a verbal working-memory task (10, 42). A PET study of auditory attention in schizophrenia found that superior temporal gyrus activation in schizophrenic subjects showed an abnormal asymmetry (underactive on the right and overactive on the left), compared to activation in comparison subjects (43).
Volumetric MRI studies have also shown that the left superior temporal gyrus and the planum temporale are abnormal in chronic (23, 44) and first-episode schizophrenic subjects (45, 46) and that the abnormalities are correlated with clinical symptoms such as thought disorder (23) and auditory hallucinations (47).
In summary, several studies now provide converging evidence that the superior temporal gyrus in schizophrenic subjects shows functional and structural abnormalities and that these abnormalities may underlie some of the hallmark symptoms of schizophrenia. In addition, these abnormalities are present at early stages of the auditory processing of relatively simple stimuli and not just at stages involving higher-order semantic or language processes.
 |
Received May 30, 2000; revision received Nov. 22, 2000; accepted Dec. 4, 2000. From the Department of Psychiatry, Harvard Medical School; the Department of Psychiatry, Brockton Veterans Affairs Medical Center; and the Department of Radiology, Surgical Planning Laboratory, Brigham and Women’s Hospital, Boston. Address reprint requests to Dr. McCarley, Department of Psychiatry, 116A, Brockton Veterans Affairs Medical Center, 940 Belmont St., Brockton, MA 02401; [email protected] (e-mail). Supported by NIMH grants MH-40799 and MH-52807 and a grant from the Commonwealth of Massachusetts Research Center to Dr. McCarley, NIMH grants MH-50740 and MH-01110 to Dr. Shenton, the Department of Veterans Affairs Medical Research Service, and the National Alliance for Research on Schizophrenia and Depression.
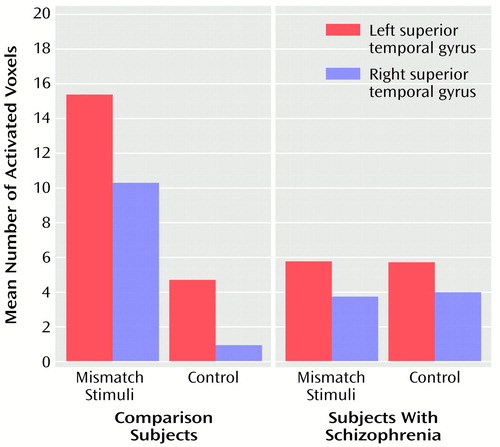
Figure 1. Number of Activated Voxels in the Left and Right Superior Temporal Gyrus of 10 Comparison Subjects and 10 Subjects With Schizophrenia During the Mismatch Stimuli and Control Conditions of an fMRI Study of Auditory Processinga
aEach epoch of the mismatch condition consisted of presentation of a 1,200-Hz tone embedded in a series of 1,000-Hz tones at a rate of three tones per second over 30 seconds such that 5% of the tones were 1,200 Hz and 95% were 1,000 Hz. Each epoch of the control condition consisted of repetition of 1,000-Hz tones at a rate of three tones per second over 30 seconds.
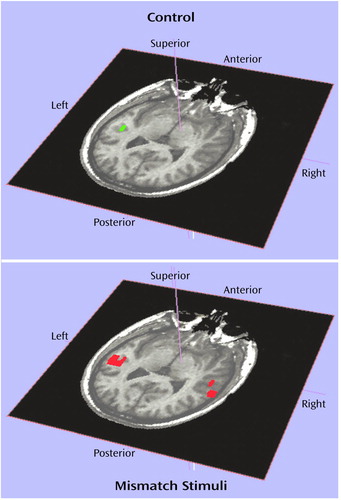
Figure 2. Activation in the Superior Temporal Plane in a Comparison Subject During the Control and Mismatch Stimuli Conditions of an fMRI Study of Auditory Processinga
aEach epoch of the mismatch condition consisted of presentation of a 1,200-Hz tone embedded in a series of 1,000-Hz tones at a rate of three tones per second over 30 seconds such that 5% of the tones were 1,200 Hz and 95% were 1,000 Hz. Each epoch of the control condition consisted of repetition of 1,000-Hz tones at a rate of three tones per second over 30 seconds.
