Opioid Use Disorder: Pernicious and Persistent
Opioid use disorder (OUD; also known as opioid dependence, opioid abuse, opioid misuse, opioid addiction) has been identified as a medical and social concern in the United States for over 150 years (1), and while the extent of the problem has waxed and waned over time, recent years have been marked by a devastating and unremitting presence of this disorder. Attempts to address OUD and the high number of annual opioid overdose deaths as well as the impact of OUD on lives, families, and communities have been particularly challenging for providers and researchers, despite ongoing pharmacotherapy innovation, the expansion of treatment capacity, and social and professional efforts to curtail the availability of opioids (especially prescription opioids). By no means is this current state an insurmountable problem, but its perniciousness and the broad social aspects of opioid misuse suggest that simplistic solutions are unlikely (2). The goal of this overview is to provide the reader with a summary of this disorder, highlighting current issues, what we know about the science and treatment, and ideas for addressing this disorder in a rapidly shifting setting.
What Is Opioid Use Disorder (OUD)?
How Is OUD Defined?
OUD is defined in DSM-5 as a “problematic pattern of opioid use leading to clinically significant impairment or distress, as manifested by at least two of eleven criteria” (3). These criteria are a mix of physiological, psychological, and social aspects of problematic use (Box 1). There is no defining feature to the disorder according to DSM-5 (4), and the severity can range from mild (2–3 criteria) to moderate (4–5 criteria) to severe (6 or more criteria). The diagnosis of OUD represents essentially an amalgamation of the DSM-IV criteria for the diagnoses of opioid abuse and opioid dependence (5), with a new criterion, craving, added to those previous criteria.
BOX 1. DSM-5-TR criteria for opioid use disordera
A problematic pattern of opioid use leading to clinically significant impairment or distress, as manifested by at least two of the following, occurring within a 12-month period:
Opioids are often taken in larger amounts or over a longer period than was intended.
There is a persistent desire or unsuccessful efforts to cut down or control opioid use.
A great deal of time is spent in activities necessary to obtain the opioid, use the opioid, or recover from its effects.
Craving, or a strong desire or urge to use opioids.
Recurrent opioid use resulting in a failure to fulfill major role obligations at work, school, or home.
Continued opioid use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of opioids.
Important social, occupational, or recreational activities are given up or reduced because of opioid use.
Recurrent opioid use in situations in which it is physically hazardous.
Continued opioid use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance.
Tolerance, as defined by either of the following:
A need for markedly increased amounts of opioids to achieve intoxication or desired effect.
A markedly diminished effect with continued use of the same amount of an opioid.
Note: This criterion is not considered to be met for those taking opioids solely under appropriate medical supervision.
Withdrawal, as manifested by either of the following:
The characteristic opioid withdrawal syndrome (refer to Criteria A and B of the criteria set for opioid withdrawal).
Opioids (or a closely related substance) are taken to relieve or avoid withdrawal symptoms.
Note: This criterion is not considered to be met for those individuals taking opioids solely under appropriate medical supervision.
aReprinted from American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision. Washington, DC, American Psychiatric Association, 2022. Copyright © 2022 American Psychiatric Association. Used with permission.
What Is the Pathophysiology of OUD?
Considerable work has helped us to better understand the pathophysiology of the disorder. Characteristic opioid effects (e.g., analgesia, euphoria, reward) are largely mediated through the mu-opioid receptor, a G protein–coupled receptor. These receptors are located throughout the central and peripheral nervous system. In the brain, they are most densely expressed in the striatum, thalamus, and cingulate. Activation of the mesocortical dopamine system, which is indirectly affected by mu-opioid receptor activation, influences the rewarding effects of drugs, including opioids. Single-nucleotide polymorphisms that influence mu-opioid expression (e.g., OPRM1) predict risk for developing opioid use disorder and how individuals experience opioid effects (i.e., how strong opioid effects are, how good opioids feel) (6).
Chronic opioid exposure leads to mu-opioid receptor desensitization (7). This desensitization is a product of several secondary messenger processes, described in more detail elsewhere (8). Briefly, chronic morphine exposure causes phosphorylation of opioid receptors, which facilitates beta-arrestin binding at opioid receptors. Desensitization occurs because of beta-arrestin binding, which blocks intracellular processes induced by G protein–mediated signaling. Desensitization is the process that contributes to the development of tolerance, wherein the cells have reduced capacity, no longer responding to usual drug doses.
Importantly, chronic exposure to opioids leads to several changes in other neurotransmitter systems, which are implicated in the development of tolerance, withdrawal, and craving (9). For example, a decrease in dopamine D2 receptors in the dorsal striatum and left caudate nucleus is observed among individuals with a history of opioid use disorder (10, 11). During states of opioid withdrawal, D2 receptors function in a state of supersensitivity, which may contribute to the expression of craving and protracted withdrawal (12–14). These conformational changes persist after acute opioid withdrawal subsides. Corticotropin-releasing factor and norepinephrine-related systems, both implicated in stress responses, also undergo neuroadaptations following chronic exposure to opioids. Antagonizing these systems mitigates withdrawal and drug taking and seeking in opioid-dependent rodents (15, 16).
Taken together, several cellular and intracellular systems are implicated in the development of opioid dependence and the expression of opioid withdrawal and craving. Delineating how these systems interact and influence opioid tolerance, withdrawal, and craving is important for developing innovative pharmacotherapies that address unmet treatment needs (17).
Where Do We Stand on the Current Epidemiology of OUD?
The Substance Abuse and Mental Health Services Administration (SAMHSA) conducts an annual survey of drug use in the United States, the National Survey on Drug Use and Health (NSDUH). The most recent results available from NSDUH are for 2020, and at that time it was estimated that there were 9,490,000 persons with past-year opioid misuse and 2,702,000 who met the criteria for OUD (18).
A particularly tragic feature of this illicit opioid use has been an alarming and persistently high number of opioid-related overdose deaths. Opioid overdoses over the past 20-plus years have been characterized as occurring in four major overlapping waves (19, 20). The first wave was dominated by liberal availability of prescription opioids in the 1990s in response to calls to relax the prescribing of these medications for the treatment of pain (21), which resulted in diversion, misuse, and overdose deaths. This was followed by a second wave, starting around 2010, characterized by heroin overdoses. The third wave started shortly thereafter, around 2013, and has been dominated by illicit fentanyl and its analogues. While opioids have been the focus of these overdose deaths, rising rates of other drug use (particularly stimulants) since 2015 and the addition of other constituents to the fentanyl supply (e.g., xylazine) (22, 23) are significant characteristics of the current fourth wave. The mechanisms and causal relationships that these drug combinations may have with overdoses are not fully understood, although overdoses that involve both a stimulant and an opioid have clearly increased in recent years (24). These waves and the corresponding patterns of drug-specific overdoses from 2015 through July 2021 are depicted in Figure 1. Overdose death rates over this period show a shallow decline in heroin-related overdose deaths but a relatively sharp incline of synthetic opioid–related deaths and a corresponding general acceleration of opioid overdose deaths overall. Stimulant-related overdose deaths have also steadily increased along with opioid overdose deaths in recent years.
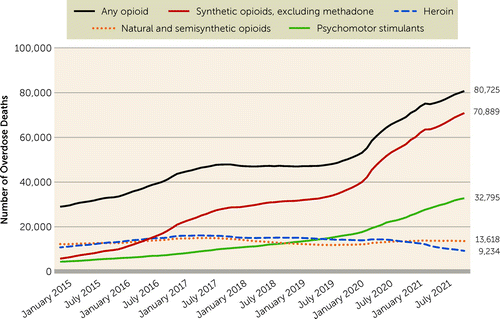
FIGURE 1. Drug overdose deaths in the United States, 2015 through July 2021, by drug toxicology reporta
aData Source: National Center for Health Statistics, National Vital Statistics System, Provisional drug overdose death counts, 2022.
The current overwhelming use of fentanyl (25, 26) deserves special mention. Addressing the liberal availability of prescription opioids associated with the first wave of overdoses was relatively easy, as the primary source (medical professionals) could be mitigated through regulatory agencies, to which providers generally readily respond. However, the sources of the second and especially the third wave have been much more problematic. As fentanyl and its analogues can be manufactured in a laboratory essentially anywhere (i.e., there is no need to have an area to cultivate poppy plants, the usual source of heroin), and the cost of producing fentanyl is relatively low, controlling the source as a means for decreasing use is much more challenging. This would suggest that reducing demand rather than supply will be necessary to make a meaningful and sustained impact on opioid misuse.
