Use of the Selective Serotonin 3 Receptor Antagonist Ondansetron in the Treatment of Neuroleptic-Induced Tardive Dyskinesia
Abstract
OBJECTIVE: The authors examined the efficacy, tolerability, and safety of ondansetron, a selective serotonin 3 receptor antagonist, in patients with tardive dyskinesia. METHOD: Twenty patients with schizophrenia who had neuroleptic-induced tardive dyskinesia were given 12 mg/day of ondansetron for 12 weeks in an open-label study. RESULTS: Administration of ondansetron resulted in a statistically significant improvement in tardive dyskinesia and psychotic symptoms. CONCLUSIONS: Ondansetron may be an effective and safe therapy to control tardive dyskinesia and psychosis in patients with schizophrenia.
Tardive dyskinesia consists of abnormal, involuntary, irregular choreoathetoid movements of the muscles of the head, limbs, and trunk and often is irreversible (1). Several different suppressive agents have been tried with limited success (2). Serotonin (5-HT) modulates striatal dopamine release and could influence dyskinetic movements (2, 3). Excess dopamine may amplify 5-HT release in the brain. Improvement of tardive dyskinesia with cyproheptadine, a 5-HT antagonist, has been noted (4). Because ondansetron is a selective 5-HT3 antagonist used to prevent nausea and vomiting in cancer patients (5), we examined its efficacy, safety, and tolerability in a short-term, open-label trial in patients with tardive dyskinesia.
METHOD
We studied 20 inpatients with DSM-IV-diagnosed schizophrenia. Five of the patients were men, and 15 were women; their mean age was 69.8 years (range=42–81, SD=10.1). All of the patients met Research Diagnostic Criteria for at least mild tardive dyskinesia, and all had been experiencing the dyskinesia for at least 6 months. The mean duration of tardive dyskinesia was 9.8 years (range=1–30 years). All patients had stable psychopathology and had been receiving a stable psychotropic drug regimen for at least 6 months before study entry. Tardive dyskinesia had been stable for at least 3 months before entry to the study as measured by Abnormal Involuntary Movement Scale (AIMS) (6) scores. All patients had no serious medical or neurological disease, and all provided written informed consent.
Oral ondansetron was administered in a dose of 8 mg/day at 8:00 a.m. in the first week of the study; a dose of 12 mg/day (8 mg at 8:00 a.m. and 4 mg at 8:00 p.m.) was administered during weeks 2–12. Doses of all psychotropic medications were kept unchanged throughout the study.
Tardive dyskinesia was assessed with the AIMS. Patients were rated at baseline and after 4, 8, and 12 weeks. Severity of movements was rated on a scale of 0 to 4. Assessments were videotaped and then reviewed in random order at the completion of the study by four blind raters (P.S., T.M., H.S., and N.G.) who scored each session; their scores were then averaged for each subject and session. The intraclass correlation coefficient (ICC) (7) for total AIMS scores, based on 25 randomly selected ratings, was 0.83 (df=2, 15, p=0.001).
Psychosis was assessed by two trained raters using the Positive and Negative Syndrome Scale (8) and the severity subscale of the Clinical Global Impression scale (CGI) (9) at baseline and after 12 weeks. For the Positive and Negative Syndrome Scale, ICC=0.79 (df=2, 19, p=0.002); for the CGI severity subscale, ICC=0.86 (df=3, 18, p<0.001).
Total AIMS scores at baseline and after 4, 8, and 12 weeks of treatment were analyzed by using one-way analysis of variance (ANOVA) with repeated measures. Total Positive and Negative Syndrome Scale as well as CGI scores at baseline and after 12 weeks were compared by using ANOVA with repeated measures.
RESULTS
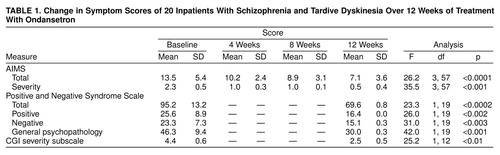
A significant overall reduction of AIMS total scores after treatment with ondansetron was noted (table 1). The mean severity score decreased significantly during the study (table 1). A clinically significant improvement in the severity of tardive dyskinesia, defined as a reduction of 75% or greater, occurred in 13 patients. The mean reduction in severity score was 78%.
The total AIMS scores of the 10 patients who had experienced tardive dyskinesia for 10 years or less (mean=9.6, SD=4.0) was 25% lower than the ratings of 10 patients who had experienced tardive dyskinesia for more than 10 years (mean=12.8, SD=4.2) (F=8.2, df=3, 54, p<0.01). AIMS scores were not influenced by the patients’ age (F=0.17, df=3, 54, p=0.91) or sex (F=0.15, df=3, 54, p=0.93).
The patients’ total scores on the Positive and Negative Syndrome Scale showed a significant decrease from baseline to week 12 (table 1). There were significant improvements in scores on the Positive and Negative Syndrome Scale subscales as well as on the CGI severity subscale from baseline to week 12 (table 1).
DISCUSSION
These findings show that ondansetron is beneficial in treating tardive dyskinesia as well as psychotic symptoms in patients with schizophrenia. The drug was generally very well tolerated and caused no major side effects. A literature search did not reveal any effects of ondansetron on the metabolism of the other medications.
