Comparison of Aerobic Exercise, Clomipramine, and Placebo in the Treatment of Panic Disorder
Abstract
OBJECTIVE: The purpose of this study was to compare the therapeutic effect of exercise for patients with panic disorder to a drug treatment of proven efficacy and to placebo. METHOD: Forty-six outpatients suffering from moderate to severe panic disorder with or without agoraphobia (DSM-III-R criteria) were randomly assigned to a 10-week treatment protocol of regular aerobic exercise (running), clomipramine (112.5 mg/day), or placebo pills. RESULTS: The dropout rate was 31% for the exercise group, 27% for the placebo group, and 0% for the clomipramine group. In comparison with placebo, both exercise and clomipramine led to a significant decrease in symptoms according to all main efficacy measures (analysis of variance, last-observation-carried-forward method and completer analysis). A direct comparison of exercise and clomipramine revealed that the drug treatment improved anxiety symptoms significantly earlier and more effectively. Depressive symptoms were also significantly improved by exercise and clomipramine treatment. CONCLUSIONS: These results suggest that regular aerobic exercise alone, in comparison with placebo, is associated with significant clinical improvement in patients suffering from panic disorder, but that it is less effective than treatment with clomipramine.
Despite the fact that exercise is often recommended as a coping strategy for neurotic disorders in the lay public, surprisingly few studies have examined the therapeutic potential of exercise in psychiatric disorders (1, 2). We know of only one clinical study on exercise for patients with anxiety disorders (3) and two case reports suggesting an anxiolytic effect of acute bouts of exercise (4, 5). To our knowledge, there have been no controlled, randomized studies examining the effects of aerobic exercise in panic disorder as defined by DSM-III-R.
Several pharmacological and psychological therapies have been shown to be effective in panic disorder and agoraphobia. Tricyclic antidepressants such as imipramine and clomipramine have been the mainstay of pharmacotherapy (6–11). Benzodiazepines such as alprazolam (12), irreversible monoamine oxidase inhibitors (13), and selective serotonin reuptake inhibitors such as fluvoxamine (14) have also proven effective in blocking panic attacks. On the other hand, many patients are concerned about side effects or a possible relapse after drug discontinuation and are in search of a nonpharmacological treatment that produces longer-lasting benefits.
Several studies found indexes of reduced cardiopulmonary fitness in patients with panic disorder (15–19). Having these studies in mind, we hypothesized that aerobic exercise might be of particular benefit in the treatment of panic disorder and agoraphobia. The present study was designed to compare putative therapeutic effects of exercise with a treatment of well-documented high efficacy (clomipramine) and with placebo.
METHOD
Patients with a diagnosis of panic disorder and agoraphobia according to the DSM-III-R and ICD-10 criteria (age range=18–50 years) were recruited by physician referral and from the Outpatient Anxiety Disorders Unit of the Department of Psychiatry, University of Göttingen, Germany. Diagnoses were made by an experienced psychiatrist using the Structured Clinical Interview for DSM-III-R (20). The exclusion criteria were pregnancy, lactation, substantial medical illness, bipolar affective disorder, severe major depression, psychotic symptoms, drug dependency (alcohol, benzodiazepine, or other), anorexia or bulimia nervosa, body weight below 80% of ideal body weight, and regular aerobic exercise comparable to the exercise treatment protocol. All patients were in good physical health and had normal results on a physical examination, ECG, and routine laboratory tests (renal, hepatic, pancreatic, hematologic, and thyroid function) before inclusion in the study.
Ninety-six consecutive patients were eligible for the study in accordance with the inclusion and exclusion criteria. The main reasons for ineligibility were concomitant physical disorders or current substance abuse. The main reasons for patients' refusal to participate were 1) the possibility of receiving only placebo treatment, 2) the necessity to undergo clinical tests (e.g., bicycle ergometry), and 3) the possibility of drug treatment. Forty-nine patients gave written informed consent after the whole procedure had been explained. Two patients dropped out of the study before actual treatment began. One patient had to be excluded because most of his symptoms had subsided at baseline. Thus, 46 patients were randomly assigned to the 10-week treatment protocol.
