Prader-Willi Syndrome
Prader-Willi syndrome is a genetic disorder characterized by mental retardation, dysmorphic features, and behavioral dysfunction, most notably food-related problems such as hyperphagia, food seeking, and a high risk for obesity (1). Although food-related symptoms are a hallmark of the disorder, other psychiatric manifestations are common and can lead to significant interference in the affected individual’s development and functioning. Characteristic symptoms may include temper tantrums, oppositional behavior and stubbornness, emotional lability, and obsessive ruminations or compulsive behaviors such as skin-picking (2). In light of its distinctive behavioral, psychiatric, and genetic findings, Prader-Willi syndrome has emerged as a model for genetic and neurodevelopmental disorders. A girl with Prader-Willi syndrome illustrates the characteristics of the syndrome and its clinical management and suggests how Prader-Willi syndrome can serve as a heuristic model for other neuropsychiatric conditions.
CASE PRESENTATION
Miss A, an 11-year-old girl with a clinical and DNA-based diagnosis of Prader-Willi syndrome, was seen in psychiatric consultation at the Yale Child Study Center. She was referred for behavioral problems that had steadily worsened over the preceding 2 years. Her explosive episodes had led to severe problems at home and in school, where constant supervision had become necessary.
Presenting History
Miss A was described as an engaging and sweet child; nonetheless, her maladaptive behaviors had been episodically present throughout most of her life. Starting when she was 5 years ofage, temper tantrums, stubborn and oppositional traits, and a negativistic stance had become commonplace. In previous years such behaviors had been managed at home and in school through simple behavioral interventions, like time outs. In the months before the consultation, Miss A’s disruptive behaviors had steadily escalated in intensity and frequency, and her parents and school staff found it increasingly difficult to manage her outbursts. These incidents occurred two or three times a week and were characterized by screaming, kicking and hitting, tearing things off the wall, damaging or tossing materials, and at times trying to forcefully grab or hit her teacher or classmates. On one occasion she ran out of the schoolyard and dangerously close to oncoming traffic. The episodes were not usually directed at any particular individual but became severe enough to require her being physically restrained or observed constantly. She had often been suspended for the remainder of the school days, only to be returned home, where all symptoms typically subsided just as rapidly as they had started.
Miss A’s behavioral problems appeared to be commonly triggered by transitions or subtle changes in the daily schedule of activities and routines. Vacations and short days in school had been typically challenging, as had been teacher substitutions or changes in class composition. Her symptomatic deterioration could also be traced in part to a change in the work schedule of her mother, who had recently started working longer hours, as well as regularly on weekends.
In addition to her explosive behaviors, Miss A demonstrated a rigid preference for sameness and order. She exhibited an intense “need to ask or tell,” as when asking her parents time and again which roads would be driven on the way back home from school, even though the same path was always followed. Short-lived repetitive behaviors were also present and included multiple washings of her hair, as well as the more unusual washing, shampooing, and applying of lotions to her pet dog over a period of several weeks. Food-related behaviors such as stealing, sometimes seen in Prader-Willi syndrome, had, in general, not been major problems in her life, but foraging and hoarding of food intermittently occurred, culminating with an isolated instance at school in which she had eaten refuse out of a wastebasket.
Skin picking had been an intermittent problem that had waxed and waned since Miss A was age 5 and that had at times led to circumscribed scarring and localized infection. She had also had at least one discrete episode of self-injurious behavior. At age 10, and during a short trial of fluoxetine (20 mg/day), she had experienced behavioral disinhibition characterized by significantly worsened skin picking and by cutting her fingertips with disposable razors that she had managed to secure and hide.
Developmental History
Miss A was born at term, the product of an uncomplicated pregnancy characterized by decreased fetal movements. She was markedly hypotonic at birth, appeared lethargic and hypoaroused, and had a weak, almost imperceptible cry. Physical examination revealed hypoplastic genitalia. She had a weak suck and did not appear hungry, and her poor oral intake led to failure to thrive and lack of weight gain, with hospitalization becoming necessary for 3 weeks after her birth. It was not until the age of 6 weeks that she was able to suck adequately; before then she had required gavage feedings, or intake through the squirting of milk into her mouth.
Initial workup failed to reveal a neuromuscular abnormality to explain Ms. A’s extreme hypotonia. Her neonatal history of diffuse idiopathic hypotonia and hypoplastic genitalia led to a clinical diagnosis of Prader-Willi syndrome within her first month of life. Genetic testing soon thereafter corroborated the diagnosis, revealing an interstitial deletion on the long arm of chromosome 15. By 4 months of age, she displayed many of the dysmorphic features characteristic of Prader-Willi syndrome, including a narrow forehead, a broad nasal bridge, slightly upslanting, almond-shaped palpebral fissures, a downturned mouth with a thin upper lip, and narrow hands and feet that were disproportionately small for her size (figure 1and figure 2).
By 2 years of age, Miss A’s hypotonia had essentially resolved, but developmental delays were evident. Expressive language and gross and fine motor milestones lagged significantly behind the development of social skills and receptive language, both of which were only minimally delayed. Contributing to her difficulties in expressive language and articulation, Miss A produced small amounts of highly viscous saliva, which appeared thick and stringy, crusting around the edges of her mouth. As a consequence, dental problems and cavities became manifest at an early age.
Past Medical History
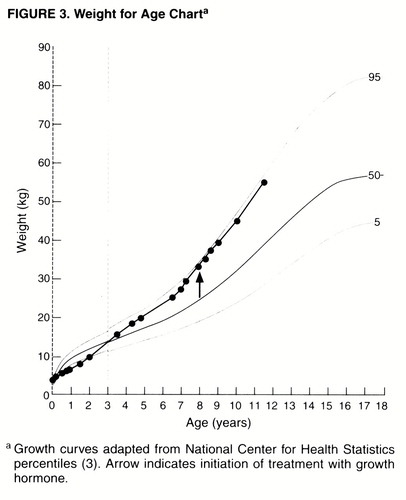
The pattern of Miss A’s appetite and oral intake changed diametrically by the age of 3 years. At birth she had weighed 3.8 kg and measured 53 cm in height, both within the normal range (75th and 50th percentiles for age, respectively). However, as a consequence of her early feeding difficulties, her weight dropped within the first 6 months of life, remaining well below the fifth percentile until her second birthday (figure 3). Between the ages of 2 and 3 she went from below the fifth percentile in weight to above the 50th, and by the age of 5 years she was closer to the 90th percentile. In close collaboration with a multidisciplinary Prader-Willi syndrome clinic that included her pediatrician and a nutritionist, Miss A’s family worked aggressively to limit further weight gain. Interventions included portion controls, calorie counts, a regular exercise schedule, and at times locking cabinet doors to limit her access to food.