Finally, the onset of the COVID-19 pandemic coincided with a sharp increase in the rates of opioid overdose deaths. While the monthly percentage changes in opioid overdoses have more recently shown some decline, the rate of opioid overdose deaths remains greater than rates prior to the COVID-19 pandemic onset (27). The COVID-19 pandemic occasioned improvements in treatment accessibility with technology-based remote monitoring and psychosocial support. These methods are described below in more detail in the section on novel treatment targets and approaches.
What Do We Know About Comorbidity?
Other medical conditions are frequently present in the person who has an OUD. These comorbidities may be broadly categorized into three groupings: mental health, other substance use, and somatic disorders. While there are certainly persons who present with OUD and no comorbidities, this tends to be the exception rather than the rule. The prevalence of these comorbidities can vary as a function of changing circumstances (e.g., the availability and popularity of other drugs, the rise of a new infectious disease such as HIV infection or COVID-19), and local factors such as access to treatments can vary the profile of common comorbidities. Each of these three categories is briefly reviewed here. For more information on these topics, see the comprehensive reviews by Buresh et al. (28), Santo et al. (29), and Karamouzian et al. (30).
Estimates of other mental health disorders in persons with OUD have varied widely over time, geographical region, and study (31), and the sample sizes of many of these reports have been relatively small and somewhat selective (e.g., persons presenting for a particular type of treatment at a single site). In general, there tend to be higher rates of depression, anxiety, and personality disorders found in persons with OUD (31, 32). Similarly, rates of other comorbid substance use disorders can also vary widely across similar parameters of time and location, although nicotine, illicit simulant (cocaine and methamphetamine), and alcohol use disorders tend to predominate (30). Finally, other somatic disorders, especially infectious illnesses such as HIV and hepatitis C, and chronic pain are commonly found among persons with OUD (28). The high level of comorbidities speaks to the need for a careful assessment of the OUD patient and to the fact that there can often be complexity in the treatment and that comprehensive and coordinated care is often needed.
What Treatments Are Currently Available?
Four medications approved by the U.S. Food and Drug Administration (FDA) are currently available for the treatment of OUD: buprenorphine, lofexidine, methadone, and naltrexone. A fifth medication, levo-alpha-acetylmethadol (LAAM), is a full mu-opioid agonist similar to methadone in its actions but with a longer duration of action than methadone. It is FDA approved but was withdrawn from the market in 2003 due to limited sales that occurred in the context of concerns regarding QTc prolongation with its use.
Lofexidine is an alpha-2 adrenergic agent akin to clonidine, and is approved for medically supervised opioid withdrawal, which is a time-limited modality of care (33, 34). Of the remaining three medications, all are generally used for maintenance treatment (i.e., ongoing therapy with typically no specified medication end date). Methadone is a full mu-opioid agonist that has good oral bioavailability and a relatively long duration of action that permits once-daily dosing. It has been used for the treatment of OUD in the United States since the early 1960s and is a Schedule II drug. Currently, it is provided through a system of specialty clinics referred to as Opioid Treatment Programs (OTPs), although some relaxation of its prescribing for OUD has been considered in the context of the COVID-19 pandemic. Methadone is usually provided in a liquid form when dispensed by an OTP, although tablet forms are also marketed. Numerous clinical trials have demonstrated that it is effective and can provide benefit for both opioid use and other non–drug use outcomes (e.g., psychosocial functioning) (35). As a full-agonist opioid, an overdose with methadone risks respiratory depression and death, depending on the individual’s level of opioid tolerance.
Buprenorphine can be provided through office-based treatment in the United States and was approved in 2002 as a Schedule III medication initially provided as a daily sublingual tablet. Its primary therapeutic effect is mediated by its action as a partial mu agonist, although it also acts at other opioid receptors (36). This partial agonism means that its potential maximal effect is less than that of a full-agonist opioid such as methadone. The initially approved tablet form of buprenorphine was combined with naloxone to decrease the risk of parenteral abuse (37). A variety of other forms have since been approved, including a transmucosal film strip and, most recently, a long-acting injectable form that is administered once a month. An implantable form that provided 6 months of buprenorphine was withdrawn from the market in 2020, reportedly due to cost.
There are special considerations when inducting an individual with chronic fentanyl use onto buprenorphine. If the dosing of buprenorphine is not appropriately timed after the last use of fentanyl, there is an increased risk of precipitated withdrawal (38). Several strategies have emerged to address this issue, including micro- and macro-dosing induction (see Greenwald et al. for a review [39]). Therefore, while dosing adjustments are needed during buprenorphine initiation, buprenorphine remains an important medication for treating OUD.
While methadone and buprenorphine exert agonist effects at the mu receptor, naltrexone is an opioid antagonist. This medication was initially approved in 1984 for the treatment of opioid dependence, and unlike the two agonist-based treatments, it has not had special stipulations to its prescribing and is an uncontrolled medication. It is marketed as a daily oral drug as well as a once-monthly injection. In a person who is not opioid tolerant, methadone and buprenorphine have acute subjective effects that can be perceived as positive and pleasurable (40, 41), while naltrexone under these circumstances does not produce such acute effects (42). However, unlike methadone and buprenorphine, naltrexone requires the patient to have been opioid abstinent prior to the first dose, as an acute dose of an antagonist in an opioid-dependent person will otherwise precipitate the opioid withdrawal syndrome. The efficacy of naltrexone has been shown to be similar to that of buprenorphine once the patient is successfully stabilized on it (43).
In addition to these pharmacological approaches, it has been recommended that nonpharmacological treatments be used in combination with pharmacotherapies for OUD treatment (44). However, data to support this combination approach have not been robust, with the exception being the use of contingency management (45). Contingency management is an intervention that delivers modest incentives contingent upon a desired behavior (e.g., providing drug-free urine samples, attending appointments). Sixty clinical trials were analyzed in a recently published meta-analysis that demonstrated the efficacy of incentives to promote various health behaviors among persons receiving OUD treatment (45). According to the meta-analysis, incentives had a medium to large effect on improving a variety of treatment outcomes, including decreasing illicit opioid use, increasing medication adherence, and increasing therapy attendance. To increase accessibility and decrease burden on clinics, a contingency management–based app targeting OUD has been developed and cleared by the FDA (reSET-O), as discussed below.
What Novel Treatment Targets and Approaches Are on the Horizon?
In recent years, there has been considerable expansion of the OUD treatment system’s capacity, especially in certain parts of the United States, such as the Northeast, the mid-Atlantic, and the West Coast regions. An analysis in 2020 showed that only 9.2% of the U.S. population was more than 10 miles from a buprenorphine provider (46), although this does not address whether providers had capacity to take on new patients or whether transportation to providers was readily available. Methadone treatment capacity also has expanded as the Affordable Care Act has provided more funding for persons with substance use disorders (47). However, despite increases in the treatment system capacity, rates of OUD and overdose have not significantly diminished, as reviewed above. This suggests that other approaches to treatment need to be considered and has led to calls for additional and novel approaches to OUD treatment. These may be conceptualized as new symptom targets for medications, nonpharmacologic treatments, and social approaches that seek to develop new systems of care.
Novel targets for OUD treatment have included outcomes such as craving (48, 49), sleep (50), and mood symptoms (51), all of which were identified as important in a 2018 FDA patient-focused drug development meeting on OUD, in which patient and family input was solicited. While craving in particular has received considerable attention, challenges in the definition of craving and psychometrically sound measures of this construct will need to be addressed as critical steps in identifying agents that may be effective in decreasing this symptom (51). In contrast to craving assessments, psychometrically sound measures that assess sleep impairments such as insomnia have a strong base that can be used in studies of OUD (52, 53), and there is preliminary work that suggests that orexin receptor antagonists may have broadly beneficial effects in persons with OUD (54).
Psychedelics have been proposed as potential treatments for a wide variety of psychiatric disorders (55, 56), and some survey work suggests that they may be useful in decreasing drug use (57), with general beneficial effects on quality of life. However, controlled trials of psychedelics for the treatment of OUD are lacking at this time, although there do appear to be studies planned, as registered at ClinicalTrials.gov.
Another potentially fruitful approach that involves nonpharmacological treatments has been the use of technological innovations. These fall into two broad categories: software applications (apps) that provide real-time assistance in treating OUD, and devices that may assist a person in their treatment of OUD (58). Regarding the former, the FDA has authorized a prescription digital app (reSET-O), which is a behaviorally based intervention that has been shown to decrease health care costs and have clinically beneficial outcomes in patients with OUD (59, 60). Other, similar interventions are in development, including by DynamiCare, which has an app that delivers a contingency management intervention with remote self-testing for substance use (61). Such treatments appear to be optimally used when integrated with medications such as buprenorphine, allowing providers to offer concurrent prescriptions for an effective medication and a nonpharmacological treatment. An alternate device approach is the use of automated dispensing delivery systems (pillboxes) to ensure that patients do not misuse controlled substances such as buprenorphine and methadone (62–64). Such boxes provide greater flexibility in home dosing of medications such as methadone, decreasing the need for daily or near daily clinic visits.
Finally, novel social approaches for treating OUD can include harm reduction interventions, such as safe injection facilities, needle exchange programs, fentanyl test strips, and the provision of heroin (or other opioids, such as hydromorphone) by prescription for supervised self-administration. These approaches have had varying levels of uptake in the United States but have been studied and utilized more extensively in other countries (65–68). As expansion of treatment capacity has not been associated with a decrease in opioid overdose numbers in the United States, substantive changes to treatment infrastructure (e.g., harm reduction efforts such as safe injection facilities or heroin maintenance) have been given significant consideration but have not had widespread social acceptance. By linking harm reduction approaches as a mechanism to engage persons in treatment (69), it may be possible to improve the social acceptance of these interventions.
Final Thoughts
The continued misuse of opioids and intersecting overdose deaths is tragic for individuals, families, and communities. There is no single approach that will eradicate OUD, and the persistence of opioid misuse over past generations suggests that it will likely continue to some degree into the future. However, this persistence should not mean a passive acceptance of the current state, and continued scientific, clinical, and social efforts should focus on decreasing initiation and continued misuse of this class of drugs.
Interventions that focus on treating persons with OUD continue to evolve, and effective treatments are available. Increased understanding of the biologic underpinnings of opioid misuse and corresponding tailored and innovative treatments have value and are needed. Importantly, decreasing initiation of use is also critical and can sometimes get lost in the attention on treatment of persons with an active OUD. At times it can seem like efforts to stem OUD are akin to frantically bailing out a sinking ship while failing to plug the leaking hole. In relation to OUD, steps to plug the “hole” include giving individuals and communities the resources and purpose that help to divert persons away from drug use and decrease their vulnerability to drug experimentation and use (70).
Addressing the social factors that can play a significant role in the initiation and early maintenance of drug use is needed. Further, identifying innovative but scalable methods to decrease reliance on opioids as analgesics should be prioritized in clinical settings. While impacting and improving the health of communities and the opportunities people have to engage in positive social activities will not end all drug use, it can shift that use and help to stem the tide of this particularly dangerous and damaging disorder.
1. : The American Disease: Origins of Narcotic Control, 3rd ed. New York, Oxford University Press, 1999 Google Scholar
2. : The Least of Us: True Tales of America and Hope in the Time of Fentanyl and Meth. New York, Bloomsbury Publishing, 2021 Google Scholar
3. : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013 Crossref, Google Scholar
4. : Hegemony, homogeneity, and DSM-5 SUD. Drug Alcohol Depend 2021; 221:108660Crossref, Medline, Google Scholar
5. : Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994 Google Scholar
6. : A systematic review of GWAS identified SNPs associated with outcomes of medications for opioid use disorder. Addict Sci Clin Pract 2021; 16:70Crossref, Medline, Google Scholar
7. : Opioid receptor desensitization: mechanisms and its link to tolerance. Front Pharmacol 2014; 5:280Crossref, Medline, Google Scholar
8. : The mechanisms involved in morphine addiction: an overview. Int J Mol Sci 2019; 20:E4302Crossref, Medline, Google Scholar
9. : Non-opioid neurotransmitter systems that contribute to the opioid withdrawal syndrome: a review of preclinical and human evidence. J Pharmacol Exp Ther 2019; 371:422–452Crossref, Medline, Google Scholar
10. : The polymorphism of dopamine D2 receptor TaqIA gene is associated with brain response to drug cues in male heroin-dependent individuals during methadone maintenance treatment. Drug Alcohol Depend 2019; 198:150–157Crossref, Medline, Google Scholar
11. : Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology 1997; 16:174–182Crossref, Medline, Google Scholar
12. : Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther 1995; 272:781–785Medline, Google Scholar
13. : Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci 1999; 11:1037–1041Crossref, Medline, Google Scholar