1. Naatanen R, Gaillard AWK, Mantysalo S: Early selective-attention effect on evoked potential reinterpreted. Acta Psychol 1978; 42:313–329Crossref, Medline, Google Scholar
2. Naatanen R Gaillard AWK: The N2 deflection of ERP and the orienting reflex, in EEG Correlates of Information Processing: Theoretical Issues. Edited by Gaillard AWK, Ritter W. Amsterdam, North Holland, 1983, pp 119–141Google Scholar
3. Naatanen R: The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci 1990; 13:201–288Crossref, Google Scholar
4. Cowan N: On short and long auditory stores. Psychol Bull 1984; 96:341–370Crossref, Medline, Google Scholar
5. Javitt DC, Grochowski S, Shelley A, Ritter W: Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr Clin Neurophysiol 1998; 108:143–153Crossref, Medline, Google Scholar
6. Hirayasu Y, Potts GF, O’Donnell BF, Kwon JS, Arakaki H, Akdag SJ, Levitt JJ, Shenton ME, McCarley RW: Auditory mismatch negativity in schizophrenia: topographic evaluation with a high-density recording montage. Am J Psychiatry 1998; 155:1281–1284Google Scholar
7. Salisbury DF, Farrell DC, Shenton ME, Fischer IA, Zarate C, McCarley RW: Mismatch negativity is reduced in chronic but not first episode schizophrenia (abstract). Biol Psychiatry 1998; 45(suppl):25SGoogle Scholar
8. Umbricht D, Javitt D, Bates J, Pollak S, Lieberman J, Kane J: Auditory event-related potentials (ERP) in first episode and chronic schizophrenia (abstract). Biol Psychiatry 1997; 41(suppl):46SGoogle Scholar
9. Renshaw PF, Levin JM, Kaufman MJ, Ross MH, Lewis RF, Harris GJ: Dynamic susceptibility contrast magnetic resonance imaging in neuropsychiatry—present utility and future promise. Eur Radiol 1997; 7(suppl 5):S216–S221Google Scholar
10. Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE: Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry 1998; 55:1097–1103Google Scholar
11. Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM: Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry 1997; 154:1676–1682Google Scholar
12. Yurgelun-Todd DA, Waternaux CM, Cohen BM, Gruber SA, English CD, Renshaw PF: Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry 1996; 153:200–205Link, Google Scholar
13. Opitz B, Mecklinger A, Friederici AD, von Cramon DY: The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cereb Cortex 1999; 9:379–391Crossref, Medline, Google Scholar
14. Opitz B, Mecklinger A, Von Cramon DY, Kruggel F: Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology 1999; 36:142–147Crossref, Medline, Google Scholar
15. Spitzer RL, Williams JBW, Gibbon M, First MB: User’s Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
16. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
17. Wells WM III, Viola P, Atsumi H, Nakajima S, Kikinis R: Multi-modal volume registration by maximization of mutual information. Med Image Anal 1996; 1:35–51Crossref, Medline, Google Scholar
18. Kanwisher N, McDermott J, Chun MM: The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17:4302–4311Google Scholar
19. Prohovnik I, Wexler BE, Stevens A, Skudlarski P, Gore JC: Motion assessment of schizophrenics during fMRI (abstract). Neuroimage 1999; 9:662Google Scholar
20. Buckner RL, Koutstaal W, Schacter D, Wagner A, Rosen B: Functional-anatomic study of episodic retrieval using fMRI I: retrieval effort versus retrieval success. Neuroimage 1998; 7:151–162Crossref, Medline, Google Scholar
21. Kastner S, DeWeerd P, Desimone R, Ungerleider LG: Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 1998; 282:108–111Crossref, Medline, Google Scholar
22. Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW: Parcellation of the human prefrontal cortex in schizophrenia: a quantitative MRI study. Psychiatry Res Neuroimaging 1997; 76:29–40Crossref, Medline, Google Scholar
23. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
24. Shenton ME, Kikinis R, McCarley RW, Saiviroonporn P, Hokama H, Robatino A, Metcalf D, Wible CG, Portas CM, Iosifescu DV, Donnino R, Goldstein JM, Jolesz FA: Harvard Brain Atlas: a teaching and visualization tool. IEEE Biomedical Visualization 1995; 61:209–229Google Scholar
25. Gering D, Nabavi A, Kikinis R, Grimson WEL, Hata N, Everett P, Jolesz F, Wells WM III: An integrated visualization system for surgical planning and guidance using image fusion interventional imaging, in Medical Image Computing and Computer-Assisted Intervention. Edited by Taylor C, Colchester A, Goos G, Hartmanis J, Van Leeuwen J. New York, Springer-Verlag, 1999, pp 809–819Google Scholar
