Impact of COVID-19 Telehealth Policy Changes on Buprenorphine Treatment for Opioid Use Disorder
Abstract
Objective:
The authors examined the impact of COVID-19-related policies reducing barriers to telehealth delivery of buprenorphine treatment for opioid use disorder (OUD) on buprenorphine treatment across different modalities (telephone, video, and in-person visits).
Methods:
This was a national retrospective cohort study with interrupted time-series analyses to examine the impact of policy changes in March 2020 on buprenorphine treatment for OUD in the Veterans Health Administration, during the year before the start of the COVID-19 pandemic (March 2019 to February 2020) and during the first year of the pandemic (March 2020 to February 2021). The authors also examined trends in the use of telephone, video, and in-person visits for buprenorphine treatment and compared patient demographic characteristics and retention in buprenorphine treatment across the two periods.
Results:
The number of patients receiving buprenorphine increased from 13,415 in March 2019 to 15,339 in February 2021. By February 2021, telephone visits were used by the most patients (50.2%; 4,456 visits), followed by video visits (32.4%; 2,870 visits) and in-person visits (17.4%; 1,544 visits). During the pre-pandemic period, the number of patients receiving buprenorphine increased significantly by 103 patients per month. After the COVID-19 policy changes, there was an immediate increase of 265 patients in the first month, and the number continued to increase significantly, at a rate of 47 patients per month. The demographic characteristics of patients receiving buprenorphine during the pandemic period were similar to those during the pre-pandemic period, but the proportion of patients reaching 90-day retention on buprenorphine treatment decreased significantly from 49.6% to 47.7%, while days on buprenorphine increased significantly from 203.8 to 208.7.
Conclusions:
The number of patients receiving buprenorphine continued to increase after the COVID-19 policy changes, but the delivery of care shifted to telehealth visits, suggesting that any reversal of COVID-19 policies must be carefully considered.
The COVID-19 pandemic sparked a rapid, unprecedented expansion of telehealth-delivered care, including delivery of buprenorphine treatment for opioid use disorder (OUD), an effective treatment that can help reduce mortality in this vulnerable patient population (1, 2). Key policy changes were implemented in March 2020, at the beginning of the pandemic, to decrease barriers to telehealth delivery of buprenorphine treatment in order to sustain treatment for patients with OUD across the United States. These key changes included 1) eliminating the initial in-person visit requirement for telehealth-delivered buprenorphine treatment under the public health emergency exception of the 2008 Ryan Haight Online Pharmacy Consumer Protection Act; 2) expanding coverage of telehealth services, including to patients at home, by the Centers for Medicare and Medicaid Services and other insurance companies; and 3) guidance from the Substance Abuse and Mental Health Services Administration and the Drug Enforcement Administration allowing prescribing of buprenorphine treatment through telephone visits (rather than requiring video) (3), an important modality given disparities in access to Internet and digital technologies.
At the same time, further acceleration of overdose mortality since the start of the COVID-19 pandemic suggests that there may either be an increase in treatment need or a decrease in treatment utilization in this population, which is one that may be particularly vulnerable to the impacts of the pandemic, underscoring the importance of examining trends in buprenorphine treatment since the start of the pandemic (4). Previous research has examined shifts in delivery of buprenorphine treatment services early in the pandemic (5, 6). However, studies are needed to examine longer-term patterns across telehealth modalities, including telephone and video, and changes in the characteristics of patients who are receiving treatment, to inform future policies as well as current debates on reversing the temporary policy changes implemented during the COVID-19 pandemic. It is also imperative to examine these trends within the Veterans Health Administration (VHA) system. The VHA is the largest addiction treatment provider in the country and provides a view of treatment across the United States. Ensuring access to buprenorphine is a leading VHA priority (7).
