Neuropsychology of First-Episode Schizophrenia: Initial Characterization and Clinical Correlates
Abstract
OBJECTIVE: Neuropsychological impairments are well documented in schizophrenia and are important targets of treatment. Information about the severity and pattern of deficits after treatment for the first psychotic episode and about relationships between these deficits and syndromal characteristics remains limited. METHOD: Comprehensive neuropsychological assessments including 41 individual tests were given to 94 patients with first-episode schizophrenia after initial stabilization of psychosis and to a comparison group of 36 healthy volunteers. Profiles of neuropsychological deficits and the relationship of deficits to sex and handedness were examined. Correlations of neuropsychological deficit with a broad range of historical and clinical characteristics, including outcome, were explored. RESULTS: Patients had a large generalized neuropsychological deficit (1.5 standard deviations compared to healthy volunteers). Patients also had, superimposed on the generalized deficit, subtle relative deficits (less than 0.5 standard deviation compared to their own average profile) in memory and executive functions. Learning/memory dysfunction best distinguished patients from healthy individuals; after accounting for this difference, only motor deficits further distinguished the groups. Patients with higher neuropsychological ability had only memory deficits, and patients with lower ability had both memory and executive deficits. No sex differences were observed beyond the normal advantage for men in motor speed. Dextral patients had less severe generalized deficit. Severity of residual symptoms was associated with greater generalized deficit. Executive and attentional deficits were most linked to global functional impairment and poor outcome. CONCLUSIONS: The results document a large generalized deficit, and more subtle differential deficits, in clinically stabilized first-episode patients. Learning/memory deficits were observed even in patients with less severe generalized deficit, but the pattern was unlike the amnestic syndrome and probably reflects different mechanisms. Executive and attentional deficits marked the more severely disabled patients, and may portend relatively poor outcome. Failure to develop typical patterns of cerebral dominance may increase the risk for greater generalized deficit.
Neuropsychological deficits are recognized as an important pathologic dimension in schizophrenia. These deficits are often severe and pervasive, but estimates of severity have varied, knowledge about when deficits first appear is incomplete, and the interpretation of deficit patterns remains controversial. Some researchers have emphasized the generalized deficit and highlighted the psychometric hurdles that must be overcome to identify differential deficits (1–4). Others have emphasized relative deficits on tasks that are putatively sensitive to dysfunction of specific brain systems, including 1) the frontal lobes (5), 2) the temporal and/or mesiotemporal regions (6, 7), and 3) integrated frontotemporal or frontolimbic systems (8–13). Although attempts to localize brain dysfunction in schizophrenia on the basis of neuropsychological tests have had multiple problems (14), specific deficit patterns may nevertheless pose significant constraints on models of pathophysiology.
Furthermore, substantial interest is currently focused on the capacity of novel treatments to ameliorate neuropsychological deficits. Given suggestions that neuropsychological deficits are linked to disability (15), treatments that are beneficial for cognition may also attenuate other functional limitations. It is therefore crucial to have solid information about the severity and patterns of deficits in schizophrenia and their relations to clinical state, treatments, and social-vocational outcome.
Most studies examining neuropsychological deficits in schizophrenia have involved chronic patients, and the findings may reflect the long-term illness or treatment experienced by these patients. These study groups may also be biased toward poorer treatment response and outcome. Some results from groups of first-episode patients have been reported (4, 7, 16–20). These studies have shown that deficits are present at illness onset, but their conclusions have been limited by a variety of factors, such as small size of the study group, lack of standardized treatments, patients’ lack of clinical stability at the time of assessment, or lack of longitudinal clinical follow-up.
This report provides comprehensive neuropsychological characterization of patients after the first episode of illness, when most patients satisfied criteria for clinical stabilization after standardized treatment with conventional antipsychotics. The strategy of assessing first-episode patients after clinical stabilization aimed to maximize measurement of stable trait-like neuropsychological characteristics and to minimize transient effects associated with acute psychosis and its treatment. The results of a longitudinal neuropsychological follow-up over 5 years and of neuropsychological tests administered before treatment, as well as descriptions of correlations with anatomic measures from magnetic resonance imaging, are being reported separately. We previously reported preliminary neuropsychological findings in a subgroup of the patients described here (8, 16, 21, 22) and recently published papers about treatment response and outcome in the overall group of 118 patients from which the patients described here were selected (23, 24). The study reported here examined 1) the magnitude of neuropsychological deficit in patients with schizophrenia after clinical stabilization of the first episode of psychosis, 2) the pattern of neuropsychological deficits in this group, and 3) the nature of any neuropsychological differences between groups defined by sex or handedness. We further examined relations of neuropsychological deficits with a broad range of historical, symptomatic, treatment response, and outcome characteristics to provide descriptive information about the possible selectivity and magnitude of these associations.
METHOD
Subjects
Subjects were participants in the Prospective Study of Psychobiology in First-Episode Schizophrenia at Hillside Hospital in Glen Oaks, N.Y. (23–26). Patients who were admitted to the hospital inpatient service for a first episode of psychotic illness and who had received less than 12 prior weeks of cumulative lifetime neuroleptic treatment were recruited for the study. Patients satisfied Research Diagnostic Criteria (RDC) (27, 28) for schizophrenia or schizoaffective disorder, on the basis of structured interviews with the Schedule for Affective Disorders and Schizophrenia (SADS) (29) and reviews of patients’ histories. Patients with a current or past serious neurologic or endocrine disorder were excluded. After a complete description of the study was provided to the subjects, we obtained their written informed consent. Further details of ascertainment and treatment have been published elsewhere (23–26). The healthy comparison group was recruited through announcements in local newspapers and within the medical center. These subjects were selected to be similar to the patients on distributions of sex and age. They were free of RDC mental disorders, as determined by using the SADS Lifetime Version interview, physical examination, and urinalysis (25). None of the subjects had a current substance use disorder or a history of substance dependence, chronic neurologic or medical illness, or drug treatment known to affect the brain.