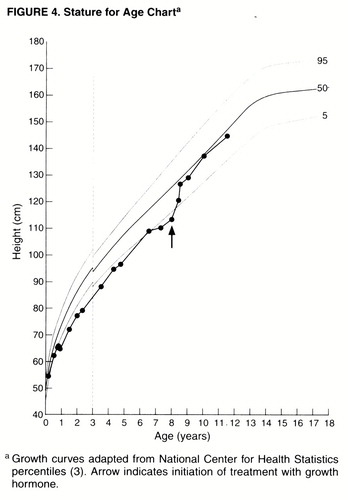
Miss A’s normal height at birth was similarly followed by a deceleration of linear growth during the first months of life. Her height remained below the fifth percentile until her eighth year (figure 4). Treatment with growth hormone (GH) was started at the age of 8 and resulted in a dramatic increase in height and height velocity within just 6 months. By the age of 11, her height was close to the population mean. GH treatment led not only to linear growth, but to other discrete and noticeable changes in body habitus as well, with a more uniform distribution of body fat, higher muscle tone, and a stronger facial bone structure (figure 2).
Miss A underwent a thorough neurologic evaluation at age 10 because of the exacerbation of behavioral problems. The possibility of a concurrent seizure disorder was raised after she was described as “dazed and confused” and having little recollection of her acts in the wake of some of her more aggressive outbursts. Results of noncontrast computed tomography were reported within normal limits, as were results of an EEG. Treatment with valproate was nevertheless recommended in an effort to target behavioral and mood lability.
When Miss A was seen in psychiatric consultation at 11 years of age, her height was 57 cm (50th percentile), and her weight was 55.5 kg (>95th percentile). She displayed pubertal changes, including breast development and mild facial acne. At a recent pediatric visit she was noted to have Tanner stage III breast development and Tanner stage III/IV pubic hair. Menses had not yet started.
Psychometric Assessments
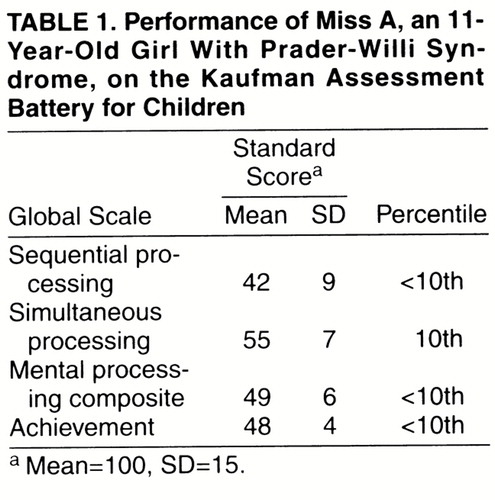
Miss A’s intellectual ability was assessed with the Kaufman Assessment Battery for Children (4). Her performance on the test is presented in table 1.
The mental processing composite on the Kaufman Assessment Battery for Children reflects Miss A’s overall performance in the moderate range of mental retardation. She had difficulty with tasks requiring serial order processing, being better able to process information presented in a holistic fashion. She showed significant strengths in her ability to complete visual patterns and to reason by analogy on visual-spatial tasks. Frequent perseveration and a short attention span became evident during testing, as did impairments in short-term auditory and visual memory. Her achievement was strongest in reading subtests (e.g., reading/decoding standard score of 73).
Miss A had difficulty during tasks that required working memory, attention to detail, and synthesizing conceptual information presented verbally. She had problems with distractibility, hyperactivity, and impulsivity. Difficulties with impulse control, perseveration, and sequencing skills suggested global executive functioning impairment. This was exemplified in her performance on the Trailmaking test, which showed significant impairment relative to her estimated IQ.
Adaptive skills (i.e., Miss A’s ability to meet the demands of everyday life) were assessed with the Vineland Adaptive Behavior Scales (5). Standard scores on the Vineland scales (mean=100, SD=15) included 42 in communication, 40 in socialization, and 32 in daily living skills; her adaptive behavior composite was 35. Overall, these data indicate adaptive functioning that was lower than anticipated for her cognitive level and largely a reflection of her behavioral difficulties.
Psychiatric Diagnoses
While Miss A presented with significant behavioral problems, her assessment did not lead to a single, overarching DSM-IV diagnosis. Initial diagnoses included expressive language disorder, generalized anxiety disorder, and impulse control disorder not otherwise specified on axis I; moderate mental retardation on axis II; and Prader-Willi syndrome on axis III.
Review of Treatment Interventions
The early age at which Miss A’s Prader-Willi syndrome diagnosis was established, together with her parents" advocacy and proactive involvement, allowed for her comprehensive and multimodal treatment from the start. Individually tailored interventions in Prader-Willi syndrome can maximize development, education, and growth, help in the stabilization of behavioral difficulties, and minimize the syndrome"s complications—most notably those related to obesity and its associated medical morbidity (6). The role of Miss A’s pediatrician in her care was critical toward these goals; the pediatrician worked in the well-orchestrated collaboration of numerous specialists and paraprofessionals, including nutritionist, clinical geneticist, pediatric endocrinologist and neurologist, and clinical psychologist, as previously highlighted.
Collaboration with appropriate institutions was similarly important. Miss A’s family was referred early on to three not-for-profit public advocacy groups serving the needs of individuals with Prader-Willi syndrome and their families (the national Prader-Willi Syndrome Association, 5700 Midnight Pass Rd., Suite 6, Sarasota, FL 34242, telephone 1-800-926-4797, as well as its local state chapter; and the Prader-Willi Foundation, Inc., 223 Main St., Port Washington, NY 11050, telephone 1-800-253-7993). The involvement of Miss A’s family with these organizations gave them a sense of not being alone, provided them with critical support and information, and was helpful in securing maximum available interventions and services. In light of her developmental delays and special needs, Miss A was concurrently referred to her local Birth to Three chapter and later on to the Department of Mental Retardation. Through the involvement of all of these agencies, Miss A was enrolled in developmentally appropriate school settings.