The objective of this study was to examine the effects of COVID-19-related policy changes reducing barriers to telehealth delivery of buprenorphine treatment for patients with OUD across the VHA. We examined shifts in overall treatment receipt, treatment delivery modalities (video, telephone, and in-person), treatment retention, and the demographic characteristics of patients receiving treatment.
Methods
We examined national VHA data to compare trends in buprenorphine treatment across the 12 months before and after federal-level COVID-19 policy changes were implemented in March 2020 to decrease barriers to telehealth-delivered buprenorphine treatment for OUD during the pandemic (3). The pre-pandemic period was defined as the 12 months from March 2019 through February 2020, and the pandemic period was defined as the 12 months from March 2020 through February 2021. Data were obtained from VHA’s Corporate Data Warehouse. This study was deemed exempt from review by the VA Ann Arbor Healthcare System Institutional Review Board.
Cohort Definition
Monthly rolling cohorts of VHA patients with an OUD diagnosis during the 2-year study period were examined. Patients in each monthly cohort included VHA patients age 18 or older with at least one VHA outpatient encounter with an OUD diagnosis in the 12 months prior to and including the month of interest.
Outcomes
We examined the monthly number of patients receiving buprenorphine treatment, defined as patients with days’ supply of buprenorphine for OUD covering at least 1 day that month. Because we found a decreasing trend in the monthly number of patients with a diagnosis of OUD during the 2-year study period, we also examined, as a sensitivity analysis, the proportion of patients each month receiving buprenorphine treatment among those with OUD. A patient was counted in each month that they received treatment during the study period even if they started and stopped treatment multiple times during the study period. In addition to monthly number of patients receiving buprenorphine treatment, as in other studies (5), we also examined two mutually exclusive monthly measures: the number of patients continuing buprenorphine treatment, defined as those who received any buprenorphine for OUD in the previous 3 months, and the number of patients newly initiating buprenorphine, defined as those with no buprenorphine fills covering any days in the previous 3 months.
Using approaches similar to those in our previous work (8), we examined visit modalities for buprenorphine treatment (i.e., telephone, video, or in-person) monthly throughout the study period (see Table S1 in the online supplement for modality definitions). Visits for buprenorphine treatment were defined as visits with the same provider as the person prescribing buprenorphine treatment for OUD.
For each unique patient who received buprenorphine for OUD in the pre-pandemic and pandemic periods, we collected data on demographic characteristics (age, sex, race/ethnicity, and rural/urban residence), number of treatment visits, total days with supplied buprenorphine, and 90-day retention on buprenorphine (9–11). The number of treatment visits for buprenorphine was totaled for each patient within each pre-pandemic and pandemic period, and total days of buprenorphine supplied for each patient was determined by aggregating total days’ supply of buprenorphine within each pre-pandemic and pandemic period. We defined 90-day retention as having at least one episode of buprenorphine treatment for at least 90 total days, allowing gaps in treatment of no more than 30 days. Thus, patients who met the 90-day retention criteria could have gaps in treatment up to 30 days, but they must have had at least 90 total days of treatment starting from the date of the first fill.
Statistical Analysis
Monthly numbers of patients receiving buprenorphine treatment for OUD over the study period were examined using interrupted time-series analyses to estimate changes in buprenorphine prescribing following COVID-19-related policy changes implemented in March 2020. This method adjusted for baseline levels and trends to more robustly examine change in treatment after the policy change (12, 13) and also accounted for correlation of data over time, using the autoregression procedure (13). Patients who received buprenorphine for OUD in the pre-pandemic and pandemic periods were compared on demographic characteristics, retention in buprenorphine treatment, and number of treatment visits, using a generalized estimating equation with an indicator for the pandemic period to account for correlation of patients included in both periods.
Results
The total number of VHA patients receiving buprenorphine for OUD increased from 13,415 in March 2019 to 15,339 in February 2021 (14%) (Figure 1). During this same interval, the number of patients receiving any substance use disorder treatment in the VHA (not just OUD treatment) decreased from 138,745 to 129,806 (a 6% decrease). Interrupted time-series analysis showed that in the 12-month pre-pandemic period, buprenorphine treatment was increasing at a rate of 103 patients per month (p<0.001) (Table 1). In the 12-month period after the start of COVID-19-related policy changes, the number of patients receiving treatment continued to increase, but at a rate of 47 patients per month (p<0.001), which was lower than the pre-pandemic rate of increase (p<0.001). The number of patients continuing buprenorphine increased at a rate of 107 per month (p<0.001) during the 12 months prior to the policy changes, and the rate continued to increase at 53 patients per month (p<0.001) after the policy changes, although the rate of increase was lower than in the pre-pandemic period (p<0.001). The number of patients newly initiating buprenorphine decreased overall across both periods, but the rate of decrease was not statistically significant, and no significant difference in the rates was seen between the pre-pandemic and pandemic periods.
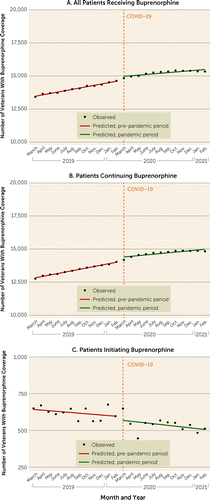
FIGURE 1. Trends in number of patients in the Veterans Health Administration receiving buprenorphine for opioid use disorder before and after COVID-19-related telehealth policy changes (March 2019–February 2021)a
aThe pre-pandemic period was defined as the 12 months from March 2019 through February 2020, and the pandemic period as the 12 months from March 2020 through February 2021.
| Pre-Pandemic Period | Pandemic Period | Pandemic Compared to Pre-Pandemic Period | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Monthly Rateb | p | Immediate Change in Number of Patients | p | Monthly Rateb | p | Rate Difference | p |
| All buprenorphine patients | 102.96 | <0.001 | 264.72 | 0.003 | 47.07 | <0.001 | −55.89 | <0.001 |
| Patients initiating buprenorphine | −4.17 | 0.16 | −24.38 | 0.60 | −5.62 | 0.23 | −1.45 | 0.79 |
| Patients continuing buprenorphine | 107.13 | <0.001 | 289.10 | 0.006 | 52.69 | <0.001 | −54.44 | 0.001 |
TABLE 1. Interrupted time-series analyses examining number of patients in the Veterans Health Administration receiving buprenorphine for opioid use disorder before and after COVID-19-related telehealth policy changes (March 2019–February 2021)a
We also conducted sensitivity analyses examining trends in monthly proportions of patients receiving buprenorphine among those with OUD each month. In the 12-month period after the start of COVID-19-related policy changes, the proportion of patients receiving treatment continued to increase, but this time at a rate not significantly different from the pre-pandemic trend (see Table S2 in the online supplement). The proportion of patients continuing buprenorphine also increased but was not significantly different from the pre-pandemic trends. For the proportion of patients newly initiating buprenorphine, similar to what we found in the primary results, there was no significant difference in the rates during the pandemic period compared to the pre-pandemic period.
Among patients receiving a buprenorphine treatment visit each month, the proportion of telehealth visits (telephone and video) increased dramatically, from 11.9% (1,025 of 8,589 visits) in March 2019 to 82.6% (7,326 of 8,870 visits) in February 2021 (Figure 2). Of all buprenorphine treatment visits, the proportion of telephone visits increased and remained the most prevalent modality in February 2021 (50.2%; 4,456 of 8,870 visits), followed by video visits (32.4%; 2,870 visits) and in-person visits (17.4%; 1,544 visits). Among 4,720 patients newly initiating buprenorphine in the 1-year period after the start of the pandemic, 64.4% (3,039) of the patients received phone visits, 40.5% (1,910) video visits, and 20.4% (962) in-person visits. Note that some patients could have received more than one type of modality. Among 13,462 patients continuing buprenorphine in the 1-year period after the start of the pandemic, 86.7% (11,667) of the patients received phone visits, 46.7% (6,287) video visits, and 6.0% (807) in-person visits. In the pandemic period, the proportion of patients receiving video or telephone visits did not differ substantially by rural/nonrural residence (see Table S3 in the online supplement).