Procedure
Neuropsychological tests were planned for 6 months after study entry if the patient had already achieved remission or a stable level of residual symptoms. We selected 6 months because pilot data showed asymptotic levels of symptom remission at that point. Patients who did not satisfy the criteria for symptom remission or stable residual symptoms at 6 months were tested as soon as possible after they satisfied the criteria. Remission was defined as 1) a rating not greater than 3 on the positive psychotic symptoms items of the SADS Change Version (SADS-C) psychosis and disorganization dimensions, 2) a rating of 3 (mild) or less on the Clinical Global Impression (CGI) severity items, 3) a rating of 2 (much improved) or 1 (very much improved) on the CGI improvement items, and 4) maintenance of this level of response for 8 weeks. Stability of residual symptoms required no changes greater than 1 point on positive psychotic symptom items of the SADS-C psychosis and disorganization dimensions or on global ratings on the Scale for the Assessment of Negative Symptoms (SANS) for two consecutive biweekly rating periods.
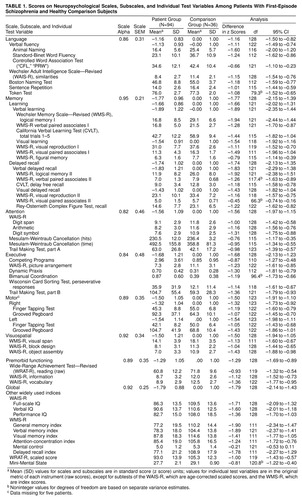
Test administration was typically divided into two 3–4 hour sessions within 1 week, and two counterbalanced test sequences were employed across subjects to minimize possible order effects. The battery included 41 tests from which variables were selected to characterize six neuropsychological domains represented by the following scales: language, memory, attentional, executive, motor, and visuospatial. A global neuropsychological scale that represented the mean of these six scales was constructed. A scale for measuring premorbid intellectual ability was also constructed, based on the idea that certain tests of general knowledge, vocabulary, and reading skill are less vulnerable to deterioration, as our previous reports have shown (16, 30). Loading of test variables on scales was based on a priori assessment of content validity, by using methods similar to those used previously by our group (8, 30–32) and others (3, 6, 7, 17). Scores for each scale were computed by averaging z scores on contributing variables. These z scores were based on performance of the healthy comparison group, which by definition had mean scale scores of zero and standard deviations set to one. All scales were computed so that higher values indicated better performance. At each stage of scale construction, contributing variables were restandardized before means were computed over all nonmissing data. The values for widely used test variables were not restandardized; the original scores, t tests for the difference between groups, and the 95% confidence intervals (CIs) for the difference in original score units, are provided for descriptive purposes.
Additional scaling procedures were applied to improve psychometric properties, and confirmatory factor analysis was used to assess validity of the assignments of variables to scales. First, each test variable was examined for extreme values, and in several instances these deviant scores were replaced by scores within the tails of their underlying distributions (this procedure affected two tests and a total of seven patients’ scores). Second, we examined each variable for possible sex differences. Only the Finger Tapping Test showed a significant difference, with men having higher scores, and therefore variables from this test were standardized separately in each sex. Third, the distributions were examined both within and between groups, with special attention to problems involving heteroscedasticity, and variance-stabilizing algorithms were applied (in most cases power transformations) to optimize homogeneity of variance between groups for each variable (Levene’s test was used as a criterion). Fourth, reliability analyses were conducted for each scale, using the initial a priori variable assignments, and any test variable that decreased the internal consistency of the composite (as assessed by Cronbach’s coefficient alpha) was eliminated. Fifth, the scales were computed, and at each stage of scale construction, further tests on homogeneity of variance were conducted and transformations were applied where indicated, as was done for the individual test variables. Finally, the validity of variables’ assignments to scales, which was solely on a rational basis, was examined using confirmatory factor analysis (33) This analysis supported the rational grouping of neuropsychological variables into the scales described here (comparative fit index=0.92 [χ2=1593, df=29, p<0.001] for the comparison with the null model; comparative fit index=0.86 [χ2=1494, df=24, p<0.001] for the conparison with the single-factor model). The final coefficient alpha for each scale, and the standard error of measurement, are provided for each scale (table 1). Scales with higher reliability are more sensitive to group differences than less reliable scales. In this study the standard error of measurement varied from 0.21 to 0.46 across scales, offering relatively high uniformity and precision in the estimate of true scores compared to other reports in the literature. Although alternate models might yield superior results, systematic exploration of these models is beyond the scope of this paper. Further details of scale construction, and the code used to specify all transformations, are available on request, as are raw data for those desiring to test alternate models for scale composition.
Hand preference was assessed using a modified 20-item Edinburgh Inventory (34). The total number of right- and left-hand items were scored, and the laterality quotient was computed as (total right–total left)/(total right+total left). Thus laterality quotients could range from 1.00 (all items right) to –1.00 (all items left). Subjects with a laterality quotient greater than 0.70 were classified as dextral; the rest were classified as nondextral (35).
Data Analysis
The primary tests of group differences used profile analysis by multivariate analysis of variance (MANOVA), with group as a between-subjects factor and neuropsychological scale as a within-subject factor. Multivariate analysis of covariance (MANCOVA) was used to assess effects of possible moderating variables. Deviations from flatness in the patient profiles, if suggested by significant effects of the interactions of group and scale, were assessed by contrasting the mean for each individual scale with the mean of all other scales using paired t tests. This procedure allowed us to determine which scales showed impairment relative to each of the other scales in the analysis of data from the patient group. We also used the method recommended by Chapman and Chapman (36), involving standardized residual scores. In contrast to the profile analysis by MANOVA (which examines the extent to which the average score on Scale X differs from the mean score on other scales), standardized residual score analysis considers the statistical interdependence of the scales (i.e, examines the extent to which a subject’s score on Scale X is deviant, given that subject’s scores on the other scales). Prediction equations were based on data from the comparison group.