Patients were not allowed to undergo additional psychological treatment during the study. Patients taking psychotropic medication were required to discontinue this medication at least 3 weeks before baseline. During this time, only promethazine (25–50 mg) was allowed in the case of severe panic attacks. However, all patients were given the opportunity to talk to one of the study therapists at any time. Urine analysis for benzodiazepine intake was done at baseline and at the end of the treatment period. Patients who no longer met the DSM-III-R criteria for panic disorder and agoraphobia during the week before treatment and patients who had shown an improvement of 2 or more points on the Clinical Global Impression (CGI) scale between the screening interview and baseline were excluded from the study.
At baseline, patients were randomly assigned to the clomipramine/placebo group (N=30) or the exercise group (N=16). The study therapists (A.B., G.P., and A.G.) were not blind to this assignment. Patients in the drug group were further randomly assigned to receive either clomipramine (N=15) or placebo (N=15). The assignment was done by the hospital pharmacist; investigators and subjects remained blind to this assignment.
Patients in the drug treatment groups received capsules containing either 37.5 mg of clomipramine or placebo and were asked to take one capsule per day during the first week, two capsules per day during the second week, and three capsules per day (i.e., 112.5 mg of clomipramine or placebo) during the third week and thereafter. In the case of adverse events, patients were given the opportunity to call someone from the staff at any time or come to the outpatient department. All side effects were noted, and patients were reassured that most symptoms would be temporary and not dangerous. The study was conducted in accordance with the guidelines in Good Clinical Practice for Trials of Medical Products in the European Community: Declaration of the World Medical Association (Helsinki, 1989).
Patients in the exercise group were trained according to a specific training protocol that is based on general recommendations for effective aerobic exercise. Every patient was asked to find a 4-mile route (park or forest) that was easily accessible from his or her home. During the first week, it was sufficient to walk this route three or four times alone or with an accompanying person. During the second week, the patients had to start short running periods (2–4 minutes). Within the next 4 weeks, a gradual prolongation of these running periods had to be attempted. During the final 4 weeks of training, all patients were encouraged to run the whole distance without any breaks. However, the main emphasis was put on the requirement to “complete the route” at least three times a week, even if longer walking periods could not be avoided. Once a week all patients in the running group and a trainer (T.M.) would meet at a sports ground in order to run together.
All patients were required to complete activity diaries, which had to be presented at the weekly meetings with the therapists. The study therapists limited their interaction with the subjects to discussion of clinical history, recent important events, panic attacks during the last week, side effects of medication, and exercise-related problems. Therapists had to restrict their interaction to general support and avoid any specific cognitive or exposure techniques. The timing of these talks (once a week, then once a fortnight after week 6) was similar in all treatment groups.
The following set of clinician- and self-rated measures was completed at the screening interview, at baseline, and after 2, 4, 6, 8, and 10 weeks: the Hamilton Anxiety Rating Scale (21), observer-rated and patient-rated versions of the Panic and Agoraphobia Scale (22), observer and patient versions of the CGI (23), and the Fear Questionnaire (24). Additional scales were administered at baseline and week 10: the Beck Anxiety Inventory (25), the Beck Depression Inventory (26), and the Montgomery-Åsberg Depression Rating Scale (27).
The Panic and Agoraphobia Scale is a new scale for measuring severity in patients with panic disorder and agoraphobia. It contains 13 items with a 0–4 Likert rating scale and is divided into five subscores (intensity and frequency of panic attacks, agoraphobia, anticipatory anxiety, disability, health-related concerns). Before and after treatment, patients were also asked to rate on a 12-item list how effective they considered different therapies or activities to be in the treatment of panic disorder and agoraphobia. Rater and patient versions of the CGI were used as a global rating of psychopathology and overall severity of the condition. The following scales were defined as the main efficacy measures before the study began: the Hamilton anxiety scale, the observer-rated and patient-rated versions of the Panic and Agoraphobia Scale, and the rater version of the CGI. All other scales were treated as secondary outcome measures.
All statistical analyses were performed on an intent-to-treat basis; that is, data from all randomly assigned patients were included in the analysis if they had both a baseline observation and at least one postbaseline observation. In order to consider the influence of dropouts, calculations were performed on the basis of the last available results of each patient (last-observation-carried-forward method). As a second step, calculations were also performed for those patients who had actually completed the 10-week treatment protocol (completer analysis).
Data were analyzed by two-factor repeated measures analysis of variance (ANOVA) with baseline status as a covariate. The first repeated factor, group, was defined by the three treatment groups: exercise, clomipramine, and placebo. The second repeated factor, time, was defined by six time points (baseline and weeks 2, 4, 6, 8, and 10). A conventional univariate repeated measures approach was used to investigate the main effects of group and time and the interaction between group and time. Adjustments were made according to the Greenhouse-Geisser method (28), provided that the corresponding epsilon value was less than 1. If the interaction was significant, post hoc tests were done to compare the main treatment effects with each other; we used the Bonferroni-Holm method to correct p values. If a significant interaction between group and time was evident, treatment effects were also examined at each postbaseline time point with the use of Tukey-Kramer adjustments for multiple comparisons.
Categorical data were compared by Fisher's exact test. Results with p values less than 0.05 were interpreted as significant. All data analysis was done with SAS, version 6.11 (29).
RESULTS
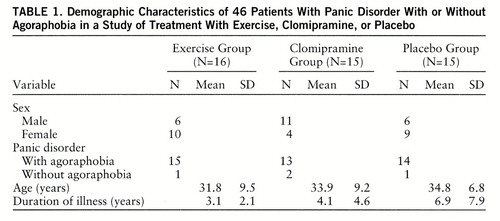
The randomization procedure produced comparable groups clinically and demographically for the three treatments (table 1). There were no statistical differences in terms of age, sex distribution, or duration of illness.
One patient in the exercise group dropped out of the study within the first week of treatment. Her first attempts to run had convinced her that exercise would not be a desirable and appropriate method to relieve her anxiety problems. Since no postbaseline assessment could be obtained, this patient was not included in the analysis. Two other patients from the exercise group and four patients from the placebo group decided to leave the treatment protocol (all between week 6 and week 8) because their symptoms had not improved. Two further patients from the exercise group could not continue training after week 6 and week 8 because of intermittent disease that was not exercise-related. In summary, the overall dropout rate was 31% for the exercise group and 27% for the placebo group. There were no dropouts in the clomipramine group.
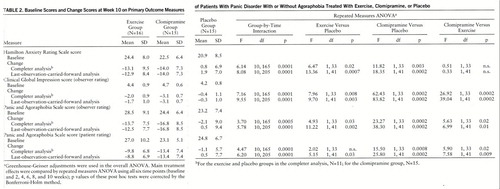
There were no significant differences between the three treatment groups in scores on any of the psychometric scales at baseline. Table 2 gives the mean baseline scores on all primary outcome measures and the mean change from baseline score after 10 weeks of treatment (completer and last-observation-carried-forward analyses). Repeated measures ANOVA in which all six time points were used revealed a significant group-by-time interaction for all primary outcome measures in both the completer and the last-observation-carried-forward analyses. Post hoc comparisons showed that exercise was significantly more effective than placebo for all of these measures except the patient-rated version of the Panic and Agoraphobia Scale, in which a significant difference between exercise and placebo was evident only in the last-observation-carried-forward analysis. Clomipramine was significantly more effective than placebo as measured by the rater version of the CGI and the observer-rated and patient-rated versions of the Panic and Agoraphobia Scale.
Figure 1 illustrates the time course of the treatment effects (completer analysis). A significant treatment effect of clomipramine was already evident after 4 weeks (rater version of the CGI and observer-rated and patient-rated versions of the Panic and Agoraphobia Scale) and more pronounced after 6, 8, and 10 weeks of treatment (Hamilton anxiety scale, rater version of the CGI, and observer-rated and patient-rated versions of the Panic and Agoraphobia Scale). The effect of exercise was significantly different from that of placebo after 8 weeks and at the end of the treatment phase (Ham~ilton anxiety scale, rater version of the CGI, and observer-rated version of the Panic and Agoraphobia Scale). Direct comparison of the two active treatment conditions revealed that clomipramine was superior to exercise after 4 weeks (rater version of the CGI) and 6 and 8 weeks (rater version of the CGI and observer-rated and patient-rated versions of the Panic and Agoraphobia Scale). At the end of the treatment period, the superiority of clomipramine was evident only in the rater version of the CGI.
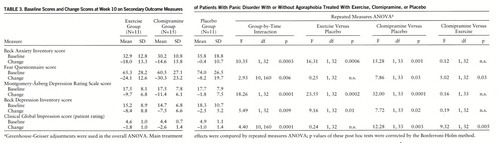
Table 3 gives the mean baseline scores on all secondary outcome measures and the mean change from baseline score after 10 weeks of treatment. A significant group-by-time interaction was evident for all secondary outcome measures. Post hoc comparisons showed that clomipramine was significantly more effective than placebo on all secondary measures of anxiety (Beck Anxiety Inventory and Fear Questionnaire) and depression (Beck Depression Inventory and Montgomery-Åsberg scale), as well on the patient-rated CGI. In contrast, exercise treatment was associated with significant clinical improvement only on the Beck Anxiety Inventory, the Beck Depression Inventory, and the Montgomery-Åsberg scale. Clomipramine's therapeutic effect was significantly superior to the effects of exercise when scores on the Fear Questionnaire and the patient version of the CGI were used as efficacy criteria.
Side effects were documented at every weekly appointment. In the exercise group, no side effects except transient muscle or joint complaints were noted. With the exception of one patient, these complaints completely disappeared with continuous participation in the exercise program and did not lead to dropout. Patients receiving clomipramine or placebo had significantly more side effects than those assigned to exercise treatment. The most common complaints were dry mouth, sweating, mild tremor, dizziness, tachycardia, nausea, constipation, diarrhea, and, rarely, impaired erection or ejaculation. These side effects were most pronounced during the first 4 weeks of treatment, followed by a gradual decline during the remaining 6 weeks.
DISCUSSION
The therapeutic effects of a 10-week protocol of regular aerobic exercise was compared to treatment with clomipramine or placebo in patients with panic disorder and agoraphobia. Exercise was associated with significant improvements in all primary outcome measures in comparison with placebo treatment. Clomipramine was confirmed to be a highly effective treatment for nearly all symptoms of panic disorder and agoraphobia. In comparison with exercise, clomipramine improved anxiety symptoms significantly more effectively and significantly earlier (i.e., after 4 weeks of treatment). To our knowledge, this is the first placebo-controlled, randomized study to examine the effects of aerobic exercise in panic disorder with or without agoraphobia as defined by the DSM-III-R or ICD-10 criteria.
The dropout rate was 31% for the exercise group, 27% for the placebo group, and 0% for the clomipramine group, which is an important outcome measure in itself. Despite the fact that side effects of the active drug were quite disturbing in some patients, none of them discontinued drug treatment. It can be assumed that this was mainly because our patients were thoroughly informed about the typical side effects of clomipramine and their transitory nature.
One of the potential criticisms of our study is that the duration of 10 weeks might be too short for the evaluation of exercise effects. In fact, several patients had considerable difficulty starting running on a regular basis in the first 4 weeks of the trial. Most patients suffered from moderate or severe agoraphobia, and some of them could only perform their weekly exercise task with a friend or relative accompanying them, at least during the first weeks of the treatment phase. It could take a longer period of time before exercise fully deploys its effects. However, exercise diaries and results from spiroergometric testing before and after treatment (data not shown) support the assumption that compliance in the exercise group was satisfactory. It is interesting that only two patients reported a panic attack during a running session. Both continued running and experienced a remission of the attack within 15 minutes. This observation is consistent with the report of Stein et al. (30) that only one of 16 patients with panic disorder and agoraphobia panicked during submaximal exercise testing on a bicycle ergometer.
Treatment effects in the placebo group were surprisingly small. Other studies on panic disorder have reported higher responses to placebo (12). This has been explained as nonspecific psychological effects. In the present study, we tried to restrict our psychological interventions to general support only. A second reason for the low response to placebo might be related to the 3-week observation period before the treatment phase. Other studies reporting higher placebo effects have used a shorter placebo washout period. Moreover, in some of these studies, patients with mild panic disorder and agoraphobia were included; in contrast, we recruited only patients with at least a moderate severity rating on the observer version of the CGI.
A clear limitation of our study design is that it is not possible to establish true double-blind conditions in a study that compares a behavioral treatment approach with intake of medication. When the patient is informed that he or she has been randomly assigned to a running regimen, it becomes obvious that exercise is hypothesized to reduce anxiety symptoms (although many of our patients found it difficult to believe this). On the other hand, adequate clinician ratings require more than mere checking of symptoms. It is our experience that in an unstructured talk, patients would readily mention circumstances which would suggest to the rater that the patient was participating in an exercise program or was taking medication.
Nevertheless, we think that our results cannot be explained solely on the basis of expectation and bias for the following reasons. 1) During the first 4 weeks, patients in the exercise condition did not show more improvement than patients in the placebo condition. 2) The patients' statements before treatment about whether exer~cise would reduce anxiety symptoms did not correlate with the individual response to exercise as measured by all main efficacy scales. 3) We did not observe significant discrepancies between self-ratings and observer ratings; there was a significant correlation between the patient- and the observer-rated versions of the Panic and Agoraphobia Scale when baseline ratings were compared with the ratings after the 10-week exercise protocol (r=0.66, N=11, p<0.02).
We cannot exclude the possibility that nonspecific effects such as increased social interaction might contribute to the beneficial effect of exercise. However, the short discussions before running were focused on sports-related topics in order to avoid some additional “group therapy.”
In the one clinical study on exercise for patients with “anxiety neurosis” or “neurotic depression” (3), 52 patients underwent an 8-week trial of walking or jogging (30 minutes, three to four times per week). There was no placebo control group. A significant decline in anxiety and depression scores was observed in both the walking and the jogging groups. Treatment effects at the end of the trial did not correlate with improved cardiopulmonary fitness. However, a follow-up examination after 6 months revealed a significant correlation between maximal oxygen consumption and low anxiety ratings. Exercise programs in clinical groups have been used predominantly in the treatment of depression. All studies have shown more or less positive effects, but most of these studies have lacked the rigor of controlled clinical trials (31–35).
The mechanisms by which exercise exerts psychological changes are not clear. From a cognitive behavior perspective, running would represent some kind of exposure treatment in which the patient is confronted with feared internal stimuli such as palpitations, shortness of breath, dizziness, and sweating. While experiencing panic attacks, patients tend to misinterpret these bodily sensations as an expression of a life-threatening organic disease. Exercise typically provokes very similar symptoms, which can now be experienced as a physiological reaction. Some cognitive behavioral techniques include interoceptive conditioning, a technique that helps the patient to reattribute certain somatic cues to nonpathological vegetative functions (36, 37).
In this study, some patients initially did not dare to run intensively outside their weekly group sessions, because they feared heart attacks or “dangerous” increases in blood pressure. Discussing these concerns with the therapist could represent an important therapeutic factor in itself. We cannot exclude the possibility that such exercise-related corrections of dysfunctional cognitions help patients to interpret other perceived dangers in a more harmless way.
These clinical observations have led us to the assumption that abstaining from exercise is part of the phobic avoidance behavior. In fact, our patients explained that they had reduced their exercise because of a fear that they would suffer from organic heart disease or that exercise could precipitate dangerous heart attacks. Reduced cardiopulmonary fitness in patients with panic disorder has been reported in several studies (15–19). It is not clear whether these deficits represent a primary biological disposition or whether all observations are a consequence of exercise avoidance.
Earlier exercise studies have suggested that exercise as an active coping strategy gives patients a “sense of mastery” that reliance on drug treatment does not. Since exercise is indeed associated with other health benefits, such as a reduction of risk factors for heart disease and arteriosclerosis, it might help patients to develop a more optimistic view of their physical fitness. Running may also lead to distraction from depressive or anxiety-related thoughts.
Anxiolytic or antidepressant effects of exercise might also be related to adaptive changes in the central nervous system, which are reviewed elsewhere (38–42). However, it is not clear whether these neuroendocrine adaptations are related to the psychological effects of exercise.
In conclusion, regular aerobic exercise alone seems to be associated with significant clinical improvement in patients suffering from panic disorder. The role of exercise in treating panic may be particularly valuable for patients who are unable or unwilling to take medication. The majority of patients suffering from panic disorder and agoraphobia are young and have no contraindications for aerobic or other forms of exercise. On the basis of these results, further trials using exercise in the treatment of anxiety disorders are warranted. Exercise could be easily integrated into cognitive behavioral treatments. It is not clear whether the combination of exercise and drug treatment will lead to a potentiation of treatment effects. Further studies should also evaluate clinical characteristics associated with a favorable response to medication or exercise. Subgroups of patients may respond preferentially to exercise. So far, there have been no long-term follow-up studies, and there are no data to answer the important question of whether the risk of relapse can be reduced in vulnerable individuals by continuing regular exercise.
 |
 |
 |
Received Feb. 5, 1997; revisions received Aug. 5 and Oct. 10, 1997; accepted Nov. 14, 1997. From the Department of Psychiatry and the Department of Sports Medicine, University of G<148>ttingen; the Department of Neurology, University of Würzburg; and the Department of Sports Medicine, University of Saarbrücken, Germany. Address reprint requests to Dr. Broocks, Department of Psychiatry, University of Göttingen, von-Siebold-Strasse 5, D-37075 Göttingen, Germany. Supported by a public grant from the Volkswagen Foundation.
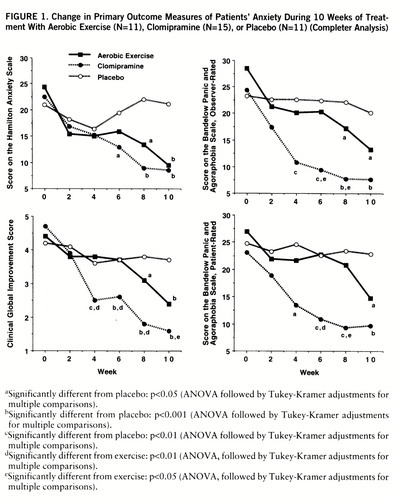
FIGURE 1. Change in Primary Outcome Measures of Patients' Anxiety During 10 Weeks of Treatment With Aerobic Exercise (N=11), Clomipramine (N=15), or Placebo (N=11) (Completer Analysis)
aSignificantly different from placebo: p<0.05 (ANOVA followed by Tukey-Kramer adjustments for multiple comparisons).
bSignificantly different from placebo: p<0.001 (ANOVA followed by Tukey-Kramer adjustments for multiple comparisons).
cSignificantly different from placebo: p<0.01 (ANOVA followed by Tukey-Kramer adjustments for multiple comparisons).
dSignificantly different from exercise: p<0.01 (ANOVA, followed by Tukey-Kramer adjustments for multiple comparisons).
eSignificantly different from exercise: p<0.05 (ANOVA, followed by Tukey-Kramer adjustments for multiple comparisons).
1 Hughes JR: Psychological effects of habitual aerobic exercise: a critical review. Prev Med 1984; 13:66–78Crossref, Medline, Google Scholar
2 de Coverley Veale DMW: Exercise and mental health. Acta Psychiatr Scand 1987; 76:113–120Crossref, Medline, Google Scholar
3 Sexton H, Maere A, Dahl NH: Exercise intensity and reduction in neurotic symptoms—a controlled follow-up study. Acta Psychiatr Scand 1989; 80:231–235Crossref, Medline, Google Scholar
4 Orwin A: Treatment of a situational phobia—a case for running. Br J Psychiatry 1974; 125:95–98Crossref, Medline, Google Scholar
5 Muller B, Armstrong HE: A further note on the running treatment for anxiety. Psychotherapy: Theory, Research and Practice 1975; 12:385–387Crossref, Google Scholar
6 Cross-National Collaborative Panic Study, Second Phase Investigators: Drug treatment of panic disorder: comparative efficacy of alprazolam, imipramine, and placebo. Br J Psychiatry 1992; 160:191–202; correction, 1992; 161:724Google Scholar
7 Johnston D, Troyer I, Whitsett S: Clomipramine treatment of agoraphobic women: an eight-week controlled trial. Arch Gen Psychiatry 1988; 45:453–459Crossref, Medline, Google Scholar
8 LeCrubier Y, Bakker A, Dunbar G, Judge R, Collaborative Paroxetine Panic Study Investigators: A comparison of paroxetine, clomipramine, and placebo in the treatment of panic disorder. Acta Psychiatr Scand 1997; 95:145–152Crossref, Medline, Google Scholar
9 Mavissakalian M, Michelson L: Agoraphobia: relative and combined effectiveness of therapist-assisted in vivo exposure and imipramine. J Clin Psychiatry 1986; 47:117–122Medline, Google Scholar
10 Modigh K, Westberg P, Eriksson E: Superiority of clomipramine over imipramine in the treatment of panic disorder: a placebo-controlled trial. J Clin Psychopharmacol 1992; 12:251–261Crossref, Medline, Google Scholar
11 Sheehan DV, Raj AB, Sheehan KH, Soto S: Is buspirone effective for panic disorder? J Clin Psychopharmacol 1990; 10:3–11Google Scholar
12 Ballenger JC, Burrows GD, DuPont RL Jr: Alprazolam in panic disorder and agoraphobia: results from a multicenter trial, I: efficacy in short-term treatment. Arch Gen Psychiatry 1988; 45:413–422Crossref, Medline, Google Scholar
13 Sheehan DV, Ballenger J, Jacobsen G: Treatment of endogenous anxiety with phobic, hysterical, and hypochondriacal symptoms. Arch Gen Psychiatry 1980; 37:51–59Crossref, Medline, Google Scholar
14 Black DW, Wesner R, Bowers W, Gabel J: A comparison of fluvoxamine, cognitive therapy, and placebo in the treatment of panic disorder. Arch Gen Psychiatry 1993; 50:44–50Crossref, Medline, Google Scholar
15 Taylor CB, King R, Ehlers A, Margraf J, Clark D, Hayward C, Roth WT, Agras S: Treadmill exercise test and ambulatory measures in panic attacks. Am J Cardiol 1987; 60:48J–52JCrossref, Medline, Google Scholar
16 Roth WT, Telch MJ, Taylor CB: Autonomic characteristics of agoraphobia with panic attacks. Biol Psychiatry 1986; 21:1133–1154Crossref, Medline, Google Scholar
17 Roth DL: Acute emotional and psychophysiological effects of aerobic exercise. Psychophysiology 1989; 26:593–602Crossref, Medline, Google Scholar
18 Martinsen EW, Strand J, Paulsson G, Kaggestad J: Physical fitness level in patients with anxiety and depressive disorders. Int J Sports Med 1989; 10:58–61Crossref, Medline, Google Scholar
19 Miyakoda H, Kitamura H, Kinuguwa T, Saito M, Kotake H, Mashiba H: Cardiac neurosis: exercise tolerance and the role of sympathetic activity. Jpn J Med 1990; 29:493–499Crossref, Medline, Google Scholar
20 Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1985Google Scholar
21 Hamilton M: Diagnosis and rating of anxiety. Br J Psychiatry Special Publication 1969; 3:76–79Google Scholar
22 Bandelow B: Assessing the efficacy of treatments for panic disorder and agoraphobia, II: the Panic and Agoraphobia Scale. Int Clin Psychopharmacol 1995; 10:73–81Google Scholar
23 Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Rockville, Md, US Department of Health, Education, and Welfare, 1976, pp 217–222Google Scholar
24 Marks IM, Matthews AM: Brief Standard Self-Rating for Phobic Patients. Behav Res Ther 1979; 17:263–267Crossref, Medline, Google Scholar
25 Beck AT, Epstein N, Brown G, Steer RA: An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–897Crossref, Medline, Google Scholar
26 Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
27 Montgomery SA, Åsberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
28 Greenhouse SW, Geisser S: On methods in the analysis of profile data. Psychometrika 1959; 32:95–112Crossref, Google Scholar
29 SAS/STAT User's Guide, version 6, 4th ed. Cary, NC, SAS Institute, 1989Google Scholar
30 Stein JM, Papp LA, Klein DF: Exercise tolerance in panic disorder patients. Biol Psychiatry 1992; 32:281–287Crossref, Medline, Google Scholar
31 Martinsen EW, Medhus A, Sandvik L: Effects of aerobic exercise on depression: a controlled study. Br Med J (Clin Res) 1985; 291:109Crossref, Medline, Google Scholar
32 McCann IL, Holmes DS: Influence of aerobic exercise on depression. J Pers Soc Psychol 1984; 46:1142–1147Crossref, Medline, Google Scholar
33 Greist JH, Klein MH, Eischens RR, Faris J, Gurman AS, Morgan WP: Running as treatment for depression. Compr Psychiatry 1979; 20:41–54Crossref, Medline, Google Scholar
34 Pappas GP, Golin S, Meyer DL: Reducing symptoms of depression with exercise. Psychosomatics 1990; 31:112–113Crossref, Medline, Google Scholar
35 Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, Neimeyer RA: Running versus weightlifting in the treatment of depression. J Consult Clin Psychol 1987; 55:748–754Crossref, Medline, Google Scholar
36 Barlow DH, Craske MG, Cerny JA, Klosko JS: Behavioral treatment of panic disorder. Behavior Therapy 1989; 20:261–282Crossref, Google Scholar
37 Salkovskis PM, Jones DRO, Clark DM: Respiratory control in the treatment of panic attacks: replication and extension with concurrent measurement of behaviour and pCO2. Br J Psychiatry 1986; 148:526–532Crossref, Medline, Google Scholar
38 Markoff RA, Ryan P, Young T: Endorphins and mood changes in long-distance running. Med Sci Sports Exerc 1982; 14:11–15Crossref, Medline, Google Scholar
39 Janal MN, Colt EWD, Clark WC, Glusman M: Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain 1984; 19:13–25Crossref, Medline, Google Scholar
40 Post RM, Kotin J, Goodwin FK, Gordon EK: Psychomotor activity and cerebrospinal fluid amine metabolites in affective illness. Am J Psychiatry 1973; 130:67–72Link, Google Scholar
41 Broocks A, Schweiger U, Pirke KM: The influence of semistarvation-induced hyperactivity on hypothalamic serotonin metabolism. Physiol Behav 1991; 50:385–388Crossref, Medline, Google Scholar
42 Beckmann H, Ebert MH, Post R, Goodwin FK: Effect of moderate exercise on urinary MHPG in depressed patients. Pharmaco~psychiatry 1979; 12:351–356Crossref, Google Scholar