Psychotropic medications prescribed by a consulting child psychiatrist became a part of Miss A’s regimen starting at the age of 10. Methylphenidate had been implemented to treat symptoms of distractibility and short attention span, as well as excessive daytime sleepiness. Methylphenidate (10 mg orally b.i.d.) was remarkably effective in targeting both symptom clusters. A second trial of a low-dose selective serotonin reuptake inhibitor (SSRI) was implemented after our initial consultation. Fluvoxamine (25 mg/day) was well tolerated and resulted in the amelioration of separation and social anxieties, the brightening of Miss A’s mood, and the curbing of some of her disruptive behaviors and more intrusive obsessive ruminations. To target her mood lability, Miss A also continued treatment with valproate, 375 mg/day p.o. (yielding a low-therapeutic serum level of 56 mg/dl). GH therapy was maintained at 1.5 mg/day, administered subcutaneously, 6 of 7 days per week.
DISCUSSION
Diagnosis and Natural History
The clinical diagnosis of Prader-Willi syndrome is made on the basis of widely accepted consensus criteria (7) (appendix 1). This diagnostic scheme relies on a point system that divides signs and symptoms into major and minor criteria. Major criteria are assigned 1 point, with half a point for minor criteria. In addition, there are a number of supportive criteria that do not contribute to the overall total but are helpful in increasing diagnostic certainty. In older children, adolescents, and adults, the diagnosis of Prader-Willi syndrome requires a total of 8 points, with at least 5 from the list of major criteria. In children under the age of 3, diagnosis requires a total of 5 points, with 4 from the major criteria group.
As her background history reveals, Miss A met all major and most of the minor criteria for Prader-Willi syndrome; there were also several other supportive findings. Genetic testing confirmed her clinical diagnosis. While her history and diagnosis are typical and representative of Prader-Willi syndrome, it may be misleading to assume that all cases have such classic presentations. As in Miss A’s case, Prader-Willi syndrome can be diagnosed in the neonatal period, but at other times it may not become evident until much later. The syndrome may first be suspected in adolescents or adults who present with mild mental retardation, obesity, and behavioral difficulties.
One complaint that was not present in Miss A’s history and does not appear among the formal diagnostic criteria but deserves comment is rectal digging. The problem is common for both children and adults with Prader-Willi syndrome and may present a number of risks, ranging from potential fecal contamination to rectal incompetence and bleeding (8). The symptom may be related to the skin picking that is nearly ubiquitous in Prader-Willi syndrome. Rectal digging is usually not the most severe problem associated with the disorder but can often be the cause of embarrassment to patients and families. As a result, many times the presence of the symptom will not be mentioned during evaluation and treatment and will not be disclosed unless specifically inquired for.
Physical and behavioral manifestations in Prader-Willi syndrome are highly interrelated. While most physical findings are neither life threatening nor progressive, the sequelae of the syndrome’s behavioral traits are not nearly so benign. In the absence of aggressive intervention, morbid obesity at a young age is common. Food-related behaviors in Prader-Willi syndrome may lead to a host of serious medical consequences, including the range of cardiovascular diseases, diabetes mellitus, sleep disturbances, and respiratory compromise. It is these sequelae that represent the most common causes of premature morbidity and mortality among patients with Prader-Willi syndrome.
Genetics of Prader-Willi Syndrome
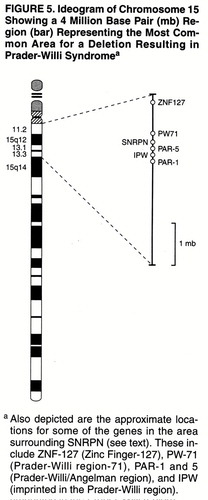
Prader-Willi syndrome is a sporadic genetic disorder with an estimated prevalence of 1 in 15,000. It represents the most common diagnosable genetic obesity syndrome (6). Prader-Willi syndrome results from characteristic abnormalities involving chromosome 15, although the specific gene or genes responsible for the syndrome have not yet been determined conclusively. The search for such a gene or genes has led to the identification of a genetic region of interest and a candidate gene, the small nuclear ribonuclear polypeptide N (SNRPN), which codes for the polypeptide involved in RNA splicing (9, 10). Other genes in the Prader-Willi syndrome critical region (as those depicted in figure 5) are likely to be involved. In fact, Prader-Willi syndrome may be considered a contiguous gene syndrome in which a number of nearby loci contribute to its overall clinical phenotype (11). Genetic research in Prader-Willi syndrome has also uncovered two important genetic mechanisms, genomic imprinting and uniparental disomy, that have considerable relevance to the broader understanding of human genetics (12).
Genomic imprinting and uniparental disomy. A genetic abnormality was documented in Prader-Willi syndrome over two decades after the syndrome was originally identified (1). High-resolution chromosomal banding allowing for the examination of million base-pair regions of DNA revealed that a substantial portion of patients with Prader-Willi syndrome had large deletions in the proximal long arm of chromosome 15 (13), particularly at bands 11–13 (denoted del 15q11-q13).
A perplexing finding was that an apparently identical deletion of chromosome 15 led to another, and markedly distinct, mental retardation condition, Angelman syndrome (14, 15). Patients with Angelman syndrome show profound mental retardation, marked language abnormalities, intractable seizures, and physical features impossible to confuse with those of Prader-Willi syndrome. The mystery of seemingly identical genetic lesions leading to two vastly different clinical entities was resolved when it was recognized that in cases of Prader-Willi syndrome, deletions were found only on the chromosome 15 that originated from the father (16). Conversely, in the case of Angelman syndrome, such deletions occurred only in the maternally derived chromosome. The finding of functional nonequivalence of the parental genomes was unexpected.
This phenomenon, termed genomic imprinting, describes a situation in which a chromosome retains a “memory” or imprint of its parental origin. The relevance of such a mechanism was emphasized when it was determined that in cases of Prader-Willi syndrome in which no deletion could be found, patients had two normal-appearing maternal copies of chromosome 15 and no paternal contribution, a condition termed maternal uniparental disomy. Moreover, some patients with Angelman syndrome who did not have identifiable deletions were also found to have paternal uniparental disomy (17, 18). In short, Prader-Willi syndrome and Angelman syndrome demonstrated for the first time that the difference between maternal and paternal chromosomes in certain specific regions could be highly relevant. The presence of two normal copies of one parental origin can not make up for the absence of the “correct” counterpart chromosome for those particular regions.
One suspected molecular mechanism for imprinting is methylation, a process by which methyl groups bind to cytosine residues, often organized in CpG islands (12). Allele-specific methylation differences have been documented for a number of imprinted genes, and high degrees of methylation often serve to silence gene expression (19). Methylation is an epigenetic phenomenon, not resulting in permanent DNA changes, but rather in developmentally timed alterations to the DNA structure that lead to uniparental mono-allelic gene expression. Early in development the preimplantation embryo shows bi-allelic expression and little evidence of methylation. Later in development, tissue-specific patterns of gene expression appear (mono-allelic or bi-allelic expression). For example, the normally imprinted insulin-like growth factor gene shows bi-allelic expression in cells of the choroid plexus (20). The Prader-Willi syndrome region is differentially methylated depending on the chromosome"s parent of origin: extensively so in the case of maternally derived chromosomes and sparingly in that of paternally derived ones.
The clustering of imprinted genes within specific chromosomal regions that replicate asynchronously has led to the search for DNA regulatory regions, “imprinting centers,” that can extend their effects over large stretches of DNA. In the case of Prader-Willi syndrome and Angelman syndrome, a putative bipartite imprinting center has been identified in the region of the SNRPN promoter (21). Consistent with this interpretation, mutations in this region result in bi-allelic expression of normally imprinted genes in the Prader-Willi syndrome region. Whether or not the characterization of this region will share features in common with other inactivation control regions such as those found on the X chromosome awaits further study (22).
Genetic diagnosis and subtyping. The earliest approach to identifying deletions in Prader-Willi syndrome used high-resolution cytogenetic examination, which involves arresting chromosomal division at metaphase, followed by staining and viewing the preparation under a light microscope. Deletions large enough to be seen in this fashion, such as in the case of Miss A, account for 50%–60% of Prader-Willi syndrome cases. Cytogenetic analysis remains useful for identifying these deletions, as well as translocations or ring chromosomes that may also lead to Prader-Willi syndrome.
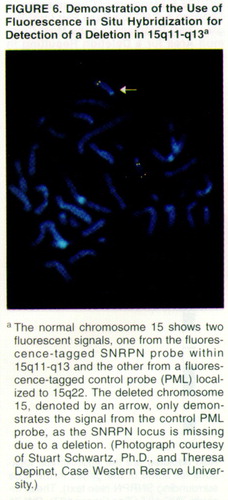
In a significant minority of Prader-Willi syndrome cases, deletions are too small to be visualized in this manner. In such instances, DNA probes are available to search 15q11-q13. Probes with nucleotide sequences complementary to those in the Prader-Willi region, including SNRPN, can be visualized when linked to a fluorescent marker, a technique called fluorescent in situ hybridization (figure 6).
Despite the much greater resolution afforded by fluorescent in situ hybridization, the approach cannot identify cases of uniparental disomy. Several alternative techniques are able to do so. One older technique relies on restriction fragment length polymorphisms that are able to trace the parent of origin and distinguish between two normal-appearing chromosomes in a suspected uniparental disomy patient. This approach requires blood from the patient and both parents. A more recent alternative technique relies on restriction enzymes that cut DNA differently depending on the methylation status of its regions. The technique requires blood from the patient only and allows for a highly sensitive assay for uniparental disomy, while also being able to identify most deletion cases (23).
Taken together, these commonly used approaches are effective tools in determining the genetic anomalies associated with Prader-Willi syndrome. However, given the multiplicity of techniques available, clinicians must note that a negative finding on certain examinations does not necessarily preclude either a clinical or a laboratory diagnosis of Prader-Willi syndrome (23).
The ability to reliably categorize patients on the basis of these techniques raises the question of whether different types of genetic processes lead to distinct clinical phenotypes. While subjects with either the paternal deletion or maternal uniparental disomy share the syndrome"s cardinal features, those with uniparental disomy may show milder symptom expression in both the physical and behavioral arenas (24-28).
Neuropsychiatric Aspects of Prader-Willi Syndrome
As reflected in Miss A’s presentation, maladaptive behaviors and psychiatric symptoms in Prader-Willi syndrome are often disproportionately debilitating relative to the cognitive impairment associated with the syndrome. While persons with Prader-Willi syndrome have, on average, mild to moderate levels of cognitive delay, their behavioral and psychiatric dysfunction may overwhelm the patient, the family, and other caregivers or lead to the need for highly restrictive levels of care.
Neurobiological substrates. Core features of Prader-Willi syndrome such as hypogonadism, short stature, and appetite dysregulation have led to suspect hypothalamic dysfunction as a putative neural substrate involved in the disorder. Minor and supportive criteria such as temperature and pain instability and early adrenarche also suggest hypothalamic localization. At present, however, there is no clear evidence for gross anatomical lesions in general, or in the hypothalamus or the pituitary in particular (29), nor are there any published functional neuroimaging findings.
The notion that the hypothalamus may play a central role in the syndrome has been supported by the single published neuropathological investigation of Prader-Willi syndrome (30). In postmortem samples, Swaab and colleagues found a significantly lower number of small oxytocin-secreting neurons in the hypothalamic paraventricular nucleus in Prader-Willi syndrome sufferers than in comparison subjects. Arginine-vasopressin neurons, a closely related neuronal population, appeared normal in Prader-Willi syndrome.
Psychiatric phenomenology. While food-related difficulties are the most widely recognized behavioral manifestations of the syndrome, subjects with Prader-Willi syndrome commonly show the types of difficulties that brought Miss A to psychiatric attention: temper tantrums, emotional lability, stubbornness, skin picking, and obsessive-compulsive symptoms. In addition, they may be at increased risk for depressive disorders (6). All told, these behavioral complaints are sufficiently common to qualify as diagnostic criteria, and they suggest that there may be a distinctive “behavioral phenotype” associated with the disorder.
Dykens and Kasari (31) compared 43 children with Prader-Willi syndrome aged 4 to 19 years to age- and gender-matched children with Down’s syndrome or nonspecific mental retardation. While all three groups showed stubbornness and temper tantrums, seven behaviors predicted Prader-Willi syndrome group membership with over 90% accuracy: skin picking, obsessions, fatigue, underactivity, impulsivity, speech problems, and talking too much. These same behaviors, along with overeating, also emerged as highly characteristic of a separate sample of Prader-Willi syndrome youngsters relative to subjects with Smith-Magenis syndrome (32), further showing the distinctiveness of the Prader-Willi syndrome behavioral phenotype.
High rates of non-food-related obsessive-compulsive behaviors were further examined in a study of 91 subjects with Prader-Willi syndrome aged 5 to 47 years (33). As assessed by the Yale-Brown Obsessive Compulsive Scales, subjects showed an average of three different obsessions and compulsions. Prominent symptoms included hoarding objects, ordering and arranging objects according to certain rules, “just right” phenomena, and repetitive asking or telling. Symptoms were similar in both type and severity to age- and gender-matched patients with obsessive-compulsive disorder (OCD); the subjects with Prader-Willi syndrome were more likely to hoard, and patients with OCD were more likely to show checking compulsions.
Few studies have looked at the relative risk for other types of psychopathology in patients with Prader-Willi syndrome. A recent survey of clinicians familiar with the syndrome estimated the types of psychiatric disorders seen in Prader-Willi syndrome and suggested that as many as 50% of patients may have depressive and anxious symptoms sufficient to warrant a DSM-IV diagnosis (6). Although such findings have not yet been substantiated by rigorous study, they do suggest that psychiatric disorders present a formidable challenge to patients with Prader-Willi syndrome and their families. A detailed understanding of the common psychiatric and behavioral manifestations of Prader-Willi syndrome may allow families and patients to identify such difficulties and seek treatment early in their course and may help in coping with symptoms that may otherwise be attributed to lazy, stubborn, or manipulative behaviors.
Cognitive profiles. Subjects with Prader-Willi syndrome span the full range of intelligence, from average ability to severe retardation, and the mean IQ of 70 is high relative to those with other genetic mental retardation syndromes. Adaptively, however, even high-functioning individuals rarely function at a level commensurate with their IQs, because of interference from food-related and other behavioral problems. Many individuals with Prader-Willi syndrome show a distinctive, although not necessarily unique, profile of cognitive strengths and weaknesses. Academically, reading/decoding and comprehension may exceed arithmetic skills, although uneven academic performance may not be striking enough to meet learning disability criteria (see reference 34 for review). As in Miss A’s case, some people with Prader-Willi syndrome show relative strengths in spatial-perceptual organization and visual processing tasks; these strengths may relate to the proficiency in jigsaw puzzles noted as a supportive finding in the clinical criteria for Prader-Willi syndrome. By contrast, common weaknesses are noted in sequential processing and short-term memory tasks, including visual, motoric and auditory short-term memory (35-37). Not all persons with Prader-Willi syndrome show these profiles, and studies are needed that identify the range and sources of individual differences in cognitive levels and profiles.
Treatment Approach and Prognosis
The separation of medical and behavioral aspects in Prader-Willi syndrome is artificial, as exemplified in Miss A’s treatment with GH. GH therapy in children with Prader-Willi syndrome increases muscle tone and enhances growth (38, 39). Anecdotally, GH is thought to have other significant benefits, such as allowing certain patients to moderately increase daily caloric intake without incurring substantial weight gain, thus mitigating some of the difficulties associated with maintaining highly restrictive diets. Through GH’s combined effects on muscle mass and tone, and in increasing height and decreasing central obesity, it can have a substantial effect in normalizing body habitus (figure 2). As in Miss A’s case, these combined results can have an enormous impact on the psychological well-being of Prader-Willi syndrome patients and their families, as well as decreasing their risks for medical morbidity.
In contrast to the clear evidence supporting the use of GH, there is a paucity of studies of anorectic agents in the management of Prader-Willi syndrome, and anecdotal reports have been uniformly discouraging. When phentermine, fenfluramine, and dexfenfluramine were available, there was little to suggest their efficacy in Prader-Willi syndrome, especially over time. Now that the severe cardiovascular consequences of these agents are known (40), their use in Prader-Willi syndrome is not supportable.
From the standpoint of non-food-related behavioral problems in Prader-Willi syndrome, there are few guidelines regarding psychopharmacology. The relatively high rate of anxious and depressive presentations among Prader-Willi syndrome patients has led to the frequent use of SSRIs. Marked improvements in the repetitive, aggressive, and affective symptoms of Prader-Willi syndrome through use of these medications have been reported in small open studies (41-43), although controlled trials are lacking. One frequent observation has been that doses expected for body weight appear to be poorly tolerated in Prader-Willi syndrome. As in Miss A’s case, a number of patients have shown exacerbations of aggressive, repetitive, and compulsive behaviors during initiation or upward dose titration of medication. Despite the generally positive experience with SSRIs for a variety of complaints common to Prader-Willi syndrome, there is unfortunately little to suggest that these medications have an impact on eating patterns or weight gain.
The role of antiepileptic agents, opiate antagonists, and benzodiazepines, as sometimes used in the treatment of impulsive behavior in patients with mental retardation, is not well documented in Prader-Willi syndrome (44). Clinically, neuroleptics are widely used and are an option for treatment-refractory patients, but they should be reserved as second-line agents because of their potential for unwanted side effects, including further weight gain.
Even with the clinical success seen with SSRIs and GH, the mainstay of management for behavioral difficulties—both food- and non-food-related—remains behavior modification. Programs such as token economies or star systems may be useful (44). Efforts to restrict food intake by careful dietary planning, close supervision, and limiting food access must extend out of the home and into school, work, and community settings. Hoarding food and stealing money to buy food are extremely common complaints in Prader-Willi syndrome, and vigilance is required even when patients are otherwise extremely well behaved. Exercise programs are also important and must be appropriate to the cognitive level and physical skills of the patient.
