Risk-Benefit Decision Making for Treatment of Depression During Pregnancy
Abstract
OBJECTIVE: The Committee on Research on Psychiatric Treatments of the American Psychiatric Association identified treatment of major depression during pregnancy as a priority area for improvement in clinical management. The goal of this article was to assist physicians in optimizing treatment plans for childbearing women. METHOD: The authors’ work group developed a decision-making model designed to structure the information delivered to pregnant women in the context of the risk-benefit discussion. Perspectives of forensic and decision-making experts were incorporated. RESULTS: The model directs the psychiatrist to structure the problem through diagnostic formulation and identification of treatment options for depression. Reproductive toxicity in five domains (intrauterine fetal death, physical malformations, growth impairment, behavioral teratogenicity, and neonatal toxicity) is reviewed for the potential somatic treatments. The illness (depression) also is characterized by symptoms of somatic dysregulation that compromise health during pregnancy. The patient actively participates and provides her evaluation of the acceptability of the various treatments and outcomes. Her capacity to participate in this process provides evidence of competence to consent. Included in the decision-making process are the patient’s significant others and obstetrical physician. The process is ongoing, with the need for incorporation of additional data as the pregnancy and treatment response progress. CONCLUSIONS: The conceptual model provides structure to a process that is frequently stressful for both patients and psychiatrists. By applying the model, clinicians will ensure that critical aspects of the risk-benefit discussion are included in their care of pregnant women.
Women of childbearing age frequently suffer from major depression. Estimates of the lifetime risk in community samples have varied from 10% to 25%, with the peak prevalence between 25 and 44 years of age (1). Nine percent of pregnant women have illnesses that fulfill the Research Diagnostic Criteria for depression (2). In a large sample of childbearing-age women who sought treatment at an urban psychiatric hospital (3), the proportion whose illness had begun during pregnancy or within 3 months of birth was 9%. When the sample was restricted to women who had ever experienced a pregnancy, one out of seven women who sought care was experiencing an episode that began during pregnancy or the postpartum period.
Many women have difficulty obtaining pharmacologic care during pregnancy. This problem was highlighted in the lay press in a U.S. News and World Report article: “The Baby or The Drug? It’s a Choice That Many Pregnant Women Often Face—But Shouldn’t” (4). The Committee on Research on Psychiatric Treatments of the American Psychiatric Association identified treatment of major depression during pregnancy as a priority area for improvement in clinical management. To assist physicians in developing treatment plans for childbearing women with major depression, we reviewed data from recent prospective studies of antidepressant treatment during pregnancy (5). Delivered in the context of a risk-benefit discussion, this new information increases our sophistication in formulating drug therapy for pregnant women with depression.
Our work group developed a decision-making model designed to structure the information delivered to pregnant women in the context of the risk-benefit discussion. Perspectives of forensic and decision-making experts were incorporated. Our goal here is to present the model to clinicians, as thoughtful clinical decisions will maximize the likelihood of healthy pregnancy outcomes.
Model for Clinical Decision Making During Pregnancy
The treatment of depressed pregnant women requires skilled decision making by the psychiatrist. The appropriateness of somatic therapy during pregnancy is a case-specific judgment that is the result of a complex discussion between the physician and patient. The patient’s sense of caring for herself and her baby is promoted by participating in treatment selection and monitoring. The communication inherent in the informed consent process enhances the treatment alliance, has therapeutic value, indicates recognition of the patient’s responsibility for her own care, and provides an opportunity for ongoing assessment of the woman’s decision-making competence.
Informed consent for treatment is essential. We present a model of clinical decision making in Figure 1. The discussion is framed by the physician’s clinical expertise and should display respect for the patient’s values and treatment preferences. Patients consistently prefer that problem-solving tasks be performed by the physician (7). Examples of such tasks are formulating diagnoses, identifying and reviewing the likely occurrence of risks and benefits, and presenting treatment options. However, many patients want active involvement in decision-making tasks, such as evaluating the acceptability of possible outcomes and selecting treatment (7). Psychiatrists assist patients in problem solving by structuring choices and supporting them in making decisions. The physician and patient contribute expertise to the process.
The following questions about the patient’s history are relevant in the decision-making process (8): Which disorder is being treated? What has been the clinical course of this patient’s illness? What is her intent about continuing this pregnancy? Which treatments has this patient experienced, and what was the result? What is the quality of the patient’s psychosocial support as she manages the pregnancy? Who are her significant others, and how do they understand her illness and behave during episodes? Does her obstetrical physician know about her psychiatric history? Does she have obstetrical health concerns, and which tests (such as ultrasound, amniocentesis) have been done or are planned? What drugs has she taken during the pregnancy? Has she used alcohol or cigarettes (9)? After discussion of the decision-making model, we provide case examples to demonstrate its application.