RESULTS
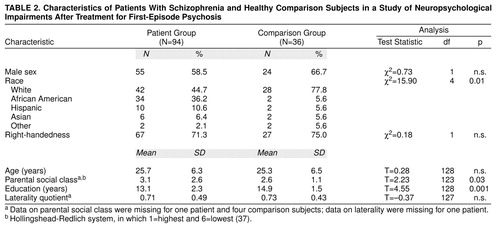
The 94 patients completing neuropsychological exams satisfied RDC for schizophrenia (N=70; subtypes included: paranoid=54, disorganized=4, catatonic=1, and undifferentiated=11) or schizoaffective disorder (N=24). Characteristics of the patient group and the comparison group are provided in table 2 The groups were well matched on distributions of sex, age, and hand preference, but they differed in racial/ethnic group composition, parental social class, and education. Possible effects of these differences were examined in subsequent analyses.
The 94 patients, who completed at least one comprehensive neuropsychological examination, were a subset of the 118 patients described by Robinson et al. (23, 24), reflecting an 80% completion rate. To determine if the 94 patients were representative of the larger group, we compared the 94 patients who completed the neuropsychological examinations with the 24 patients who did not. The two groups were similar in age, education, racial/ethnic group composition, parental social class, RDC diagnosis, duration of symptoms before study entry, measures of symptoms at baseline, and course/outcome characteristics (all p values >0.05). Compared to the patients who completed the neuropsychological examinations, those who did not were more likely to be women (75%, N=18, compared to 41%, N=39; χ2=8.6, df=1, p=0.003) and to have been married (42%, N=10, compared to 16%, N=15; χ2=24.0, df=3, p=0.00002).
Among the 94 patients, nine patients did not satisfy stabilization criteria at 6 months when they were initially scheduled for examination. They were reexamined during the next year, after they had achieved the criteria. Twelve patients who entered the study before the neuropsychological protocol was put in place (April 1988) were examined as soon as possible after the protocol was established. The modal time from the beginning of treatment to neuropsychological examination was 0.47 years (median=0.61 years); 79% (N=74) of the patients were seen within the first year of any treatment.
Figure 1 shows the mean neuropsychological profile for the patients relative to the healthy comparison group. The patient group was more impaired than the comparison group on every neuropsychological dimension measured. Mean effect sizes (in z score units, reflecting the number of standard deviations below the comparison group means) ranged from –1.11 to –1.75; 95% CIs ranged from a minimum deficit of –0.82 to a maximum deficit of –2.13 (main effect of group: F=87.2, df=1, 123, p<0.0001, N=125). The overall profile mean for the patients was –1.53, indicating a generalized deficit of approximately 1.5 standard deviations.
The patient profile deviated significantly from flatness; in other words, the means for some scales reflected more impairment than the means for other scales (group-by-scale interaction: Wilks lambda=0.91; F=2.39, df=5, 119, p<0.04, N=125). Specifically, the memory (paired t=3.44, df=88, p=0.001, N=90) and executive (paired t=–2.36, df=88, p=0.02, N=90) scales showed significantly more impairment, and the language scale (paired t=5.64, df=88, p<0.001, N=90) showed significantly less impairment, compared with the remaining scales.
Additional analyses examined possible effects of parental social class (by using MANCOVA), racial/ethnic group composition (by examining results for white subjects, the only racial/ethnic group large enough for analysis), and diagnostic subgroups (by examining results for patients with RDC schizophrenia only compared to the healthy subjects). None of these analyses produced findings that differed substantively from those of the original analyses. No significant differences in neuropsychological profiles were found between the patients with schizophrenia and those with schizoaffective disorder.
Analysis of standardized residual scores revealed a significant group effect (Wilks lambda=0.77, F=5.84, df=6, 118, p=0.001, N=125), with patients showing significant residual deficits on memory (F=5.70, df=1, 123, p=0.02, N=125) and motor (F=6.65, df=1, 123, p=0.01, N=125) scales. Further descriptive statistics and effect sizes for individual tests are provided in table 1.
Because the generalized deficit of the patients was so large, we examined differences in neuropsychological profiles between groups of patients with low and high levels of general ability (median split on the global neuropsychological scale). The high-ability group (N=43) had a mean deficit of –0.83 standard deviations (95% CI=–0.98 to –0.70), and the low-ability group (N=46) had a mean deficit of –2.22 standard deviations (95% CI=–2.36 to –2.07), relative to the comparison group. MANOVA showed that these groups differed in profile (Wilks lambda=0.65; F=9.03, df=5, 83, p<0.001). The high-ability group showed relative deficits (compared to their own profile mean) only on the memory scale (mean deficit=–1.07, 95% CI=–1.26 to –0.87). The low-ability group showed relative deficits on both the memory scale (mean deficit=–2.48, 95% CI=–2.68 to –2.28) and the executive scale (mean deficit=–2.50, 95% CI=–2.75 to –2.25). The low-ability group also showed a higher mean language scale score than would be expected based on their profile mean (mean deficit=–1.60, 95% CI=–1.82 to –1.38).
Effects of sex were assessed using MANOVA with diagnostic group (patient group or comparison group) and sex as between-subject factors and the six neuropsychological scales as within-subject factors. The main effect of sex (F=0.69, df=1, 121, p=0.40, N=125), and all of the interactions involving sex were nonsignificant (all F values <1). The main effect of group and the effect of the interaction of group and scale were essentially identical to the original analysis. In addition, we examined the sex difference on the scale that measured premorbid functioning. Significantly greater impairment was found in the female patients compared to the male patients (t=2.89, df=92, p<0.005, N=94); no sex differences were observed in the comparison group.
Handedness effects could not be assessed using MANOVA due to a violation in the assumption of multivariate homogeneity of variance (Box’s M=121; approximate F=1.61, df=63, 3297, p<0.002). We therefore examined differences using t tests. No significant effects of handedness were found in the comparison group. In contrast, the 67 dextral patients performed approximately 0.5 standard deviations higher on each scale compared to the 27 nondextral patients. These groups differed in global neuropsychological scale score by 0.47 scaled score units (mean deficit for dextral patients=–1.65, SD=0.87; mean deficit for nondextral patients=–2.12, SD=0.84; t=2.4, df=92, p=0.02). Differences were also observed on the scales for the neuropsychological domains (range of differences on individual scales=0.35–0.63, range of t values=1.6–2.6, df=92, range of p values=0.11–0.01). No significant differences in the distribution of handedness were found among male and female patients.
To examine the possibility that a subgroup of patients with mixed hand preference might be particularly impaired (38), we inspected scatterplots of neuropsychological scale scores with respect to the laterality quotient. We then examined the effects of adding a quadratic term to the linear relation between the laterality quotient and the global neuropsychological scale score within the patient group. The overall regression analysis was significant (F=6.2, df=2, 90, p<0.003, N=94), but the significance was mostly due to the linear term (beta=0.41, T=3.5, p<0.007), supporting the original analysis. The quadratic term contributed less (beta=–0.18, T=–1.5, p=0.13), and the sign of this term was opposite that predicted if patients with mixed handedness performed more poorly. There were no significant correlations of laterality quotient with neuropsychological scale scores in the comparison group.
We explored relationships between neuropsychological scale scores and demographic, historical, and clinical variables, including: 1) age at time of examination, 2) age at onset of first psychotic symptoms, 3) ratings on the NIMH modification of the Premorbid Adjustment Scale (39), 4) global ratings of extrapyramidal symptoms made after 8 and 16 weeks of treatment, 5) global measures of course made by physicians after patients completed the first year of the study, 6) ratings on the Social Adjustment Scale made after 2 years in the study, 7) ratings of the deficit syndrome (40), and 8) medications prescribed at the time of testing. Further descriptions and operational criteria are published elsewhere (16, 23–26, 41, 42). Because the goal of these analyses was descriptive, and given the large number of tests conducted, only relations with an effect size equivalent to r>0.30 were interpreted as significant. Use of this threshold protected against type I error and helped ensure that reported findings are likely to be replicated. A sample size of 94 yields 85% power to detect an effect of this size with alpha set at 0.05 (two-tailed). The probability of observing r>0.30 in this sample is approximately 0.005 (two-tailed). The standard error of these correlations is approximately 0.10. Thus we interpreted only effects with probability less than 0.005 (two-tailed), and we can be 95% confident that the true population correlations were within 0.2 of those reported. Some of these results are described below, and others are shown in table 3.
There were significant correlations between lower neuropsychological scores and poorer Premorbid Adjustment Scale ratings, which were most consistent for social-personal adjustment (e.g., with global neuropsychological scale score, r=–0.34, df=88, p=0.0009) but were not specific to any single neuropsychological function. There were moderate relations of neuropsychological impairment with extrapyramidal symptoms assessed over the first 16 weeks of treatment; patients who did develop extrapyramidal symptoms (N=51) had slightly poorer functioning than those who did not develop extrapyramidal symptoms (N=40) (global neuropsychological scale score: F=5.26, df=1, 89, p=0.01), but did not differ in profile. There were no significant correlations of neuropsychological scales with age in either the patient group or the comparison group, with age at onset in the entire patient sample, or with age of onset among men or women patients.
We examined correlations of scores on each neuropsychological scale with symptom ratings at two time points: 1) at study entry, before treatment (baseline ratings); and 2) close to the time of the neuropsychological examination (typically within 2 weeks of the examination). Ratings on the SADS-C psychosis and disorganization dimensions and on the SANS were examined. There were no correlations greater than 0.30 between neuropsychological scale scores and ratings of symptoms in the SADS-C psychosis and disorganization dimensions at baseline. Correlations with baseline SANS ratings were somewhat more robust; specifically, the SANS global score for affective flattening had correlations >0.30 with scores on the neuropsychological scales for memory (r=–0.31) and attention (r=–0.36) and on the global neuropsychological scale (r=–0.30) (df=91; all p values <0.01, two-tailed). Neuropsychological scale scores correlated more strongly with symptom ratings at the time of the neuropsychological examination (table 3). Neuropsychological scale scores tended to correlate most with global clinical assessments and ratings of negative symptoms. Neuropsychological deficits explained approximately 5% to 25% of the variance in ratings of course and general social/vocational outcome after 2 years (table 3).
Scores on the executive scale, compared to other neuropsychological scales, appeared to correlate strongly with several functional indices. Given the theoretical implications of differential associations with executive compared to memory deficits, we tested differences between the size of these correlations (43). Compared to memory deficits, executive deficits were more strongly correlated with the Global Assessment Scale rating (for the difference between correlations, t=2.33, df=91, p<0.01, N=94) and the mean of SANS global ratings (for the difference between correlations, t=1.66, df=91, p<0.05, N=94).
We examined relationships between neuropsychological scores and treatments prescribed and cumulative antipsychotic dose at the time of testing. Doses of antipsychotics were converted into chlorpromazine equivalents, and doses of antiparkinsonian agents were converted into benztropine equivalents. The median chlorpromazine-equivalent dose was 500 mg/day (mean=712, SD=730, with a range from 0 mg/day [nine patients were receiving no medication] to 3750 mg/day). The median benztropine-equivalent dose among the 71 patients receiving antiparkinsonian agents was 4 mg/day (mean=4.56, SD=2.11, with a range from 0 to 11.5 mg/day).
Scores on neuropsychological scales showed moderate correlations with chlorpromazine-equivalent doses (table 3). (Correlations involving medication doses excluded data for patients who were not receiving medications.). However, chlorpromazine-equivalent dose also correlated significantly with a variety of symptom ratings, including severity of hallucinations (r=0.55, df=83, p<0.001) and the CGI score (r=0.43, df=83, p<0.001). After statistically controlling for both of these clinical variables, correlations of chlorpromazine-equivalent dose with neuropsychological scale scores were partially attenuated (e.g., r=–0.30, df=80, p<0.005, for the correlation with the memory scale score, and r=–0.28, df=80, p=0.009, for the correlation with the global neuropsychological scale score; correlations with scores on other neuropsychological scales ranged from r=–0.14 to r=–0.26, df=80, p values >0.02).