The management of the overall range of behavioral problems seen in Prader-Willi syndrome may be quite taxing of families, teachers, and patients. Out-of-home placement often becomes necessary over time (6, 44). Certain “dedicated” group homes specialize in the treatment of individuals with Prader-Willi syndrome, and in cases in which obesity, other behaviors, or both are out of control, such highly structured and specialized programs may be critical. Leaving the family home for a group setting may be viewed as a transition that fulfills developmental needs not dissimilar from those of other young adults. Nonetheless, out-of-home placement is at times construed by families and patients as a sign of failure. These feelings may be exacerbated by the fact that the potentially life-threatening problems associated with Prader-Willi syndrome typically arise from behaviors usually thought of as willful and controllable. Patients with Prader-Willi syndrome may feel that they are not trying hard enough to control their urges or their temper, and their families may feel that they have failed because obesity and tantrums remain a problem. The clinician can help to place these issues into a developmental context, to reinforce the idea that blame need not be assigned, and to remain engaged with patients and their families over time.
Prader-Willi Syndrome as a Heuristic Model
Efforts to understand the epigenetic mechanisms underlying Prader-Willi syndrome have led in unexpected directions. One of the most intriguing observations is that paternally imprinted genes differentially contribute to the development of hypothalamic and septal regions of the brain, while maternally imprinted genes contribute to the development of the neocortex and striatum (45). While these observations fit nicely with the clinical phenotypes associated with Prader-Willi syndrome and Angelman syndrome, respectively, the basis of this differential contribution remains enigmatic. Remarkably, from an evolutionary point of view, genomic imprinting is a relatively recent phenomenon—seen only in mammals and not in other metazoa, including other vertebrate species.
Since vertebrate development is highly conserved and imprinting is a mechanism found mostly in mammals, it is thought by some that imprinting mechanisms are unlikely to be “necessary” for the development of the well-known structures contained within the vertebrate CNS (46). On the other hand, it is clear that among mammalian species this mechanism for adjusting gene dosage has profoundly influenced aspects of brain development and growth. On the basis of morphometric analyses, some investigators have suggested that genomic imprinting may be one of the mechanisms responsible for the nonlinear expansion of neocortical and striatal structures in primate evolution (47).
One of the more provocative proposals to account for why the mammalian genome is ornamented with as many as ~3×107 methyl groups concerns the competition between parental genomes, particularly when the paternity of the offspring is in doubt (48, 49). Although this proposal is speculative, it is consistent with the observation that imprinted genes do not appear to be strictly conserved across mammalian species (see reference 46 for a review). Indeed, there is some evidence that patterns of genomic imprinting may reflect both species-specific patterns of social and reproductive behavior and differences in brain morphometry (45). The finding in Prader-Willi syndrome of reduced oxytocinergic neurons in the paraventricular nucleus (30) may be consistent with this proposal, given the role of oxytocinergic pathways in the initiation of maternal and other affiliative behaviors (see reference 50 for a review). These apparent alterations in oxytocin levels may also play a role in the obsessive-compulsive symptoms, and in the separation distress seen in Miss A and other patients with Prader-Willi syndrome (51, 52). This set of observations has also led us to suspect that genomic imprinting may play a role in OCD unrelated to Prader-Willi syndrome, and a search of the Prader-Willi syndrome region of chromosome 15 for genes that might contribute to OCD is underway. Finally, novel therapies directed toward hypothalamic dysfunction, and possibly involving congeners of oxytocin, may prove to be of value in this syndrome.
Appendix 1. Diagnostic Criteria for Prader-Willi Syndrome
Major Criteria (1 point)
| 1. | Neonatal and infantile central hypotonia with poor suck reflex, which improves over time | ||||
| 2. | Feeding problems in infancy with need for special feeding techniques and poor weight gain or failure to thrive | ||||
| 3. | Excessive or rapid weight gain (crossing two centile channels in weight for length charts) after 12 months but before 6 years of age; central obesity in the absence of intervention | ||||
| 4. | Characteristic facial features with dolichocephaly in infancy, narrow face or bitemporal diameter, almond-shaped eyes, small mouth with thin upper lip, downturn at corners of mouth (need three or more) | ||||
| 5. | Hypoganadism—with any of the following depending on age: genital hypoplasia or delayed or incomplete gonadal maturation with delayed pubertal signs in the absence of intervention after 16 years of age | ||||
| 6. | Global developmental delay in children younger than 6 years of age; mild to moderate mental retardation or learning problems in older children | ||||
| 7. | Hyperphagia, food foraging, or obsessions with food | ||||
| 8. | Deletion of 15q11-q13 on high resolution (greater than 650 bands) or other cytogenetic or molecular abnormality of the Prader-Willi chromosome region, including maternal disomy | ||||
Minor Criteria (one-half point)
| 1. | Decreased fetal movement or infantile lethargy or weak cry in infancy, improving with age | ||||
| 2. | Characteristic behavior problems—temper tantrums, violent outbursts, and obsessive- compulsive behavior; tendency to be argumentative, oppositional, rigid, manipulative, possessive, or stubborn; perseverating, stealing, or lying (more than five required) | ||||
| 3. | Sleep disturbance or sleep apnea | ||||
| 4. | Short stature for genetic background by age 15 (in the absence of growth hormone intervention) | ||||
| 5. | Hypopigmentation—fair skin and hair compared to family | ||||
| 6. | Small hands (<25 percentile), feet (<10 percentile), or both, for height and age | ||||
| 7. | Narrow hands with straight ulnar border | ||||
| 8. | Eye abnormalities (esotropia, myopia) | ||||
| 9. | Thick, viscous saliva with crusting at corners of mouth | ||||
| 10. | Speech articulation defects | ||||
| 11. | Skin picking | ||||
Supportive Criteria (no score)
| 1. | High pain threshold | ||||
| 2. | Decreased vomiting | ||||
| 3. | Temperature instability in infancy or altered temperature sensitivity in older children and adults | ||||
| 4. | Scoliosis, kyphosis, or both | ||||
| 5. | Early adrenarche | ||||
| 6. | Osteoporosis | ||||
| 7. | Unusual skill with jigsaw puzzles | ||||
| 8. | Normal neuromuscular studies | ||||
Received March 3, 1998; accepted April 24, 1998. From the Child Study Center, Yale University School of Medicine; the Neuropsychiatric Institute, University of California, Los Angeles; and the Department of Genetics, Case Western Reserve University School of Medicine.. Address reprint requests to Dr. Martin, Yale Child Study Center, 230 South Frontage Rd., New Haven, CT 06520-7900; [email protected] (e-mail). Supported in part by a grant from the Program for Minority Research Training in Psychiatry from the American Psychiatric Association and NIMH (Dr. Martin), by NIMH grant MH-18268 (Dr. State), and by program project grant HD-03008 from the National Institute of Child Health and Human Development. Identifying information has been changed to protect patient confidentiality. The authors thank patients A and B and their families for their cooperation; the staff of the PWS Management Clinics from the Connecticut Children"s Medical Center and from the University of Connecticut Health Center; and Drs. Donald Cohen and Paul Lombroso for their comments on an earlier draft of this article.