26. Brugge JF, Merzenich MM: Patterns of activity of single neurons of the auditory cortex in monkey, in Basic Mechanisms of Hearing. Edited by Moller AR. New York, Academic Press, 1973, pp 745–766Google Scholar
27. Millen SJ, Haughton VM, Yetkin Z: Functional magnetic resonance imaging of the central auditory pathway following speech and pure-tone stimuli. Laryngoscope 1995; 105:1305–1310Google Scholar
28. Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, Hyde JS: Functional magnetic resonance imaging of human auditory cortex. Ann Neurol 1994; 35:662–672Crossref, Medline, Google Scholar
29. Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N: Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry 1991; 30:1059–1062Google Scholar
30. Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan Jr HG: Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol Psychiatry 1993; 33:513–519Crossref, Medline, Google Scholar
31. Javitt DC, Doneshka P, Grochowski S, Ritter W: Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry 1995; 52:550–558Crossref, Medline, Google Scholar
32. Oades RD, Zerbin D, Eggers C: Negative difference (Nd), an ERP marker of stimulus relevance: different lateral asymmetries for paranoid and nonparanoid schizophrenics. Pharmacopsychiatry 1994; 27:65–67Crossref, Medline, Google Scholar
33. Shutara Y, Koga Y, Fujita K, Takeuchi H, Mochida M, Takemasa K: An event-related potential study on the impairment of automatic processing of auditory input in schizophrenia. Brain Topogr 1996; 8:285–289Crossref, Medline, Google Scholar
34. Alho K, Paavilainen P, Reinikainen K, Sams M, Naatanen R: Separability of different negative components of the event-related potential associated with auditory stimulus processing. Psychophysiology 1986; 23:613–623Crossref, Medline, Google Scholar
35. Hari R, Hamalainen M, Ilmoniemi R, Kaukoranta E, Reinikainen K, Salminen J, Alho K, Naatanen R, Sams M: Responses of the primary auditory cortex to pitch changes in a sequence of tone pips: neuromagnetic recordings in man. Neurosci Lett 1984; 50:127–132Crossref, Medline, Google Scholar
36. Scherg M, Picton TW: Brain electric source analysis of the mismatch negativity, in Psychophysiological Brain Research. Edited by Brunia CMH, Gaillard AWK, Kok A. Tilburg, the Netherlands, Tilburg University Press, 1990, pp 94–98Google Scholar
37. Kropotov JD, Naatanen R, Sevostianov AV, Alho K, Reinikainen K, Kropotova OV: Mismatch negativity to auditory stimulus change recorded directly from the human temporal cortex. Psychophysiology 1995; 32:418–422Crossref, Medline, Google Scholar
38. Tiitinen H, Alho K, Huotilainen M, Ilmoniemi RJ, Simola J, Naatanen R: Tonotopic auditory cortex and the magnetoencephalographic (MEG) equivalent of the mismatch negativity. Psychophysiology 1993; 30:537–540Crossref, Medline, Google Scholar
39. Csepe V, Karmos G, Molnar M: Evoked potential correlates of stimulus deviance during wakefulness and sleep in cat—animal model of mismatch negativity. Electroencephalogr Clin Neurophysiol 1987; 66:571–578Crossref, Medline, Google Scholar
40. Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC: Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res 1994; 667:192–200Crossref, Medline, Google Scholar
41. Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann I, Singer W: Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999; 22:615–621Crossref, Medline, Google Scholar
42. Wexler BE, Stevens AA, Bowers AA, Sernyak MJ, Goldman-Rakic PS: Word and tone working memory deficits in schizophrenia. Arch Gen Psychiatry 1998; 55:1093–1096Google Scholar
43. O’Leary DS, Andreasen NC, Hurtig RR, Kesler ML, Rogers M, Arndt S, Cizadlo T, Watkins GL, Boles Ponto LL, Kirchner PT, Hichwa RD: Auditory attentional deficits in patients with schizophrenia: a positron emission tomography study. Arch Gen Psychiatry 1996; 53:633–641Crossref, Medline, Google Scholar
44. Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME: Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry 1999; 56:142–148Crossref, Medline, Google Scholar
45. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Google Scholar
46. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry2000; 57:692–699Google Scholar
47. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar