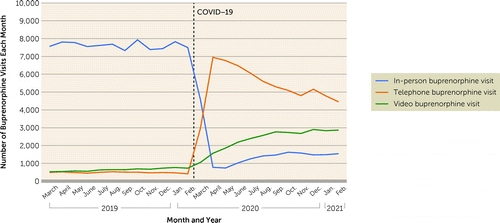
FIGURE 2. Trends in number of buprenorphine treatment visits for OUD across modalities in the Veterans Health Administration before and after COVID-19-related telehealth policy changes (March 2019–February 2021)
Compared with patients who received buprenorphine for OUD during the 1-year pandemic period, those who received buprenorphine for OUD during the 1-year pre-pandemic period were significantly more likely to live in urban areas (86% compared to 82%) and less likely to be classified as living in other/unknown areas (2% compared to 5%) (p<0.001), but they did not differ significantly in terms of age group, sex, or race/ethnicity (Table 2). During the pre-pandemic period, 49.6% of patients were retained on buprenorphine at least 90 days, compared to 47.7% during the pandemic period (p<0.05). During the pre-pandemic period, the average number of days supplied of buprenorphine treatment was 203.8, compared to 208.7 during the pandemic period (p<0.001), and the average number of treatment visits was 5.3 (SD=3.3), compared to 5.4 (SD=3.2) during the pandemic period (p=0.07).
| Characteristic | Pre-Pandemic Period (N=18,012) | Pandemic Period (N=18,182) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Age (years) | 49.4 | 13.9 | 49.3 | 13.9 | 0.72 |
| % | N | % | N | p | |
| Age group (years) | |||||
| 18–29 | 3.4 | 615 | 3.2 | 584 | 0.53 |
| 30–44 | 40.8 | 7,347 | 41.4 | 7,523 | |
| 45–64 | 37.7 | 6,788 | 37.5 | 6,819 | |
| ≥65 | 18.1 | 3,262 | 17.9 | 3,256 | |
| Female | 7.9 | 1,421 | 7.9 | 1,436 | 0.98 |
| Race/ethnicity | |||||
| White, non-Hispanic | 77.7 | 14,003 | 78.0 | 14,182 | 0.64 |
| Black, non-Hispanic | 11.4 | 2,058 | 11.0 | 2,004 | |
| Hispanic | 5.2 | 942 | 5.2 | 952 | |
| Other/unknown | 5.6 | 1,009 | 5.7 | 1,044 | |
| Rurality | |||||
| Urban | 86.0 | 15,483 | 82.4 | 14,989 | <0.001 |
| Rural | 12.0 | 2,162 | 12.2 | 2,213 | |
| Other/unknown | 2.0 | 367 | 5.4 | 980 | |
| Patients with ≥90 days buprenorphine retention | 49.6 | 8,932 | 47.7 | 8,664 | <0.05 |
| Mean | SD | Mean | SD | p | |
| Average days on buprenorphine treatment | 203.8 | 156.0 | 208.7 | 113.7 | <0.001 |
| Number of buprenorphine treatment visits | 5.3 | 3.3 | 5.4 | 3.2 | 0.07 |
TABLE 2. Characteristics and treatment utilization among patients in the Veterans Health Administration receiving buprenorphine for opioid use disorder before and after COVID-19-related telehealth policy changes (March 2019–February 2021)a
Discussion
In this study of national VHA data, we found that the number of patients receiving buprenorphine for OUD treatment continued to increase following COVID-19-related policy changes to reduce barriers to telehealth, albeit at a slower rate. However, delivery of care changed dramatically during this period; the majority of visits shifted to telehealth, with telephone visits outnumbering video visits. These findings suggest that although the COVID-19 pandemic substantially changed the way OUD care is delivered, policy changes that were rapidly implemented in order to reduce barriers to telehealth allowed delivery of this life-saving treatment to be sustained during the pandemic.