 |


1. Prader A, Labhart A, Willi H: Ein Syndrom von Adipositas, Kleinwuchs, Kryptorchismus und Oligophrenie nach myatonieartigem Zustand im Neugeborenenalter. Schweiz Med Wochenschr 1956; 86:1260–1261Google Scholar
2. Dykens EM, Cassidy SB: Prader-Willi syndrome: four decades of progress, in Neurodevelopmental and Genetic Disorders in Children. Edited by Goldstein S, Reynolds C. New York, Guilford (in press)Google Scholar
3. Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM: Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 1979; 32:607–629Crossref, Medline, Google Scholar
4. Kaufman AS, Kaufman NL: K-ABC: Kaufman Assessment Battery for Children. Circle Pines, Minn, American Guidance Service, 1983Google Scholar
5. Sparrow S, Balla D, Cicchetti D: Vineland Adaptive Behavior Scales, expanded ed. Circle Pines, Minn, American Guidance Service, 1984Google Scholar
6. Dykens EM CS: Prader-Willi syndrome: genetic, behavioral and treatment issues. Child Adolesc Psychiatr Clin North Am 1996; 5:913–927Crossref, Google Scholar
7. Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F: Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 1993; 91:398–402Medline, Google Scholar
8. Bhargava SA, Putnam PE, Kocoshis SA, Rowe M, Hanchett JM: Rectal bleeding in Prader-Willi syndrome. Pediatrics 1996; 97:265–267Medline, Google Scholar
9. Reed ML, Leff SE: Maternal imprinting of human SNRPN, a gene deleted in Prader-Willi syndrome. Nat Genet 1994; 6:163–167Crossref, Medline, Google Scholar
10. Glenn CC, Saitoh S, Jong MT, Filbrandt MM, Surti U, Driscoll DJ, Nicholls RD: Gene structure, DNA methylation, and imprinted expression of the human SNRPN gene. Am J Hum Genet 1996; 58:335–346Medline, Google Scholar
11. Nicholls RD: Genomic imprinting and uniparental disomy in Angelman and Prader-Willi syndromes: a review. Am J Med Genet 1993; 46:16–25Crossref, Medline, Google Scholar
12. Lalande M: Parental imprinting and human disease. Annu Rev Genet 1996; 30:173–195Crossref, Medline, Google Scholar
13. Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan BS, Crawford JD: Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med 1981; 304:325–329Crossref, Medline, Google Scholar
14. Kaplan LC, Wharton R, Elias E, Mandell F, Donlon T, Latt SA: Clinical heterogeneity associated with deletions in the long arm of chromosome 15: report of 3 new cases and their possible genetic significance. Am J Med Genet 1987; 28:45–53Crossref, Medline, Google Scholar
15. Magenis RE, Brown MG, Lacy DA, Budden S, LaFranchi S: Is Angelman syndrome an alternate result of del(15)(q11q13)? Am J Med Genet 1987; 28:829–838Google Scholar
16. Butler MG, Meany FJ, Palmer CG: Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 1986; 23:793–809Crossref, Medline, Google Scholar
17. Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M: Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature 1989; 342:281–285Crossref, Medline, Google Scholar
18. Knoll JH, Wagstaff J, Lalande M: Cytogenetic and molecular studies in the Prader-Willi and Angelman syndromes: an overview. Am J Med Genet 1993; 46:2–6Crossref, Medline, Google Scholar
19. Li E, Beard C, Jaenisch R: Role for DNA methylation in genomic imprinting. Nature 1993; 366:362–365Crossref, Medline, Google Scholar
20. DeChiara TM, Robertson EJ, Efstratiadis A: Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991; 64:849–859Crossref, Medline, Google Scholar
21. Saitoh S, Buiting K, Rogan PK, Buxton JL, Driscoll DJ, Arnemann J, Konig R, Malcolm S, Horsthemke B, Nicholls RD: Minimal definition of the imprinting center and fixation of chromosome 15q11-q13 epigenotype by imprinting mutations. Proc Natl Acad Sci USA 1996; 93:7811–7815Crossref, Medline, Google Scholar
22. Herzing LB, Romer JT, Horn JM, Ashworth A: Xist has properties of the X-chromosome inactivation centre. Nature 1997; 386:272–275Crossref, Medline, Google Scholar
23. Diagnostic testing for Prader-Willi and Angleman syndromes: report of the ASHG/ACMG Test and Technology Transfer Committee. Am J Hum Genet 1996; 58:1085–1088Medline, Google Scholar
24. Robinson WP, Langlois S, Schuffenhauer S, Horsthemke B, Michaelis RC, Christian S, Ledbetter DH, Schinzel A: Cytogenetic and age-dependent risk factors associated with uniparental disomy 15. Prenat Diagn 1996; 16:837–844Crossref, Medline, Google Scholar
25. Gillessen-Kaesbach G, Robinson W, Lohmann D, Kaya-Westerloh S, Passarge E, Horsthemke B: Genotype-phenotype correlation in a series of 167 deletion and non-deletion patients with Prader-Willi syndrome. Hum Genet 1995; 96:638–643Crossref, Medline, Google Scholar
26. Cassidy SB, Forsythe M, Heeger S, Nicholls RD, Schork N, Benn P, Schwartz S: Comparison of phenotype between patients with Prader-Willi syndrome due to deletion 15q and uniparental disomy 15. Am J Med Genet 1997; 68:433–440Crossref, Medline, Google Scholar
27. Gunay-Aygun M, Heeger S, Schwartz S, Cassidy SB: Delayed diagnosis in patients with Prader-Willi syndrome due to maternal uniparental disomy 15. Am J Med Genet 1997; 71:106–110Crossref, Medline, Google Scholar