Benztropine-equivalent dose was significantly correlated with poorer performance on the memory scale (table 3), but benztropine-equivalent dose was also significantly correlated with the CGI score (r=0.30, df=69, p=0.01) and had similar correlations with scores on SANS items reflecting decreased spontaneous movement (r=0.28, df=69, p=0.02) and poverty of speech (r=0.25, df=69, p=0.04). After controlling for the CGI scores and scores on these two SANS items, the correlation of benztropine-equivalent dose with memory impairment was attenuated only slightly (r=–0.25, df=66, p<0.04).
DISCUSSION
This study characterized the neuropsychological function of patients after initial stabilization of the first episode of schizophrenia or schizoaffective disorder. The patients showed a generalized deficit of approximately 1.5 standard deviations relative to the comparison group. This effect is large in both statistical and clinical terms. In the context of this generalized deficit, language function was relatively spared, memory was most impaired, and executive and motor dysfunctions also emerged as relative deficits (see below). Although these fluctuations are statistically significant, their magnitude pales in contrast to the size of the generalized deficit, prompting questions about their pathophysiological and clinical significance, as noted recently by Mohamed and colleagues (4).
Classical neuropsychological interpretation of the mean patient profile suggests a relatively nonspecific deficit pattern, which could reflect either diffuse dysfunction or disturbances in key systems (mesencephalic, diencephalic, limbic, or frontal functional systems) that have modulatory efffects on broadly distributed neural networks. Given the caveat that neuropsychological inference based on adult, focal lesion studies may be invalid in the study of schizophrenia (14, 44), the findings are nevertheless consistent with current conceptualizations of the prevailing neuropsychological deficits in schizophrenia as either “widespread” (i.e., affecting intrinsic cortical circuitry) or as affecting frontolimbic and/or brainstem systems.
The neuropsychological profile observed in this study is similar to results obtained elsewhere in groups of first-episode patients, despite major differences in treatment conditions and more subtle differences in neuropsychological tests and data analytic methods (4, 7, 17). The combined results suggest that this profile is a relatively constant feature of the syndrome early in its course. The pattern of deficit is also generally consistent with studies of chronic schizophrenia (6, 45), but the overall severity of the deficit is about 0.3 to 1.0 standard deviations greater in the groups of chronic patients compared to first-episode patients (16, 46). It remains unclear whether this discrepancy reflects sampling bias (i.e., as many first-episode patients will not go on to have chronic illness) or a deteriorating course and associated cognitive decline in some patients. Recent reports have shown little change and some improvement in first-episode groups followed up to 5 years (47, 48), but it is difficult to rule out subgroup effects given that only 15%–25% of patients are expected to decline (44). Further, despite improvement of scores, patients may not show normal gains on retesting, which might reflect functional decline (47).
Some investigators have focused on relative deficits in learning and memory to implicate mesiotemporal pathology (6, 49), but comparison of memory impairment in schizophrenia to the amnestic syndrome may be misleading. First, memory deficit was not selective enough to resemble amnesia (50); other domains (executive, motor) were equally affected. Second, the discrepancy between immediate and delayed memory was not comparable to that observed in amnesia (table 1; for detailed analysis of these discrepancies, see reference 51). Third, memory dysfunction overlapped statistically with other deficits. Memory tests thus appear sensitive to the cognitive pathology of schizophrenia, but probably tap more complex and multifactorial pathology for most patients. Despite these findings across the group, 28% of the patients may satisfy objective criteria for amnesia based on discrepancies between IQ and memory (52), and this subgroup merits further attention.
The contrast between patients of high and low neuropsychological ability indicates that learning/memory deficit is present even in patients with a less severe generalized deficit. But in more severely affected individuals, relative deficits in executive functions are also present, and these deficits may be more severe than that for memory. These findings are consistent with the hypothesis of dysfunction in an integrated frontolimbic system, with less severely affected patients showing only learning/memory deficit and more severely affected patients manifesting deficits in executive functions as well (10).
Motor dysfunction emerged as a relative deficit in analysis of residual scores (i.e., after statistically controlling for all the other scores for that subject). Our finding that motor deficits are statistically independent from memory and other deficits is similar to that of Sullivan and colleagues (53). Motor deficits may reflect in part adverse medication effects, as seen in studies of acute neuroleptic treatment (54, 55). This idea is further supported by previous findings of less severe motor deficits in studies of mostly neuroleptic-naive patients (4, 7). Although an iatrogenic contribution is possible, it is unlikely to be the sole cause of the motor deficits. First, motor dysfunction was neither highly correlated with current or cumulative antipsychotic dose, nor with extrapyramidal symptoms during early treatment. Second, longitudinal analyses in our sample show that motor deficits are present before treatment, are exacerbated acutely by antipsychotic treatment (i.e., over the first few months), and then gradually return to baseline levels with continued treatment (56). Third, motor abnormalities have been observed in high-risk samples (57–60) and in home movies of children who later developed schizophrenia (61). Although it will be important to examine motor function in patients who receive new antipsychotics with less adverse extrapyramidal effects, the motor impairments reported here likely reflect persistent deficits. Because these motor deficits are statistically independent from other neuropsychological deficits, they may reflect a distinct pathologic process.