These findings build on previous studies in other patient populations that examined telehealth delivery of buprenorphine in the initial months of the pandemic (5, 14, 15) and show that buprenorphine treatment continued to be sustained 1 year after the policy changes, primarily through a large-scale transition to telephone and video visits. This unprecedented shift allowed clinicians to continue care in a patient population that may be particularly vulnerable to COVID-19-related care disruptions. People with substance use disorders, including OUD, have a higher burden of comorbid conditions that put them at higher risk for COVID-19, which may cause a greater reduction of in-person health care (16, 17). People with OUD are also more vulnerable to psychosocial disruptions (e.g., financial challenges, job loss, exacerbation of comorbid mental health disorders) that may affect care utilization (18), which may have contributed to the lower 90-day retention we found in the pandemic period. Additional studies are needed that specifically compare retention across treatment modalities during the COVID-19 pandemic to better disaggregate the effects of the pandemic from treatment modalities on outcomes. However, it is reassuring to see that overall buprenorphine treatment utilization did not decline, as other key health care services did during the pandemic across the United States (19–21), including other VHA substance use disorder services, as found in this study. Among patients receiving buprenorphine for OUD, there was a slight increase in the proportion of patients whose residence (i.e., rural/urban) was unknown, but no other changes in patient demographic characteristics were observed (including for race/ethnicity), suggesting that the transition to telephone and video telehealth was not associated with major shifts in the demographics of the population receiving buprenorphine (22, 23).
Despite the apparent success of maintaining delivery of buprenorphine through telehealth during the pandemic, it remains critical to continue prioritizing increased access to this treatment. The study results suggest that the transition to telehealth supported ongoing increases in the number of patients who were already in treatment, specifically through increased days of treatment among those receiving buprenorphine. It is possible that making treatment more accessible through telehealth makes it easier for patients who are already in treatment to stay in treatment, which is a crucial consideration in future policy decisions. However, we did not observe increased rates of patients initiating buprenorphine treatment. This may indicate that increasing the number of new patients on buprenorphine via both in-person and telehealth visits may be a bigger challenge, one that cannot be overcome through policies allowing increased telehealth delivery alone. Clinicians have also described greater comfort using telehealth with patients they have already seen in person and feeling less comfortable initiating buprenorphine treatment via telehealth (15). If providers’ discomfort with initiating buprenorphine treatment via telehealth is preventing use of telehealth to increase access for patients with untreated OUD, additional guidance and support on telehealth delivery may be needed (24).
This study also found that telephone visits were the most commonly used treatment modality during the first year of the COVID-19 pandemic. During the pandemic, the VHA made substantial investments to support video telehealth, including providing VHA Internet-connected devices (e.g., tablets) to veterans, while also providing training (25). Despite these investments, telephone visits still greatly outnumbered video visits, suggesting that even with increased availability of video-enabled devices, there remain barriers to video visits in this patient population. These may include Internet service availability, comfort with use of technology, and knowledge about using video telehealth (26–28). Notwithstanding persistent barriers to video visits and the predominance of telephone visits for buprenorphine in the study period, the option for telephone visits for buprenorphine is currently being contested, and it is uncertain whether this modality will be supported in the long term by policy makers (29). Given the large number of patients who are currently receiving buprenorphine via telehealth, any policy changes must be thoughtfully considered, including any changes that would eliminate or reduce telephone access. It will be critical for providers to thoughtfully transition patients back to in-person care, if this is required, and a hybrid model blending in-person and virtual visits may be needed. Some patients have only known buprenorphine treatment via telehealth, with many—potentially more frequently from disadvantaged groups—engaging via telephone rather than video (30). There could be major unintended consequences, including worsening existing treatment disparities, if these modalities are suddenly disrupted.