Physician: Structure of Problem
Diagnostic Formulation
The physician provides the structure for decision making, which includes education about the diagnosis and its natural history. Common clinical presentations are the first onset of depression during pregnancy, previous depressive episodes with a new episode during gestation, and use of maintenance antidepressants to prevent recurrent episodes by women who desire a pregnancy. For patients with recurrent major depression (three or more lifetime episodes), the risk of another episode without maintenance treatment is high, with a median time to recurrence of 21 weeks (10). Maintenance interpersonal psychotherapy has been shown to increase the median time to recurrence to 54 weeks (10).
Treatment Options
The American Psychiatric Association has provided clinical practice guidelines for major depression in adults (11). Effective treatments for depression include psychotherapy, antidepressant medication, and electroconvulsive therapy (ECT). The severity of symptoms and patient choice are important factors in the decision-making process. Experienced clinicians and researchers concluded that major depression that is chronic or moderate to severe warrants a recommendation for somatic intervention (11). A useful approach is to discuss the strengths and weaknesses of each therapeutic option for major depression in general, and then describe how pregnancy modifies each option.
Somatic therapy
ECT, which involves the administration of drugs with short half-lives, has been used safely and successfully during pregnancy (12). Nonpharmacologic environmental treatments for depression, such as partial sleep deprivation (13, 14) and rapid transcranial magnetic stimulation (15), have been proposed. High-density negative air ionization and bright light therapy are effective for seasonal affective disorder (16, 17). Oren et al. found light therapy to be efficacious in an open pilot study of pregnant women with depression (unpublished data, 1999). These novel modalities are less well studied and less available than standard treatments, but avoidance of drug exposure may be valued by the patient to the extent that they are the only acceptable somatic treatment options.
Psychotherapy
Interpersonal psychotherapy has been adapted and used successfully for treating pregnant patients with depression (18). The focus of interpersonal psychotherapy on role change and interpersonal functioning is particularly relevant during pregnancy.
No treatment
Depression is a disease state that affects fetal and infant health. Maternal stress influences the fetus and birth outcomes, which are mediated by peptides derived from the activated hypothalamic-pituitary-adrenal (HPA) axis, such as adrenocorticotropic hormone and β-endorphin (19). The consequences of no treatment are an integral part of the discussion. If the patient refuses treatment, monitoring can be recommended to allow for evaluation of urgent situations (suicidality, deteriorating social and physical functions, inability to comply with obstetrical evaluations).
Independent of biomedical risk, maternal prenatal stress is significantly associated with lower infant birth weight and gestational age at birth (20). The biological dysregulation of depression is not an ideal milieu for pregnancy. In animals, maternal stress is associated with fetal hypoxia, low birth weight, smaller litter size, miscarriage, and fetal hypotension (21). Stress (without exposure to chemical agents) can cause behavioral teratogenicity. A study in which groups of pregnant rats were treated with restraint stress alone, restraint stress plus diazepam, diazepam alone, and no medication yielded interesting results (22). The offspring of mothers subjected to restraint stress alone had significant delays on a number of developmental measures, such as growth and reflexes. As adults, these offspring had significantly impaired learning ability in a swimming maze. However, the rates of development and learning ability in the restraint-plus-diazepam and diazepam-only groups were comparable to the rates for control rats. The investigators concluded that concurrent administration of an antianxiety agent during restraint prevented the adverse postnatal effects of maternal restraint stress during pregnancy. The possibility that prenatal psychopharmacologic treatment may prevent negative outcomes during human pregnancy rarely is entertained.
Physician: Likelihood of Outcomes
Fetal Toxicity
In an earlier article (5) we critically reviewed data from recent studies related to reproductive outcomes for pregnant women exposed to antidepressant treatment. Four prospective investigations (22–25) have provided new information about the effects of antidepressant exposure. This information must be incorporated in the outcomes considered in the decision-making model we propose (Figure 1). Following is a summary of our findings, which focus on the five domains of reproductive toxicity.
Intrauterine death
There is no evidence that exposure to tricyclics (23), fluoxetine (23, 24), or newer serotonin-specific reuptake inhibitors (SSRIs)—which include sertraline, paroxetine, and fluvoxamine (25)—during pregnancy increases the risk for intrauterine fetal death.
Physical malformations
There is no evidence to implicate tricyclics, fluoxetine, or newer SSRIs as causes of major birth defects in humans or animals (23–25).