14. : Persistent and reversible morphine withdrawal-induced morphological changes in the nucleus accumbens. Ann N Y Acad Sci 2006; 1074:446–457Crossref, Medline, Google Scholar
15. : Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 1995; 6:74–80Crossref, Medline, Google Scholar
16. : Involvement of noradrenergic system within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Psychopharmacology 2003; 170:80–88Crossref, Medline, Google Scholar
17. : Non-opioid neurotransmitter systems that contribute to the opioid withdrawal syndrome: a review of preclinical and human evidence. J Pharmacol Exp Ther 2019; 371:422–452Crossref, Medline, Google Scholar
18. : Results from the 2020 National Survey on Drug Use and Health: detailed tables. 2021. https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables Google Scholar
19. : High potency synthetic opioids: curbing the third wave of the opioid crisis. Neurosci Biobehav Rev 2019; 106:9–10Crossref, Medline, Google Scholar
20. : Geographic trends in opioid overdoses in the US from 1999 to 2020. JAMA Netw Open 2022; 5:e2223631 eCrossref, Google Scholar
21. : Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain 1986; 25:171–186Crossref, Medline, Google Scholar
22. : Racial/ethnic and geographic trends in combined stimulant/opioid overdoses, 2007–2019. Am J Epidemiol 2022; 191:599–612Crossref, Medline, Google Scholar
23. : Xylazine and overdoses: trends, concerns, and recommendations. Am J Public Health 2022; 112:1212–1216Crossref, Medline, Google Scholar
24. : Co-involvement of opioids in drug overdose deaths involving cocaine and psychostimulants. NCHS Data Brief 2021; 406:1–8Google Scholar
25. : Steep increases in fentanyl-related mortality west of the Mississippi River: recent evidence from county and state surveillance. Drug Alcohol Depend 2020; 216:108314Crossref, Medline, Google Scholar
26. : The rise of illicit fentanyls, stimulants, and the fourth wave of the opioid overdose crisis. Curr Opin Psychiatry 2021; 34:344–350Crossref, Medline, Google Scholar
27. : Trend analysis of drug overdose deaths before and during the COVID-19 pandemic. Am J Transl Res 2022; 14:2685–2688Medline, Google Scholar
28. : Treatment of opioid use disorder in primary care. BMJ 2021; 373:n784Crossref, Medline, Google Scholar
29. : Prevalence of mental disorders among people with opioid use disorder: a systematic review and meta-analysis. Drug Alcohol Depend 2022; 238:109551Crossref, Medline, Google Scholar
30. : Latent patterns of polysubstance use among people who use opioids: a systematic review. Int J Drug Policy 2022; 102:103584Crossref, Medline, Google Scholar
31. : The phenomics and genetics of addictive and affective comorbidity in opioid use disorder. Drug Alcohol Depend 2021; 221:108602Crossref, Medline, Google Scholar
32. : Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry 1997; 54:71–80Crossref, Medline, Google Scholar
33. : A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend 2017; 176:79–88Crossref, Medline, Google Scholar
34. : A comprehensive update of lofexidine for the management of opioid withdrawal symptoms. Psychopharmacol Bull 2020; 50:76–96Medline, Google Scholar
35. : Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database Syst Rev 2017; 4:Cd011983Medline, Google Scholar
36. : A narrative pharmacological review of buprenorphine: a unique opioid for the treatment of chronic pain. Pain Ther 2020; 9:41–54Crossref, Medline, Google Scholar
37. : Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl) 2000; 148:374–383Crossref, Medline, Google Scholar
38. : Method for successfully inducting individuals who use illicit fentanyl onto buprenorphine/naloxone. Am J Addict 2021; 30:83–87Crossref, Medline, Google Scholar
39. : A neuropharmacological model to explain buprenorphine induction challenges. Ann Emerg Med (Online ahead of print, August 5, 2022)Google Scholar
40. : Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 1994; 55:569–580Crossref, Medline, Google Scholar
41. : Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 1978; 35:501–516Crossref, Medline, Google Scholar
42. : Human abuse liability assessment of oxycodone combined with ultra-low-dose naltrexone. Psychopharmacology (Berl) 2010; 210:471–480Crossref, Medline, Google Scholar
43. : Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 2018; 391:309–318Crossref, Medline, Google Scholar
44. : Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry 2019; 76:208–216Crossref, Medline, Google Scholar