There are several limitations to this study. While the VHA is the country’s largest single addiction treatment provider, and veterans are an important population at higher risk for overdose (31) and with a prevalence of OUD similar to that of the nonveteran population (32), additional studies are needed outside the VHA. Notably, the VHA implemented infrastructure to support telehealth care delivery more broadly during COVID-19 (25), which may have resulted in trends in buprenorphine treatment delivery that differ from those in other settings. Additionally, it was not possible to include a control group unaffected by COVID-19-related buprenorphine policy changes in this interrupted time-series analysis, as these changes were enacted at the federal level and simultaneously affected all patients with OUD across the country. It is therefore possible that outside factors occurring contemporaneously with policy changes had an impact on observed changes in buprenorphine utilization. Telehealth use and the data on telehealth visits may also have varied across VHA facilities as a result of differences in facility-level policies, infrastructure, coding of telehealth visits, and other factors. Also, this study focused only on how COVID-19-related policies affected treatment receipt, treatment retention, and modality of buprenorphine treatment delivery. It is possible that a shift to telehealth will have broad implications for how OUD care is delivered. Therefore, future research should examine the effects of telehealth modalities on additional measures of treatment quality, patient experience, and outcomes. Finally, we did not examine the use of psychotherapy and other treatments commonly used in patients with OUD and other substance use disorders. Given the finding that overall substance use disorder care decreased during this study period, studies are needed to examine the impacts of the COVID-19 pandemic on treatment and outcomes in other substance use disorder patient populations.
Conclusions
We found that in the national VHA health care system, buprenorphine treatment for OUD was maintained during the COVID-19 pandemic through a rapid shift to telehealth, suggesting that any future changes to telehealth policies must be carefully considered, as they could have major implications for patient care. We found that although the number of patients receiving buprenorphine continued to increase throughout the study period, the number initiating care did not increase. This finding suggests that while maintaining policies that allow telehealth for buprenorphine delivery may be an important tool for improving retention, and therefore important for preventing overdose and other adverse outcomes, other efforts in addition to these are urgently needed to improve access for patients with untreated OUD and to better link and engage patients in treatment via telehealth or in person. Additional studies are needed in other health care systems, as well as studies examining how changes in treatment modality affect other treatment outcomes. Although the pandemic has presented acute challenges for health care and has likely substantially affected the lives of people with OUD, it may also have fostered innovations in care delivery.
1. : Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018; 169:137–145Crossref, Medline, Google Scholar
2. : Mortality associated with time in and out of buprenorphine treatment in French office-based general practice: a 7-year cohort study. Ann Fam Med 2017; 15:355–358Crossref, Medline, Google Scholar
3. : Telehealth for substance-using populations in the age of coronavirus disease 2019: recommendations to enhance adoption. JAMA Psychiatry 2020; 77:1209–1210Crossref, Medline, Google Scholar
4. : Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. 2020. https://emergency.cdc.gov/han/2020/han00438.asp Google Scholar
5. : Treatment of opioid use disorder among commercially insured patients in the context of the COVID-19 pandemic. JAMA 2020; 324:2440–2442Crossref, Medline, Google Scholar
6. : Assessment of filled buprenorphine prescriptions for opioid use disorder during the coronavirus disease 2019 pandemic. JAMA Intern Med 2021; 181:562–565Crossref, Medline, Google Scholar
7. : Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abus 2018; 39:139–144Crossref, Medline, Google Scholar