28. Dykens EM, Cassidy SB, King BH: Maladaptive behavior differences in Prader-Willi syndrome due to paternal deletion versus maternal uniparental disomy. Am J Retard (in press)Google Scholar
29. Miller L, Angulo M, Price D, Taneja S: MR of the pituitary in patients with Prader-Willi syndrome: size determination and imaging findings. Pediatr Radiol 1996; 26:43–47Crossref, Medline, Google Scholar
30. Swaab DF, Purba JS, Hofman MA: Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: a study of five cases. J Clin Endocrinol Metab 1995; 80:573–579Medline, Google Scholar
31. Dykens EM, Kasari C: Maladaptive behavior in children with Prader-Willi syndrome, Down syndrome, and nonspecific mental retardation. Am J Ment Retard 1997; 102:228–237Crossref, Medline, Google Scholar
32. Dykens EM, Smith ACM: Distinctiveness and correlates of maladaptive behavior in children and adolescents with Smith-Magenis syndrome. J Intellect Disabil Res (in press)Google Scholar
33. Dykens EM, Leckman JF, Cassidy SB: Obsessions and compulsions in Prader-Willi syndrome. J Child Psychol Psychiatry 1996; 37:995–1002Crossref, Medline, Google Scholar
34. Dykens EM: Prader-Willi syndrome: toward a behavioral phenotype, in Neurodevelopmental Disorders: Contributions to a New Framework From the Cognitive Neurosciences. Edited by Tager-Flusberg H. Cambridge, Mass, MIT Press (in press)Google Scholar
35. Curfs LM, Wiegers AM, Sommers JR, Borghgraef M, Fryns JP: Strengths and weaknesses in the cognitive profile of youngsters with Prader-Willi syndrome. Clin Genet 1991; 40:430–434Crossref, Medline, Google Scholar
36. Gabel S, Tarter RE, Gavaler J, Golden WL, Hegedus AM, Maier B: Neuropsychological capacity of Prader-Willi children: general and specific aspects of impairment. Appl Res Ment Retard 1986; 7:459–466Crossref, Medline, Google Scholar
37. Dykens EM, Hodapp RM, Walsh K, Nash LJ: Profiles, correlates, and trajectories of intelligence in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 1992; 31:1125–1130Crossref, Medline, Google Scholar
38. Angulo M, Castro-Magana M, Mazur B, Canas JA, Vitollo PM, Sarrantonio M: Growth hormone secretion and effects of growth hormone therapy on growth velocity and weight gain in children with Prader-Willi syndrome. J Pediatr Endocrinol Metab 1996; 9:393–400Crossref, Medline, Google Scholar
39. Lindgren AC, Hagenas L, Muller J, Blichfeldt S, Rosenborg M, Brismar T, Ritzen EM: Effects of growth hormone treatment on growth and body composition in Prader-Willi syndrome: a preliminary report. The Swedish National Growth Hormone Advisory Group. Acta Paediatr Suppl 1997; 423:60–62Medline, Google Scholar
40. Mark EJ, Patalas ED, Chang HT, Evans RJ, Kessler SC: Fatal pulmonary hypertension associated with short-term use of fenfluramine and phentermine. N Engl J Med 1997; 337:602–606Crossref, Medline, Google Scholar
41. Hellings JA, Warnock JK: Self-injurious behavior and serotonin in Prader-Willi syndrome. Psychopharmacol Bull 1994; 30:245–250Medline, Google Scholar
42. Warnock JK, Clayton AH, Shaw HA, O"Donnell T: Onset of menses in two adult patients with Prader-Willi syndrome treated with fluoxetine. Psychopharmacol Bull 1995; 31:239–242Medline, Google Scholar
43. Benjamin E, Buot-Smith T: Naltrexone and fluoxetine in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry 1993; 32:870–873Crossref, Medline, Google Scholar
44. Dykens EM, Hodapp RM: Treatment issues in genetic mental retardation syndromes. Professional Psychology: Research and Practice 1997; 28:263–270Crossref, Google Scholar
45. Keverne EB, Fundele R, Narasimha M, Barton SC, Surani MA: Genomic imprinting and the differential roles of parental genomes in brain development. Brain Res Dev Brain Res 1996; 92:91–100Crossref, Medline, Google Scholar
46. Keverne EB, Martel FL, Nevison CM: Primate brain evolution: genetic and functional considerations. Proc R Soc Lond B Biol Sci 1996; 263:689–696Crossref, Google Scholar
47. Jaenisch R: DNA methylation and imprinting: why bother? Trends Genet 1997; 13:323–329Google Scholar
48. Moore T, Haig D: Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet 1991; 7:45–49Crossref, Medline, Google Scholar
49. Haig D: Intragenomic conflict and the evolution of eusociality (letter). J Theor Biol 1992; 156:401–403Crossref, Medline, Google Scholar
50. Insel TR: A neurobiological basis of social attachment. Am J Psychiatry 1997; 154:726–735Link, Google Scholar
51. Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McDougle CJ, Barr LC, Cohen DJ: The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 1994; 19:723–749Crossref, Medline, Google Scholar
52. Leckman JF, Mayes LC: Maladies of love—an evolutionary perspective on some forms of obsessive-compulsive disorder, in Advancing Research in Developmental Plasticity: Integrating the Behavioral Science and Neuroscience of Mental Health. Edited by Hann DM , Huffman L, Lederhendler I, Meinecke D. Rockville, Md, National Institute of Mental Health, US Department of Health and Human Services (in press)Google Scholar