45. : Contingency management for patients receiving medication for opioid use disorder: a systematic review and meta-analysis. JAMA Psychiatry 2021; 78:1092–1102Crossref, Medline, Google Scholar
46. : Geographic proximity to buprenorphine treatment providers in the US. Drug Alcohol Depend 2020; 213:108131Crossref, Medline, Google Scholar
47. : The Affordable Care Act and opioid agonist therapy for opioid use disorder. Psychiatr Serv 2019; 70:617–620Link, Google Scholar
48. : A preliminary examination of the multiple dimensions of opioid craving. Drug Alcohol Depend 2021; 219:108473Crossref, Medline, Google Scholar
49. : Time to reconsider the role of craving in opioid use disorder. JAMA Psychiatry 2019; 76:1113–1114Crossref, Medline, Google Scholar
50. : Sleep disturbance as a therapeutic target to improve opioid use disorder treatment. Exp Clin Psychopharmacol (Online ahead of print, June 10, 2021)Google Scholar
51. : Public meeting on patient-focused drug development for opioid use disorder. 2018. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/public-meeting-patient-focused-drug-development-opioid-use-disorder Google Scholar
52. : Objective sleep outcomes in randomized controlled trials in persons with substance use disorders: a systematic review. Drug Alcohol Depend 2022; 237:109509Crossref, Medline, Google Scholar
53. : Patient-reported sleep outcomes in randomized controlled trials in persons with substance use disorders: a systematic review. Drug Alcohol Depend 2022; 237:109508Crossref, Medline, Google Scholar
54. : Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Sci Transl Med 2022; 14:eabn8238Crossref, Medline, Google Scholar
55. : Associations between classic psychedelics and opioid use disorder in a nationally representative US adult sample. Sci Rep 2022; 12:4099Crossref, Medline, Google Scholar
56. : Classic psychedelics in the treatment of substance use disorder: potential synergies with twelve-step programs. Int J Drug Policy 2021; 98:103380Crossref, Medline, Google Scholar
57. : Persisting reductions in cannabis, opioid, and stimulant misuse after naturalistic psychedelic use: an online survey. Front Psychiatry 2019; 10:955Crossref, Medline, Google Scholar
58. : Medical devices to prevent opioid use disorder: innovative approaches to addressing the opioid crisis. JAMA Psychiatry 2019; 76:351–352Crossref, Medline, Google Scholar
59. : Reduced healthcare resource utilization in patients with opioid use disorder in the 12 months after initiation of a prescription digital therapeutic. Adv Ther (Online ahead of print, July 7, 2022) Google Scholar
60. : Real-world use and clinical outcomes after 24 weeks of treatment with a prescription digital therapeutic for opioid use disorder. Hosp Pract (1995) 2021; 49:348–355Crossref, Medline, Google Scholar
61. : Digital delivery of a contingency management intervention for substance use disorder: a feasibility study with DynamiCare Health. J Subst Abuse Treat 2021; 126:108425Crossref, Medline, Google Scholar
62. : Use of an electronic pillbox to increase number of methadone take-home doses during the COVID-19 pandemic. J Subst Abuse Treat 2021; 126:108328Crossref, Medline, Google Scholar
63. : Technology-assisted methadone take-home dosing for dispensing methadone to persons with opioid use disorder during the COVID-19 pandemic. J Subst Abuse Treat 2021; 121:108197Crossref, Medline, Google Scholar
64. : Interim buprenorphine vs waiting list for opioid dependence. N Engl J Med 2016; 375:2504–2505Crossref, Medline, Google Scholar
65. : Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 2009; 361:777–786Crossref, Medline, Google Scholar
66. : Medical prescription of heroin to treatment resistant heroin addicts: two randomised controlled trials. BMJ 2003; 327:310Crossref, Medline, Google Scholar
67. : Factors associated with 60-day adherence to “safer supply” opioids prescribed under British Columbia’s interim clinical guidance for health care providers to support people who use drugs during COVID-19 and the ongoing overdose emergency. Int J Drug Policy 2022; 105:103709Crossref, Medline, Google Scholar
68. : Characterizing safer supply prescribing of immediate release hydromorphone for individuals with opioid use disorder across Ontario, Canada. Int J Drug Policy 2022; 102:103601Crossref, Medline, Google Scholar
69. : Improving treatment enrollment and re-enrollment rates of syringe exchangers: 12-month outcomes. Drug Alcohol Depend 2012; 124:162–166Crossref, Medline, Google Scholar
70. : Meaning and purpose in the context of opioid overdose deaths. Drug Alcohol Depend 2021; 219:108528Crossref, Medline, Google Scholar