8. : Comparing telemedicine to in-person buprenorphine treatment in US veterans with opioid use disorder. J Subst Abuse Treat 2022; 133:108492 Crossref, Medline, Google Scholar
9. : Mobile telemedicine for buprenorphine treatment in rural populations with opioid use disorder. JAMA Netw Open 2021; 4:e2118487Crossref, Medline, Google Scholar
10. : Buprenorphine treatment in an urban community health center: what to expect. Fam Med 2008; 40:500–506Medline, Google Scholar
11. : Treatment outcome comparison between telepsychiatry and face-to-face buprenorphine medication-assisted treatment for opioid use disorder: a 2-year retrospective data analysis. J Addict Med 2017; 11:138–144Crossref, Medline, Google Scholar
12. : Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309Crossref, Medline, Google Scholar
13. : Design characteristics and statistical methods used in interrupted time series studies evaluating public health interventions: a review. J Clin Epidemiol 2020; 122:1–11Crossref, Medline, Google Scholar
14. : A telemedicine buprenorphine clinic to serve New York City: initial evaluation of the NYC public hospital system’s initiative to expand treatment access during the COVID-19 pandemic. J Addict Med 2022; 16:e40–e43 Crossref, Medline, Google Scholar
15. : Treatment of opioid use disorder during COVID-19: experiences of clinicians transitioning to telemedicine. J Subst Abuse Treat 2020; 118:108124Crossref, Medline, Google Scholar
16. : Healthcare utilization patterns among persons who use drugs during the COVID-19 pandemic. J Subst Abuse Treat 2021; 121:108177Crossref, Medline, Google Scholar
17. : COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry 2021; 26:30–39 Crossref, Medline, Google Scholar
18. : Conceptualizing the effects of the COVID-19 pandemic on people with opioid use disorder: an application of the social ecological model. Addict Sci Clin Pract 2021; 16:4Crossref, Medline, Google Scholar
19. : Estimated impact of COVID-19 on preventive care service delivery: an observational cohort study. BMC Health Serv Res 2021; 21:1107Crossref, Medline, Google Scholar
20. : Indirect effects of the COVID-19 pandemic on people with type 2 diabetes: time to urgently move into a recovery phase. BMJ Qual Saf 2022; 31:483–485Crossref, Medline, Google Scholar
21. : Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020; 4:1059–1071Crossref, Medline, Google Scholar
22. : Disparities in access to medications for opioid use disorder in the Veterans Health Administration. J Addict Med 2021; 15:143–149Crossref, Medline, Google Scholar
23. : Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry 2019; 76:979–981Crossref, Medline, Google Scholar
24. Telehealth for Opioid Use Disorder Toolkit: Guidance to Support High-Quality Care. Providers Clinical Support System, 2021. https://pcssnow.org/telehealth-for-opioid-use-disorder-toolkit-guidance-to-support-high-quality-care/ Google Scholar
25. : Connecting Veterans to Telehealth Care. 2021. https://connectedcare.va.gov/sites/default/files/telehealth-digital-divide-fact-sheet.pdf Google Scholar
26. : Implementation of telehealth services at the US Department of Veterans Affairs during the COVID-19 pandemic: mixed methods study. JMIR Form Res 2021; 5:e29429Crossref, Medline, Google Scholar
27. : Disparities in audio-only telemedicine use among Medicare beneficiaries during the coronavirus disease 2019 pandemic. Med Care 2021; 59:1014–1022Crossref, Medline, Google Scholar
28. : Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat 2021; 124:108272Crossref, Medline, Google Scholar
29. : DEA Policy: Use of telephone evaluations to initiate buprenorphine prescribing. March 31, 2020. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-022)(DEA068)%20DEA%20SAMHSA%20buprenorphine%20telemedicine%20%20(Final)%20+Esign.pdf Google Scholar
30. : Differences in the use of telephone and video telemedicine visits during the COVID-19 pandemic. Am J Manag Care 2021; 27:21–26 Crossref, Medline, Google Scholar
31. : Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care 2011; 49:393–396Crossref, Medline, Google Scholar
32. : Comparison of opioid use disorder among male veterans and non-veterans: disorder rates, socio-demographics, co-morbidities, and quality of life. Am J Addict 2019; 28:92–100Crossref, Medline, Google Scholar