Growth impairment
The prenatal growth and birth weights of infants exposed to tricyclics (23) and newer SSRIs (25) during the first trimester were comparable to those of infants exposed to drugs identified as nonteratogens. In one study (24), fetuses exposed to fluoxetine after 25 weeks of gestation had significantly lower birth weights that were related to lower maternal weight gain. However, weight loss is common in major depression, and partially treated or nonresponsive maternal mood disorder associated with fluoxetine use could affect maternal and infant weights. Growth deficits in newborns and poor maternal weight gain associated with fluoxetine were not observed by another investigative team (26). Weight gain during pregnancy is routinely monitored by obstetricians and can be followed closely in antidepressant-treated women until data to resolve these issues are obtained.
Behavioral teratogenicity
This term refers to postbirth effects on behavior due to prenatal exposure to toxic agents. This domain remains the major area of concern about prescribing central-nervous-system-active agents during gestation. In the only published study of which we are aware (26), cognitive function, temperament, and general behavior were similar in children who were exposed prenatally to tricyclics or fluoxetine and in comparison children. We know of no prospective information about newer SSRIs or other agents.
Neonatal toxicity
Both neonatal withdrawal and direct adverse effects have been reported in the offspring of pregnant women treated with tricyclics through delivery (27–31). There have been reports of direct effects of peripartum fluoxetine treatment on infant outcomes and neonatal adjustment (24, 32), such as poor neonatal adaptation (hypotonia, difficulty feeding).
Depression Outcomes
The likely outcomes for the illness state, major depression, are part of the discussion. The depression may remit fully or partially, remain unchanged, or worsen. This information is gleaned from the literature and clinical experience and modified by the physician’s knowledge of the natural history of the illness in the individual woman.
Because of our limited knowledge, clear-cut data in all domains of reproductive toxicity are often lacking. Patients should be informed about the limits of certainty of our current information. The physician and patient must take unknown or nonquantifiable risks into account. Likely benefits should also be described objectively. For instance, the lack of certainty about the results of treatment with a previously effective medication should be acknowledged.
Patient: Characteristics Influencing Decision
Relative Values of Outcomes
In response to the information provided by the physician, the patient describes how she values each outcome. The values attributed to each treatment option and its consequences vary across patients. These values are the expertise each woman brings to her situation. For example, a woman may be less concerned about the risk of fetal abnormalities or neurobehavioral effects than about being free of depression so she can work to support herself and other children. Another woman without financial concerns may place a high value on keeping the risk of fetal toxicity as low as possible.
There are unique aspects to this decision-making process that involve potential consequences for two individuals. The mother has to consider the risk information not only for herself, but also for her fetus. The outcomes can be positive for both, negative for one but positive for the other, or negative for both. In a simplified sense, the psychiatrist and mother are looking at a 2 × 4 table (Figure 2), and only one reproductive outcome cell (positive outcome for mother, positive outcome for fetus) is desirable. The complexity increases because the cells are not mutually exclusive. For example, if an effect of an intervention is negative for the mother, it will almost certainly be negative for the fetus, since the fetus depends on the mother for life-sustaining functions. In many cases the outcomes will be a mix of positive and negative, such as for a mother who accepts drug treatment and experiences resolution of her depression but also substantial worry about possible fetal effects. According to the data we reviewed earlier (5), treatments for depression generally fit the positive mother/positive fetus outcome; however, the magnitudes of the positive valences are difficult to determine.
Perception of Risk
Understanding risk is a complex task that combines objective information (estimates of risk) with subjective information (the importance of the possible negative outcome to the individual) (33). For most patients, perceptions about risk are influenced by intuitive judgments about two characteristics of risk, uncertainty and dread (34). Patients’ perceptions of risk are often affected by media attention, which can lead to incorrect estimates of risk magnitude. Provision of information through counseling has been shown to change perception of teratogenic risk (35). Pregnant women were asked to score their potential risk for major malformations on a scale from 0% to 100%. Before counseling, women exposed to a nonteratogenic agent assigned a mean risk of 24.0% (SD=2.8%) for major malformations, which is the risk of malformation from exposure to thalidomide. The women’s perceived risk was significantly lower after the counseling session (mean=14.5%, SD=3.0%). These women accurately estimated the risk for major malformations in the general population. Estimation of risk did not correlate with the number of agents consumed by the woman or her age, parity, or socioeconomic status.
Presenting the same information about risk in different ways (for example, the rate of birth defects as opposed to the rate of normal infant births) can alter risk perceptions. It is the perception of risk that drives decision making and the course of action. The manner in which the physician provides information to the patient must effectively communicate the nature, degree, and probability of the risks from treatments and depression in an understandable and accessible format despite the patient’s substantial dread of possible outcomes (34).
Another factor that influences risk perception is voluntariness. For example, voluntarily taking a medication during pregnancy may be perceived as incurring greater risk than suffering the consequences of “involuntary” depression. Familiarity also influences risk perception, and women who have taken a medication with good response in the past are more likely to select this treatment again. Other factors that affect decision making are the permanence of the potential defect and the time of occurrence of the unwanted outcome (33). The latter factor is relevant to outcomes with delayed manifestations, such as behavioral teratogenicity.
Siminoff and Fetting (36) found that physicians’ treatment recommendations were the strongest predictor of patients’ treatment decisions. Their finding suggests the question of whether the psychiatrist should express an opinion about treatment. Some pregnant patients ask for this advice directly. Whether the psychiatrist provides an answer is also a case-specific clinical judgment.
We have reviewed factors that influence the perceptions and choices of patients. However, physicians are affected by the same factors. For example, if a patient who was treated with an antidepressant delivered a malformed baby, the prescribing physician is at risk for overestimation of the fetal risk for subsequent patients (particularly if he or she has treated few pregnant women). The physician may value freedom from worry about a malformation (or lawsuit) more than comprehensively addressing the patient’s distress. The physician’s provision of treatment involves a parallel risk-benefit decision that allows the conviction that he or she is acting responsibly on behalf of the patient. For example, physician discomfort is appropriate for a pregnant patient who has a brief depression of mild severity but demands treatment with ECT. Consultation is advisable in such situations.
Competence to Consent
The goal of informed consent is to allow patients to participate meaningfully in the selection of treatment (37). The patient’s capacity to participate in this process provides evidence of competence to consent. Informed consent is usually understood as consisting of three elements: disclosure, competence, and voluntariness. Disclosure is the obligation of the physician. In most jurisdictions, physicians must disclose all information that a reasonable patient would find relevant to a treatment decision (the information depicted in Figure 1). For a consent to be valid, the patient must have adequate decision-making capacity or, in legal terms, competence (38). All people are presumed to be competent to make decisions about their medical care in the absence of evidence to the contrary. Competence requires that the patient appreciate the nature of the situation, understand the relevant information, manipulate information rationally, state a choice, and understand the consequences of the choice. Mild depression rarely interferes with competence, and even moderate depression is not likely to do so (39). Severe depression can impair decision-making competence by interfering with the patient’s ability to attend to information disclosure. Severe impairment in psychosocial function may prevent adequate appreciation of the likely benefits of treatment. When there are concerns about a patient’s competence, consultation with an experienced evaluator is helpful. Decisions can be made for incompetent subjects by substitute decision makers authorized under state laws.
Even if the legal definition of competence is fulfilled, patients will have widely varying degrees of understanding of the information conveyed by the physician. The information is complicated, and the patient’s anxiety may interfere with her ability to assimilate it. Preparation of written material may be helpful. Patients can take the information home, read it over repeatedly, discuss it with family and friends, and return with questions. Written information allows the patient to read and review the material at a pace she can control (36).
During the discussion, the presence of the baby’s father, a family member, or a friend helps reduce the patient’s anxiety and allows questions to be raised that the patient might not consider. Consent to discuss the treatment with the woman’s obstetrician is imperative, and the result of this conversation must be noted in the record. The obstetrician is an important ally in treatment planning. He or she provides information about whether the intervention of choice presents additional benefit or risk to the patient’s pregnancy management. The psychiatrist facilitates the obstetrical management by encouraging the patient 1) to make changes beneficial to the pregnancy, such as decreasing smoking and alcohol use, 2) to comply with taking prenatal vitamins, appointments, and obstetrical recommendations, and 3) to prepare psychologically for the infant’s arrival and care.
If a negative outcome of pregnancy occurs, the potential for legal action is a common concern for physicians. This difficult consideration pits the interests of the pregnant woman against those of the fetus (40). These issues have been reviewed by the Institute of Medicine (41). Claims could be made by children against parents, the physician who prescribed the drug, the institution at which the treatment occurred, and the drug manufacturer. Failing to provide treatment also conceivably could result in a negative outcome and form the basis for legal action. However, an adverse outcome alone should not result in the imposition of liability on the treating physician. The standard of medical practice is the application of knowledge at the time of the exposure. Medical knowledge at the time of the maternal exposure could include no abnormal outcomes or abnormal outcomes that were disclosed and considered in the decision making. This would be the criterion on which the physician’s actions would be judged. Therefore, currency of knowledge about prescribing drugs during pregnancy is imperative.
If the diagnosis was reached correctly and the treatment was skillfully prescribed, the issue of liability rests on the adequacy of the informed consent process. In principle, physicians who have followed the procedure we have outlined should not be found liable for harm to the fetus or mother that results from a disclosed risk or a risk so uncommon that its disclosure would not be deemed material to a reasonable patient’s decision. Nonetheless, malpractice suits involving birth defects are often highly emotional. As the experience of many obstetricians suggests, juries often reason backward from the evident harm to the newborn to the conclusion that the physician must be at fault.