Correlations of neuropsychological measures with clinical measures had small-to-medium effect sizes; the strongest indicate approximately 25% shared variance (table 1). Neuropsychological performance had little relation to symptoms at the time of study entry, but it was correlated with symptoms after clinical stabilization. This finding suggests that symptom assessments of drug-naive patients may offer little insight about persistent deficits. Neuropsychological scales tended to correlate more strongly with negative symptoms than with positive symptoms or symptoms of conceptual disorganization, but the neuropsychological correlations with some positive symptoms were not smaller than the correlations with negative symptoms. This observation suggests that persistent, treatment-resistant positive symptoms may also index a trait-like deficit. The lack of neuropsychological correlations with disorganization symptoms should be considered with caution: this sample had low levels of these symptoms, and language function was relatively well preserved. These observations stand in contrast to findings obtained by using similar methods in a group of chronic patients with both prominent disorganization symptoms and prominent language impairment, where these domains were strongly correlated (32). Patients with these features may be underrepresented in groups of first-episode patients that include more individuals with good outcome, or these features may emerge later in the illness.
Neuropsychological measures correlated significantly with both childhood adjustment and current global functioning indices, suggesting an early developmental origin of enduring deficits. Executive deficits were the strongest predictors of impairment on the global functioning indices, while memory and attention deficits were the strongest predictors of premorbid adjustment and social-vocational outcome 2 years after study entry. These results are generally consistent with those reviewed by Green (15).
Recent attention has focused on possible sex differences in neuropsychological functioning, and the implications of these differences for models of etiology and/or pathophysiology (44, 62–64). After controlling for normal differences in motor speed (men are faster), we found no neuropsychological differences. We found, however, that within the patient group, women performed more poorly than men on the premorbid index. Because the premorbid index was loaded highly on certain tests (i.e., the information subtest of the WAIS-R) that show normal advantages for men over women (65), this finding may be an exaggeration of a normal sex difference, or fewer high-functioning women may have participated in testing. In either case, the results fail to support the hypothesis of more severe pathology among men with schizophrenia.
Handedness effects were prominent. Nondextral patients performed approximately 0.5 standard deviations below dextral patients, and the profile shape was similar. These results were not due to a subgroup with mixed-hand preference (38). We previously noted in a study of WAIS-R results that only strongly dextral first-episode patients had IQ scores above the range of chronic patients (16). These strongly dextral first-episode patients may be less likely to go on to a chronic course, or they may be more likely to show cognitive deterioration if and when their illness progresses. In either case, our results reveal a major association of nondextrality with global neuropsychological deficit. We previously hypothesized that this association might reflect a generalized failure in cerebral anatomic specialization (9), but so far there is little direct evidence linking neuropsychological deficits to absence or reversal of normal structural brain asymmetries.
To what extent can findings of neuropsychological deficit in schizophrenia be attributed to medication effects? This question is intractable outside the context of a controlled treatment trial (55), but it remains important to assess relations of treatment with neuropsychological performance to guide future studies. We found significant correlations between antipsychotic dose and impairment on every neuropsychological scale, but higher dose also correlated with severity of hallucinations and the CGI score. We are no more inclined to suggest that antipsychotic drugs cause neuropsychological deficit than to suggest that antipsychotic drugs cause hallucinations. The results indicate that patients with more severe, treatment-refractory symptoms had greater neuropsychological impairment and received higher doses. Further, given that the association of higher dose with neuropsychological impairment was reduced but not eliminated by statistically controlling for symptom severity, neuropsychological deficits may either prompt dose escalation or result from it.
A similar pattern of results was observed with antiparkinsonian agents. In line with the literature (66, 67), benztropine-equivalent dose correlated significantly with poorer performance on the memory scale, although it also correlated with poorer performance on executive, motor, and global scales, suggesting little specific effect of anticholinergic load. But benztropine-equivalent dose also correlated with global clinical ratings and items reflecting decreased spontaneous movement and poverty of speech. These findings suggest that patients with persistent akinesia and more neuropsychological deficit tend to receive more antiparkinsonian medication. Because memory deficit was still associated with benztropine-equivalent dose after controlling for extrapyramidal symptoms, there may be a separate anticholinergic effect on memory that deserves further study. Such studies must now consider the overall anticholinergic burden of regimens involving both antipsychotic agents and adjunctive treatments with high levels of anticholinergic activity.
Received April 12, 1999; revision received Sept. 13, 1999; accepted Sept. 15, 1999. From the Department of Psychiatry, Hillside Hospital, North Shore–Long Island Jewish Health System, Glen Oaks, N.Y.; the Center for Advanced Brain Imaging, Nathan S. Kline Institute for Psychiatric Research; the Department of Psychiatry, Albert Einstein College of Medicine, Bronx, N.Y.; the Department of Psychiatry, Michigan State University, East Lansing; the Department of Psychiatry, New York Presbyterian Hospital, White Plains; and the Department of Psychiatry, University of North Carolina School of Medicine, Chapel Hill. Address reprint requests to Dr. Bilder, Center for Advanced Brain Imaging, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY 10962; [email protected] (e-mail). Supported by U.S. Public Health Service grants MH-41960, MH-00537 and MH-41646 from the National Institute of Mental Health, an award from the National Alliance for Research on Schizophrenia and Depression, a Faculty Research Award from Long Island Jewish Medical Center (3-926), and a grant from the Helen and Irving Schneider family.
 |
 |
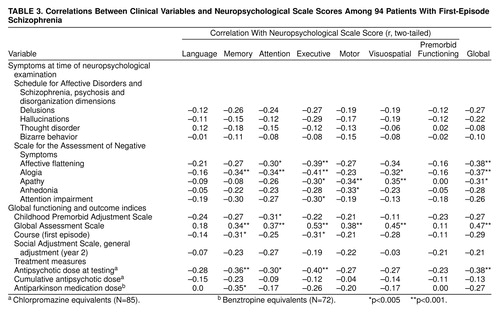 |
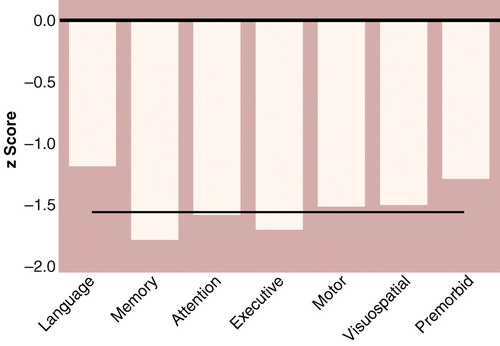
Figure 1. Deficits in Scores for Neuropsychological and Premorbid Abilities of 94 Patients With First-Episode Schizophreniaa
aRelative to scores for healthy comparison subjects; by definition, the healthy comparison group had a mean score of zero on each scale. Premorbid ability score based on tests of general knowledge, vocabulary, and reading skill. The straight horizontal line indicates the average deficit for patients across scales (–1.53).