The involvement of the serotonin system in modulating extrapyramidal motor effects is suggested by the absence of extrapyramidal symptoms with the use of clozapine, which has a strong affinity to the 5-HT1c, 5-HT2 receptor (10, 11), a high ratio of 5-HT2-to-D2 blockade, and 5-HT3 antagonism (12). The serotonin system interacts directly with dopaminergic neurons in the substantia nigra and ventral tegmental area (13).
Ondansetron and other 5-HT3 antagonists have been shown to block motor hyperactivity induced by central administration of amphetamine and dopamine (14). The ability of dopamine to inhibit the firing of medial prefrontal neurons can be potentiated by 5-HT3 agonists and is blocked by 5-HT3 antagonists (15). It has been proposed that the 5-HT3 antagonist action of clozapine could contribute to its antipsychotic action by means of this mechanism (16). These observations suggest that 5-HT3 antagonists can restore and maintain “normal” dopamine function in patients with schizophrenia and may produce an antipsychotic effect without inducing sedation or extrapyramidal symptoms.
Ondansetron appears to have an antipsychotic effect in acute schizophrenia at lower doses (4–12 mg/day) (17); a double-blind, placebo-controlled study (unpublished 1991 paper by H.Y. Meltzer), however, indicated no clinical improvement. An improvement in psychotic symptoms in patients with Parkinson’s disease has been reported (18).
In conclusion, our results suggest that ondansetron may be beneficial for tardive dyskinesia and for psychosis. Controlled double-blind studies for extended periods are required to verify our findings.
Received Nov. 3, 1998; revisions received May 13 and June 28, 1999; accepted July 6, 1999. From the Abarbanel Mental Health Center; the Department of Neurology, Sourasky Medical Center, Tel Aviv; and the Sackler Faculty of Medicine, Tel-Aviv University, Israel. Address reprint requests to Dr. Sirota, Director of Ward 6A, Y. Abarbanel Mental Health Center, 15 Keren Kayemet St., Bat Yam 59100, Israel; [email protected] (e-mail). The authors thank C.T.S. Israel for providing ondansetron tablets for this study.
 |
1. Chouinard G, Annable L, Ross-Chouinard A, Mercier P: A 5-year prospective longitudinal study of tardive dyskinesia: factors predicting appearance of new cases. J Clin Psychopharmacol 1988; 8(Aug suppl):21S–26SGoogle Scholar
2. Egan MF, Apud J, Wyatt RJ: Treatment of tardive dyskinesia. Schizophr Bull 1997; 23:583–609Crossref, Medline, Google Scholar
3. Seibyl JP, Glazer WM, Innis RB: Serotonin function in tardive dyskinesia. Psychiatr Annals 1989; 19:310–314Crossref, Google Scholar
4. Goldman D: Treatment of phenothiazine-induced dyskinesia. Psychopharmacol Bull 1976; 12:7–10Medline, Google Scholar
5. Marty M, Pouillart P, School S, Driz JP, Azab M, Brian N, Pujade-Lousaine E, Paule B, Paes D, Bons J: Comparison of the 5-hydroxytryptamine (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med 1990; 332:816–821Crossref, Google Scholar
6. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
7. Bartko JJ, Carpenter WT: On the methods and theory of reliability. J Nerv Ment Dis 1976; 163:307–317Crossref, Medline, Google Scholar
8. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
9. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Rockville, Md, US Department of Health, Education, and Welfare, 1976, pp 217–222Google Scholar
10. Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, Meltzer HY: Characterization of typical and atypical antipsychotic drugs based on in-vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther 1993; 266:1374–1384Google Scholar
11. Lieberman JA: Understanding the mechanism of action of a typical antipsychotic drug: a review of components in use and developments. Br J Psychiatry 1993; 163 (suppl 12):7–18Google Scholar
12. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP: International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 1994; 46:157–203Medline, Google Scholar
13. Barnes JM, Barnes NM, Cooper SJ: Behavioral pharmacology of 5-HT3 receptor ligands. Neurosci Biobehav Rev 1992; 16:107–113Crossref, Medline, Google Scholar
14. Costall B, Domeney AM, Naylor RJ, Tyers MB: Effects of the 5-HT3 receptor antagonist, GR38032F, on raised dopaminergic activity in the mesolimbic system of the rat and marmoset brain. Br J Pharmacol 1987; 92:881–894Crossref, Medline, Google Scholar
15. Costall B, Naylor RJ, Tyers MB: The psychopharmacology of 5-HT3 receptors. Pharmacol Ther 1990; 47:181–202Crossref, Medline, Google Scholar
16. Wang RX, Ashby CR Jr, Edwards E, Zhang JY: The role of 5-HT3-like receptors in the action of clozapine. J Clin Psychiatry 1994; 55(Sept suppl):S23–S26Google Scholar
17. Ondansetron (GR38032F): Clinical Investigators Manual (OND/CNS 0139). Greenford, Middlesex, UK, Glaxo, Medical Division, 1990Google Scholar
18. Zoldan J, Friedberg G, Livneh M, Melamed E: Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5HT3 receptor antagonist. Neurology 1995; 45:1305–1308Google Scholar