Liability risk can be minimized by careful documentation. The clinical decision making and treatment plan are documented by “thinking aloud on paper” (42), which provides evidence that the patient’s care was approached thoughtfully. Although informed consent for medication treatment is usually documented with a note in the patient’s chart, some clinicians use a consent form when prescribing drugs for pregnant women. Such forms should contain the information disclosed to the patient, a statement that the patient had the opportunity to have questions answered, and room to document any special aspects of the consent process (such as the presence of the patient’s partner). The patient’s signature serves as evidence that the information was provided, and in some states it establishes a legal presumption that informed consent was obtained.
There are two risks associated with the use of consent forms. If a form substitutes for a frank discussion with the patient, it can undercut the process of mutual decision making that lies at the core of informed consent. Forms should document, not dominate, the consent process. If the form is written in language so obscure or complex that a typical patient could not comprehend it, it may serve as evidence of the inadequacy of the consent obtained. In general, it is advisable to provide information at the comprehension level of a high school graduate. Word-processing programs now have measures of the reading ease of written material by grade level.
Decision and Action
The result of the risk-benefit discussion is a decision about the treatment and a plan for implementation. Informed consent is a process that extends across time. Figure 1 displays the dynamism of the decision-making process. As the pregnancy progresses, new information may shift the risk-benefit analysis so that a revision in the treatment plan is necessary. Following are two examples of how this decision-making model is applied in clinical practice.
Case 1
Ms. A was a 28-year-old married woman. She sought psychiatric evaluation for severe insomnia, depressed mood, panic attacks, and a 10-lb weight loss over 3 weeks. She did not smoke or use alcohol. Her psychiatrist recommended treatment with the antidepressant nortriptyline. A few days later, Ms. A was pleased to learn she was pregnant. Her psychiatrist told her by telephone to stop taking the nortriptyline because it was “not safe to use in pregnancy.” She was given no further advice. When her symptoms increased, she was enrolled in a group education program for expectant mothers. A few weeks later, her symptoms were intolerable. She saw her obstetrician, who also advised her not to take antidepressant medication because he believed that the symptoms would remit after the first trimester of pregnancy. When the symptoms intensified, her obstetrician recommended that she seek additional mental health consultation. Ms. A’s psychiatrist refused to treat her with medication unless she signed a form that he described as a statement that neither he nor the organization would be responsible if any harm came to the fetus. He did not provide a risk-benefit discussion. Ms. A was distressed by the psychiatrist’s approach. At her husband’s urging, Ms. A sought evaluation and treatment from a psychiatrist outside her insurance network.
The consulting physician met with Ms. A and her husband. They were given oral and written information about the diagnosis, major depression (43), which was in the severe range. Her score on the 17-item Hamilton Depression Rating Scale (44) was 31. The treatment options for depression were described as in Figure 1. Somatic treatments (antidepressant medication and ECT) and/or cognitive behavior therapy were offered to Ms. A. The risks of a continued lack of treatment for depression were reviewed: poor nutritional intake during pregnancy and severe fatigue and anxiety (with intermittent panic attacks) that disrupted her physical, social, and occupational functioning.
For each treatment option, there is a lower or higher probability of fetal toxicity within each domain in Figure 1. There are also four possible outcomes for the depressive episode: remission, improvement, continuation at the same symptom level, or worsening. The consulting psychiatrist explained that several prospective studies provided data about antidepressant exposure during pregnancy. Fluoxetine has been studied as a single agent, and tricyclic antidepressants have been studied as a group, as have the SSRIs sertraline, paroxetine, and fluvoxamine. The information specific to the likelihood of each reproductive toxicity domain (discussed earlier in this article and extensively in our review [5]) was given to Ms. A and her husband. The consultant, Ms. A, and her husband believed that the probability of depression remission would be greatest with somatic treatment, because of the severity of the symptoms. When all treatment options and consequences were presented, Ms. A’s valuations of different outcomes were considered. After discussion with Mr. A, Ms. A’s sister (a physician), and her obstetrician, Ms. A decided that antidepressant medication was most acceptable to her. Her obstetrician supported the decision. In a telephone call initiated by the consultant psychiatrist, the obstetrician expressed his interest in understanding the distinction between first-trimester discomforts, which subside, and major depression.
Ms. A selected nortriptyline treatment. After several weeks she experienced complete remission of symptoms, which continued throughout the pregnancy. The anticipated outcome that justified exposure to medication (remission of depression) had occurred. She delivered a healthy baby at term, with no neonatal complications. She remained in full remission during the first postpartum year, and nortriptyline was tapered successfully.