1. Heaton RK, Baade LE, Johnson KL: Neuropsychological test results associated with psychiatric disorders in adults. Psychol Bull 1978; 85:141–162Crossref, Medline, Google Scholar
2. Goldstein G: Cognitive and perceptual differences between schizophrenics and organics. Schizophr Bull 1978; 4:160–185Crossref, Medline, Google Scholar
3. Blanchard JJ, Neale JM: The neuropsychological signature of schizophrenia: generalized or differential deficit? Am J Psychiatry 1994; 151:40–48Google Scholar
4. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N: Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry 1999; 56:749–754Crossref, Medline, Google Scholar
5. Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH: Further evidence for dementia of the prefrontal type in schizophrenia? a controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry 1987; 44:1008–1014Google Scholar
6. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
7. Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–131Crossref, Medline, Google Scholar
8. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA: Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47–58Crossref, Medline, Google Scholar
9. Bilder RM, Degreef G: Morphologic markers of neurodevelopmental paths to schizophrenia, in Developmental Neuropathology of Schizophrenia. Edited by Mednick SA, Cannon TD, Barr CE, LaFosse JM. New York, Plenum, 1991, pp 167–190Google Scholar
10. Bilder RM, Szeszko PR: Structural neuroimaging and neuropsychological impairments, in The Neuropsychology of Schizophrenia. Edited by Pantelis C, Nelson HE, Barnes TRE. New York, John Wiley & Sons, 1996, pp 279–298Google Scholar
11. Flor-Henry P: Cerebral Basis of Psychopathology. Boston, John Wright-PSG, 1983Google Scholar
12. Gruzelier J, Seymour K, Wilson L, Jolley A, Hirsch S: Impairments on neuropsychologic tests of temporohippocampal and frontohippocampal functions and word fluency in remitting schizophrenia and affective disorders. Arch Gen Psychiatry 1988; 45:623–629Crossref, Medline, Google Scholar
13. Gruzelier JH: Hemispheric imbalance: syndromes of schizophrenia, premorbid personality, and neurodevelopmental influences, in Handbook of Schizophrenia, vol 5: Neuropsychology, Psychophysiology and Information Processing. Edited by Steinhauer SR, Gruzelier JH, Zubin J. New York, Elsevier, 1991, pp 599–650Google Scholar
14. Bilder RM, Goldberg E: Motor perseverations in schizophrenia. Arch Clin Neuropsychol 1987; 2:195–214Crossref, Medline, Google Scholar
15. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
16. Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA: Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull 1992; 18:437–448Crossref, Medline, Google Scholar
17. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898–903Link, Google Scholar
18. DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
19. Nuechterlein KH, Gitlin MJ, Subotnok KL: The early course of schizophrenia and long-term maintenance neuroleptic therapy. Arch Gen Psychiatry 1995; 52:203–205Crossref, Medline, Google Scholar
20. Censits DM, Ragland DJ, Gur RC, Gur RE: Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res 1997; 24:289–298Crossref, Medline, Google Scholar
21. Bilder RM, Lipschutz-Broch L, Reiter G, Geisler S, Mayerhoff D, Lieberman JA: Neuropsychological deficits in the early course of first episode schizophrenia. Schizophr Res 1991; 5:198–199Crossref, Medline, Google Scholar
22. Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA: Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res 1999; 45:680–686Google Scholar
23. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247Crossref, Medline, Google Scholar
24. Robinson DG, Woerner MG, Alvir JM, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA: Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999; 156:544–549Link, Google Scholar
25. Lieberman JA, Alvir JM, Woerner M, Degreef G, Bilder RM, Ashtari M, Bogerts B, Mayerhoff DI, Geisler SH, Loebel A, Levy DL, Hinrichsen G, Szymanski S, Chakos M, Koreen A, Borenstein M, Kane JM: Prospective study of psychobiology in first-episode schizophrenia at Hillside Hospital. Schizophr Bull 1992; 18:351–371Crossref, Medline, Google Scholar
26. Lieberman JA, Jody DN, Geisler SH, Alvir JM, Loebel A, Szymanski S, Woerner M, Borenstein M: Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry 1993; 50:369–376Crossref, Medline, Google Scholar
27. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1977Google Scholar
28. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35:773–782Crossref, Medline, Google Scholar
29. Endicott J, Spitzer RL: A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
30. Bilder RM, Degreef G, Pandurangi AK, Rieder RO, Sackeim HA, Mukherjee S: Neuropsychological deterioration and CT scan findings in chronic schizophrenia. Schizophr Res 1988; 1:37–45Crossref, Medline, Google Scholar
31. Bilder RM: Subtyping in chronic schizophrenia: clinical, neuropsychological, and structural indices of deterioration. Dissertation Abstracts International 1985; 45(11B):3651Google Scholar
32. Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK: Symptomatic and neuropsychological components of defect states. Schizophr Bull 1985; 11:409–419Crossref, Medline, Google Scholar
33. Bentler PM, Bonnett DG: Significance tests and goodness-of-fit in the analysis of covariance structures. Psychol Bull 1980; 88:588–606Crossref, Google Scholar
34. Oldfield RC: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
35. Schachter SC, Ransil BJ, Geschwind N: Associations of handedness with hair color and learning disabilities. Neuropsychologia 1987; 25:269–276Crossref, Medline, Google Scholar
36. Chapman LJ, Chapman JP: Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol 1989; 98:357–366Crossref, Medline, Google Scholar
37. Hollingshead AB, Redlich FC: Social Class and Mental Illness: A Community Study. New York, John Wiley & Sons, 1958Google Scholar
38. Green MF, Satz P, Smith C, Nelson L: Is there atypical handedness in schizophrenia? J Abnorm Psychol 1989; 98:57–61Google Scholar
39. Cannon-Spoor HE, Potkin SG, Wyatt RJ: Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 1982; 8:470–484Crossref, Medline, Google Scholar
40. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
41. Mayerhoff DI, Loebel AD, Alvir JM, Szymanski SR, Geisler SH, Borenstein M, Lieberman JA: The deficit state in first-episode schizophrenia. Am J Psychiatry 1994; 151:1417–1422Google Scholar
42. Lieberman JA, Jody D, Alvir JM, Ashtari M, Levy DL, Bogerts B, Degreef G, Mayerhoff DI, Cooper T: Brain morphology, dopamine, and eye-tracking abnormalities in first-episode schizophrenia: prevalence and clinical correlates. Arch Gen Psychiatry 1993; 50:357–368Crossref, Medline, Google Scholar
43. Meng X, Rosenthal R, Rubin DB: Comparing correlated correlation coefficients. Psychol Bull 1992; 111:172–175Crossref, Google Scholar
44. Bilder RM: Structure-function relations in schizophrenia: brain morphology and neuropsychology, in Progress in Experimental Personality and Psychopathology Research, vol 15. Edited by Walker EF, Dworkin RH, Cornblatt BA. New York, Springer, 1992, pp 183–251Google Scholar
45. Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste DV: Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
46. Hoff AL, Wieneke M, Faustman WO, Horon R, Sakuma M, Blankfeld H, Espinoza S, DeLisi LE: Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. Am J Psychiatry 1998; 155:1437–1439Google Scholar
47. Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE: Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry 1999; 156:1336–1341Google Scholar
48. Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC: Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry 1999; 156:1342–1348Google Scholar
49. Tamlyn D, McKenna PJ, Mortimer AM, Lund CE, Hammond S, Baddeley AD: Memory impairment in schizophrenia: its extent, affiliations and neuropsychological character. Psychol Med 1992; 22:101–115Crossref, Medline, Google Scholar
50. Squire LR, Zola-Morgan S: The medial temporal lobe memory system. Science 1991; 253:1380–1386Google Scholar
51. Willson DF: The Nature of Accelerated Forgetting Rates in Schizophrenia (doctoral dissertation). Jamaica, NY, St. John’s University, Department of Psychology, 1997Google Scholar
52. Goldman RS, Bates JA, Bilder RM, Reiter G, Conley J, Pappadopoulos E, Obuchowski M, Robinson D, Alvir JMA, Lieberman J, Schooler N: Severity and selectivity of memory dysfunction in first-episode schizophrenia. Schizophr Res 1999; 36:130Google Scholar
53. Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A: A deficit profile of executive, memory, and motor functions in schizophrenia. Biol Psychiatry 1994; 36:641–653Crossref, Medline, Google Scholar
54. Medalia A, Gold JM, Merriam AE: The effects of neuroleptics on neuropsychological test results of schizophrenics. Arch Clin Neuropsychol 1988; 3:249–271Crossref, Medline, Google Scholar
55. Bilder RM, Turkel E, Lipschutz-Broch L, Lieberman JA: Antipsychotic medication effects on neuropsychological functions. Psychopharmacol Bull 1992; 28:353–366Medline, Google Scholar
56. Bilder RM, Bates JA: Neuropsychological prediction of treatment response and outcome in schizophrenia, in Prediction of Neuroleptic Treatment Outcome in Schizophrenia: Concepts and Methods. Edited by Gaebel W, Awad AG. New York, Springer-Verlag Wien, 1994, 99–110Google Scholar
57. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S: Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect: a review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry 1992; 49:221–235Crossref, Medline, Google Scholar
58. Marcus J, Hans SL, Mednick SA, Schulsinger F, Michelsen N: Neurological dysfunctioning in offspring of schizophrenics in Israel and Denmark: a replication analysis. Arch Gen Psychiatry 1985; 42:753–761Crossref, Medline, Google Scholar
59. Mednick SA, Mura E, Schulsinger F, Mednick B: Perinatal conditions and infant development in children with schizophrenic parents, in Genetics, Environment, and Psychopathology. Edited by Mednick SA, Schulsinger F, Higgins J, Bell B. Amsterdam, Elsevier, 1974, pp 231–248Google Scholar
60. Neumann CS, Grimes K, Walker EF, Baum K: Developmental pathways to schizophrenia: behavioral subtypes. J Abnorm Psychol 1995; 104:558–566Crossref, Medline, Google Scholar
61. Walker E, Levine RJ: Prediction of adult-onset schizophrenia from childhood home movies of the patients. Am J Psychiatry 1990; 147:1052–1056Google Scholar
62. Goldstein JM, Seidman LJ, Santangelo S, Knapp PH, Tsuang MT: Are schizophrenic men at higher risk for developmental deficits than schizophrenic women? implications for adult neuropsychological functions. J Psychiatr Res 1994; 28:483–498Crossref, Medline, Google Scholar
63. Lewine RR, Gulley LR, Risch SC, Jewart R, Houpt JL: Sexual dimorphism, brain morphology, and schizophrenia. Schizophr Bull 1990; 16:195–203Crossref, Medline, Google Scholar
64. Lewine RR, Walker EF, Shurett R, Caudle J, Haden C: Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry 1996; 153:1178–1184Google Scholar
65. Kaufman AS: Assessing Adolescent and Adult Intelligence. Boston, Allyn & Bacon, 1990Google Scholar
66. Spohn HE, Strauss ME: Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J Abnorm Psychol 1989; 98:367–380Crossref, Medline, Google Scholar
67. Strauss ME, Reynolds KS, Jayaram G, Tune LE: Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res 1990; 3:127–129Crossref, Medline, Google Scholar