Ms. A described her depression as intolerable, and the option of no treatment was not acceptable because of the high probability that the depression would not remit. Since the depression was associated with serious weight loss and poor nutrition, her obstetrician became concerned. Ms. A did not believe that psychotherapy would be as likely to be effective, because she had difficulty concentrating. Therefore, she considered antidepressants and ECT, which were expected to be successful in treating her depression. Given these options, she chose nortriptyline, a tricyclic antidepressant, for several reasons. She was already familiar with the treatment and had had no side effects from her brief exposure, a relative had responded well to the drug, and she was reassured by the fact that nortriptyline has been used to treat depression for several decades.
Case 2
Ms. B was a 34-year-old married woman. At week 19 of gestation, she was referred for consultation by her psychiatrist and therapist because she refused to take medication during pregnancy. She previously had a good response to fluoxetine and was being treated with weekly interpersonal psychotherapy. Her treatment team was concerned that she was becoming increasingly debilitated by her severe depression. She was unable to function at her managerial position and took a leave of absence.
Upon examination by the consultant psychiatrist, Ms. B was tearful and agitated. Her score on the Hamilton Depression Rating Scale (44) was 28 (severe range). She had depressed mood and anxiety, sleep disturbance with only 2–4 hours of sleep per night, and a weight gain of only 5 lb. She had suicidal ideation but no active plan. She tearfully revealed the story of her previous stillbirth at week 24 of gestation. She noted that her distress was increasing as the gestational week of the previous stillbirth approached. The psychiatrist presented the structure of the consultation and decision-making process to Ms. B alone, since her husband was not willing to attend. She was knowledgeable about her diagnosis of major depression. The treatment options were described as in Figure 1. The risks of fetal toxicity in the five domains were reviewed. The likelihood of the depression continuing was high because of Ms. B’s history of chronic depression without pharmacologic treatment. The likelihood that the depression would remit would be greatest for the somatic treatment options (such as fluoxetine, to which she had previously responded), but the possibility of a less optimal fetal outcome was not tolerable for Ms. B. Once all treatment options and their consequences were presented, Ms. B’s valuations of the different outcomes were considered. Her obstetrician encouraged her to select a somatic treatment.
Ms. B stated that she could not accept medication or ECT. She said that she would not be able to tolerate the thought that she might have played a role in harming her baby if there was a negative outcome. Ms. B clearly stated that she understood the benefits and risks of treatment. She was pleased about her previous response to fluoxetine. She was aware that the untreated depression was posing a risk to her health and to the health of the fetus. She chose to continue weekly psychotherapy with monitoring for symptom level and suicidality, but she did not improve. She also tried morning bright light therapy but experienced no response. Unfortunately, she experienced a second stillbirth at 24 weeks’ gestation. She accepted fluoxetine treatment and continued psychotherapy after the stillbirth and eventually recovered from her depression.
Ms. B felt that any treatment option that increased the risk to the fetus was not acceptable. She dreaded another fetal demise. She viewed taking medication as an active choice that carried risk for which she was responsible. The effects of her illness on herself and the fetus were viewed as “in God’s hands.” Her thoughtful responses within the context of the risk-benefit discussion provided evidence of competence to decide about medical care on her own behalf. There was no legal or ethical way to force Ms. B to accept somatic therapy under these circumstances, which were driven by the values she brought to the decision-making process.
Conclusions
The case examples illustrate the use of the decision-making model to structure and individualize clinical treatment for pregnant women with depression. Continued research is imperative and will expand our knowledge about the effects of both antidepressant medication and major depression on maternal reproductive and fetal health. The information about the reproductive toxicity of somatic treatments and about depression that is part of the model will become more sophisticated as data accrue. However, the model presented in this article represents a stable framework into which new information can be incorporated. The physician can guide the patient through a decision-making process toward optimal childbearing outcomes for both the mother and her newborn.
Received Sept. 23, 1999; revision received March 27, 2000; accepted May 30, 2000. From Women’s Mental HealthCARE, Department of Psychiatry, and Department of Reproductive Biology, Case Western Reserve University School of Medicine; the Department of Psychiatry and Department of Obstetrics and Gynecology, University of Iowa College of Medicine, Iowa City; the Office of Quality Improvement and Psychiatric Services, American Psychiatric Association, Washington, D.C.; the Division of General Internal Medicine, National Naval Medical Center, Bethesda, Md.; the Department of Psychiatry, University of Massachusetts Medical School, Worcester; the Department of Psychiatry, School of Medicine, University of Arizona Health Sciences Center, Tucson; the Department of Psychiatry and Human Behavior, Brown University School of Medicine, Providence, R.I.; and the Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center. Address reprint requests to Dr. Wisner, Women’s Mental HealthCARE, Case Western Reserve University School of Medicine, Suite 280, 11400 Euclid Ave., Cleveland, OH 44106; [email protected] (e-mail).Supported by NIMH grant MH-60335 to Dr. Wisner.The authors thank Robert Kiwi, M.D., Barbara Gracious, M.D., and Daniel Rapport, M.D., for their comments.
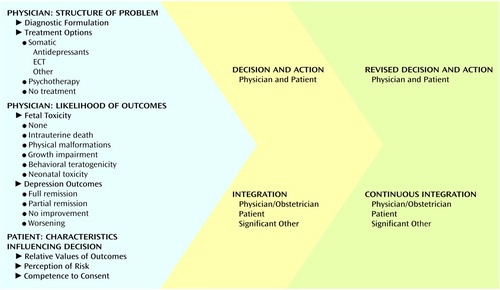
Figure 1. Model for Decisions Regarding Treatment of Depression During Pregnancya
aModified version of earlier work by Zarin and Pauker (6). Copyright Swets & Zeitlinger. Used with permission.
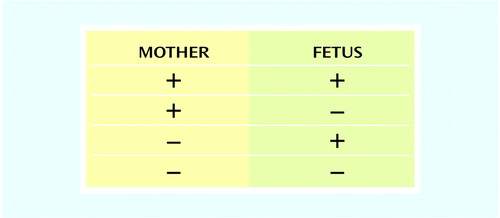
Figure 2. Possible Combinations of Positive and Negative Outcomes for Mother and Fetus From Treatments for Depression
1. Burke KC, Burke JD, Rae DS, Regier DA: Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Arch Gen Psychiatry 1991; 48:789–795Crossref, Medline, Google Scholar
2. O’Hara MW, Neunaber DJ, Zekoski EM: Prospective study of postpartum depression: prevalence, course, and predictive factors. J Abnorm Psychol 1984; 93:158–171Crossref, Medline, Google Scholar
3. Wisner KL, Peindl KP, Hanusa BH: Relationship of psychiatric illness to childbearing status: a hospital-based epidemiologic study. J Affect Disord 1993; 28:39–50Crossref, Medline, Google Scholar
4. The baby or the drug? it’s a choice that many pregnant women often face—but shouldn’t. US News and World Report, March 27, 1995, p 59Google Scholar
5. Wisner KL, Gelenberg AJ, Leonard H, Zarin D, Frank E: Pharmacologic treatment of depression during pregnancy. JAMA 1999; 282:1264–1269Google Scholar
6. Zarin DA, Pauker SG: Decision analysis as a basis for medical decision making: the tree of Hippocrates. J Med Philos 1984; 9:181–213Crossref, Medline, Google Scholar
7. Deber RB, Kraetschmer N, Irvine J: What role do patients wish to play in treatment decision making? Arch Intern Med 1996; 156:1414–1420Google Scholar
8. Wisner KL, Perel JM: Psychopharmacological treatment during pregnancy and lactation, in Psychopharmacology and Women: Sex, Gender, and Hormones. Edited by Jensvold MF, Halbreich U, Hamilton JA. Washington, DC, American Psychiatric Press, 1996, pp 191–224Google Scholar
9. Peindl KS, Day NL, Wisner KL: Alcohol use during pregnancy. Primary Psychiatry 1996; 3:43–44Google Scholar
10. Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochochinski VJ: Three year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093–1099Google Scholar
11. American Psychiatric Association: Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry 1993; 150(April suppl)Google Scholar
12. Miller LJ: Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry 1994; 45:444–450Abstract, Google Scholar
13. Parry BL, Cover H, Mostofi N, LeVeau B, Sependa PA, Resnick A, Gillin JC: Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder and normal comparison subjects. Am J Psychiatry 1995; 152:404–412Link, Google Scholar
14. Neumeister A, Goessler R, Lucht M, Kapitany T, Bamas C, Kasper S: Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biol Psychiatry 1996; 39:16–21Crossref, Medline, Google Scholar
15. Nahas Z, Bohning DE, Molloy MA, Oustz JA, Risch SC, George MS: Safety and feasibility of repetitive transcranial magnetic stimulation in the treatment of anxious depression in pregnancy: a case report. J Clin Psychiatry 1999; 60:50–52Crossref, Medline, Google Scholar
16. Terman M, Terman JS, Ross DC: A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry 1998; 55:875–882Crossref, Medline, Google Scholar
17. Terman M, Amira L, Terman JS, Ross DC: Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry 1996; 153:1423–1429Google Scholar
18. Spinelli MG: Interpersonal psychotherapy for depressed antepartum women: a pilot study. Am J Psychiatry 1997; 154:1028–1030Google Scholar
19. Sandman CA, Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Belman J, Porto M, Murata Y, Garite TJ, Crinella FM: Psychobiological influences of stress and HPA regulation on the human fetus and infant birth outcomes. Ann NY Acad Sci 1994; 739:198–210Crossref, Medline, Google Scholar
20. Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ: The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 1993; 169:858–865Crossref, Medline, Google Scholar
21. Istvan J: Stress, anxiety, and birth outcomes: a critical review of the evidence. Psychol Bull 1986; 100:331–348Crossref, Medline, Google Scholar
22. Barlow SM, Knight AF, Sullivan FM: Prevention by diazepam of adverse effects of maternal restraint stress on postnatal development and learning in the rat. Teratology 1979; 19:105–110Crossref, Medline, Google Scholar
23. Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, Donnenfeld A, McCormack M, Leen-Mitchell M, Woodland C, Gardner G, Horn M, Koren G: Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA 1993; 269:2246–2248Google Scholar
24. Chambers CD, Johnson KA, Dick LN, Felix RJ, Jones KL: Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996; 335:1010–1015Google Scholar
25. Kulin NA, Pastuszak A, Sage SR: Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. JAMA 1998; 279:609–610Crossref, Medline, Google Scholar
26. Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JGW, Kulin N, Koren G: Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 1997; 336:258–262Crossref, Medline, Google Scholar
27. Eggermont E, Raveschot J, Deneve V, Casteels-van Daele M: The adverse influence of imipramine on the adaptation of the newborn infant to extrauterine life. Acta Paediatr Belg 1972; 26:197–204Medline, Google Scholar
28. Cowe L, Lloyd DJ, Dawling S: Neonatal convulsions caused by withdrawal from maternal clomipramine. Br Med J 1982; 284:1837–1838Google Scholar
29. Webster PAC: Withdrawal symptoms in neonates associated with maternal antidepressant therapy. Lancet 1973; 2:318–319Crossref, Medline, Google Scholar
30. Wisner KL, Perel JM: Psychopharmacologic agents and electroconvulsive therapy during pregnancy and the puerperium, in Psychiatric Consultation in Childbirth Settings: Parent- and Child-Oriented Approaches. Edited by Cohen RL. New York, Plenum, 1988, pp 165–206Google Scholar
31. Shearer WT, Schreiner RL, Marshall RE: Urinary retention in a neonate secondary to maternal ingestion of nortriptyline. J Pediatr 1972; 81:570–572Crossref, Medline, Google Scholar
32. Mhanna Mj, Bennet JB, Izatt SD: Potential fluoxetine chloride (Prozac) toxicity in a newborn. Pediatrics 1997; 100:158–159Crossref, Medline, Google Scholar
33. Bogardus ST, Holmboe E, Jekel JF: Perils, pitfalls, and possibilities in talking about medical risk. JAMA 1999; 281:1037–1041Google Scholar
34. Slovic P: Perception of risk. Science 1987; 36:280–285Crossref, Google Scholar
35. Koren G, Bologa M, Long D, Feldman Y, Shear NH: Perception of teratogenic risk by pregnant women exposed to drugs and chemicals during the first trimester. Am J Obstet Gynecol 1989; 160:1190–1194Google Scholar
36. Siminoff LA, Fetting JH: Effects of outcome framing on treatment decisions in the real world: impact of framing on adjuvant breast cancer decisions. Med Decis Making 1989; 9:262–271Crossref, Medline, Google Scholar
37. Appelbaum PS, Lidz CW, Meisel A: Informed Consent: Legal Theory and Clinical Practice. New York, Oxford University Press, 1987Google Scholar
38. Grisso T, Appelbaum PS: Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. New York, Oxford University Press, 1998Google Scholar
39. Grisso T, Appelbaum PS: The MacArthur Treatment Competence Study, III: abilities of patients to consent to psychiatric and medical treatments. Law Hum Behavior 1995; 19:149–174Crossref, Medline, Google Scholar
40. Nadelson CC: Ethics, empathy, and gender in health care. Am J Psychiatry 1993; 150:1309–1314Google Scholar
41. Institute of Medicine: Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Edited by Mastroianni AC, Faden R, Federman D. Washington, DC, National Academy Press, 1994Google Scholar
42. Wettstein RM: Psychiatric malpractice, in American Psychiatric Press Review of Psychiatry, vol 8. Edited by Tasman A, Hales RE, Frances AJ. Washington, DC, American Psychiatric Press, 1989, pp 392–408Google Scholar
43. American Psychiatric Association: Let’s talk facts about depression. http://www.psych.org/public_info/DEPRES~1.HTMGoogle Scholar
44. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar



