Synaptic Variability and Cortical Gamma Oscillation Power in Schizophrenia
Abstract
Objective:
Cognitive impairments in schizophrenia are associated with lower gamma oscillation power in the prefrontal cortex (PFC). Gamma power depends in part on excitatory drive to fast-spiking parvalbumin interneurons (PVIs). Excitatory drive to cortical neurons varies in strength, which could affect how these neurons regulate network oscillations. The authors investigated whether variability in excitatory synaptic strength across PVIs could contribute to lower prefrontal gamma power in schizophrenia.
Methods:
In postmortem PFC from 20 matched pairs of comparison and schizophrenia subjects, levels of vesicular glutamate transporter 1 (VGlut1) and postsynaptic density 95 (PSD95) proteins were quantified to assess variability in excitatory synaptic strength across PVIs. A computational model network was then used to simulate how variability in excitatory synaptic strength across fast-spiking (a defining feature of PVIs) interneurons (FSIs) regulates gamma power.
Results:
The variability of VGlut1 and PSD95 levels at excitatory inputs across PVIs was larger in schizophrenia relative to comparison subjects. This alteration was not influenced by schizophrenia-associated comorbid factors, was not present in monkeys chronically exposed to antipsychotic medications, and was not present in calretinin interneurons. In the model network, variability in excitatory synaptic strength across FSIs regulated gamma power by affecting network synchrony. Finally, greater synaptic variability interacted synergistically with other synaptic alterations in schizophrenia (i.e., fewer excitatory inputs to FSIs and lower inhibitory strength from FSIs) to robustly reduce gamma power.
Conclusions:
The study findings suggest that greater variability in excitatory synaptic strength across PVIs, in combination with other modest synaptic alterations in these neurons, can markedly lower PFC gamma power in schizophrenia.
Impairments in certain cognitive processes, such as working memory, are a core clinical feature of schizophrenia (1). Working memory is associated with synchronized neural oscillatory activity at gamma band frequency (∼30–80 Hz) in the prefrontal cortex (PFC) (2–4), and the power of these oscillations during the performance of cognitive tasks is lower in individuals with schizophrenia (5–7). Thus, alterations in PFC neural circuitry are thought to contribute to impaired gamma oscillations and working memory performance in schizophrenia (8, 9).
The generation of cortical gamma oscillations appears to depend, at least in part, on the activity of a local neural circuit that includes regular-spiking excitatory pyramidal neurons and fast-spiking GABAergic parvalbumin-expressing interneurons (PVIs) (10). PVIs receive excitatory synaptic inputs from neighboring pyramidal neurons (11) and provide phasic inhibition that synchronizes the firing of those pyramidal neurons at gamma frequency (10, 12). For example, in animal models, driving excitatory inputs to PVIs generates local field potentials at gamma frequency (13, 14), whereas the loss of excitatory drive to PVIs impairs gamma oscillations (15–17). Thus, the generation of gamma oscillations in the PFC is thought to be dependent on excitatory synaptic inputs to PVIs.
Excitatory synaptic inputs to cortical neurons vary in their strength (18, 19). Furthermore, introducing variability to anatomical and physiological properties that determine synaptic strength disrupts network behaviors in computational models (20–27). These findings suggest that shifts in the normal levels of variability in excitatory synaptic strength across cortical neurons could influence how these neurons participate in the generation of network oscillations. Thus, disease-driven alterations in the variability of excitatory synaptic strength across PVIs may contribute to lower prefrontal gamma oscillation power in schizophrenia.
To explore this idea, we first examined variability in excitatory synaptic strength across PVIs by quantifying pre- and postsynaptic markers of synaptic strength to individual PVIs in postmortem human PFC from matched pairs of schizophrenia and unaffected comparison subjects. Then, we utilized a computational model network of regular-spiking excitatory and fast-spiking (a defining feature of PVIs) inhibitory neurons to simulate how variability in excitatory synaptic strength across PVIs might regulate gamma band power. Finally, we used the model network to simulate how variability in excitatory synaptic strength interacts with other synaptic parameters of PVIs that are altered in schizophrenia. Our findings suggest that greater variability in excitatory synaptic strength across PVIs is characteristic of the disease process of schizophrenia, regulates network oscillations by affecting synchronous neuronal firing, and can interact synergistically with other synaptic alterations in PVIs to robustly reduce prefrontal gamma power.
Methods
Quantifying Variability in Excitatory Synaptic Strength Across PVIs in the PFC
To quantify variability in excitatory synaptic strength across individual PVIs in the PFC of persons with schizophrenia, we reanalyzed our immunohistochemical data set from a previous study (28). This data set contains the relative protein levels of vesicular glutamate transporter 1 (VGlut1) and postsynaptic density 95 (PSD95) in excitatory inputs to PVIs (see Figure S1 and the Supplemental Methods section in the online supplement for detailed information) in the PFC (Brodmann area 9) of unaffected comparison and schizophrenia subjects (N=20 matched pairs; see Table S1 in the online supplement for summary characteristics of study subjects) and monkeys that had received oral haloperidol, olanzapine, or sham treatment for 17–27 months (N=6 matched triads). In the present analysis, given that both VGlut1 and PSD95 protein levels are correlated with the amplitude of AMPA-mediated excitatory postsynaptic currents (29, 30), the relative levels of VGlut1 protein and of PSD95 protein in all excitatory inputs to each PVI were averaged to index the mean strength of excitatory synaptic inputs to each PVI. Then the variability in mean excitatory synaptic strength across individual PVIs for each subject in both the human and monkey cohorts was computed as the coefficient of variation (CV) of either VGlut1 or PSD95 levels or the combined levels of VGlut1 and PSD95 levels (VGlut1 + PSD95 levels). The same method was used to compute the variability in excitatory synaptic strength across calretinin interneurons, which are not thought to contribute directly to the generation of gamma oscillations (31, 32).
Statistical Tests for Empirical Immunohistochemical Data
Two analysis of covariance (ANCOVA) models were used to compare the dependent variables between schizophrenia and unaffected comparison subject groups. The paired ANCOVA model included subject pair as a blocking factor and postmortem interval and tissue storage time as covariates. This model accounts for the matching of subject pairs for sex and age and for the parallel tissue processing of subject pairs but is not a true statistical paired design. Thus, we also used an unpaired ANCOVA model, which included age, sex, race, postmortem interval, and tissue storage time as covariates. Nonsignificant covariates were excluded in the final reported analyses. The paired and unpaired ANCOVA analyses produced comparable levels of statistical significance on all dependent variables. Thus, the results from the paired ANCOVA analysis are reported in the main text, and the results from the unpaired ANCOVA analysis are provided in Table S2 in the online supplement. For the antipsychotic-exposed monkeys, an ANCOVA was used to assess the main effect of antipsychotic treatment with triad as a blocking factor. The effect size was calculated by Cohen’s d (33) to assess the magnitude of difference in all dependent measures between subject groups.
Computational Model Network of Excitatory and Fast-Spiking Inhibitory Cells
We simulated gamma oscillations using a pyramidal interneuron gamma (PING) network that can model the effect of various properties of excitatory and inhibitory synapses on gamma band power (34, 35) (see the Supplemental Methods section in the online supplement for detailed information). In brief, the PING network consisted of 80 regular-spiking excitatory (RSEs) and 20 fast-spiking inhibitory (FSIs) quadratic integrate-and-fire cells (36). Cells were connected to every other cell in the network (all-to-all connection). Each excitatory synaptic connection contained AMPA and NMDA conductance, and each inhibitory synaptic connection contained GABA conductance (Figure 1A). Parameters used to model the regular-spiking property of RSEs (Figure 1B, left panel), the fast-spiking property of FSIs (Figure 1B, right panel), and synaptic conductance between these cells are described in the Supplemental Methods of the online supplement. External excitatory synaptic currents were applied to RSEs to initiate network activity. For each simulation trial, power spectral density was taken on the network activity (i.e., the sum of all excitatory synaptic currents into RSEs) to compute peak gamma power and frequency (Figure 1C, D) (35). Results of each experiment are the average of 200 trials.
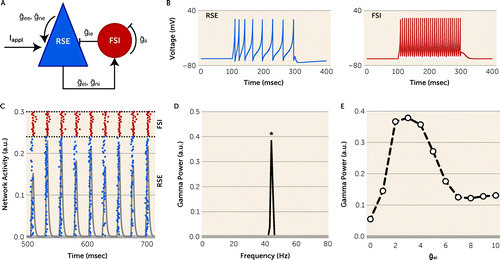
FIGURE 1. Properties of pyramidal interneuron gamma (PING) model networka
a Panel A is a schematic diagram of the network architecture illustrating recurrent connectivity among regular-spiking excitatory cells (RSEs) and fast-spiking inhibitory cells (FSIs). ḡee and ḡne indicate mean AMPA and NMDA conductance to RSEs, respectively. ḡei and ḡni indicate mean AMPA and NMDA conductance to FSIs, respectively. ḡie and ḡii indicate mean GABA conductance to RSEs and FSIs, respectively. Iappl indicates current applied to RSEs to initiate network activity. Panel B shows membrane properties of an RSE (left) and an FSI (right) under current injection. Panel C is an example of a raster plot (blue dots indicate RSEs, N=80; red dots indicate FSIs, N=20) and network activity (gray) across time during gamma oscillations. a.u.=arbitrary units. Panel D shows a power spectral density analysis from the network behavior shown in panel C. Asterisk indicates the frequency with peak power for this trial. Panel E is a plot showing the effect of ḡei on gamma power computed as an average over 200 trials. ḡei and gamma power form an inverted U curve with a peak at ḡei=2–4.
Simulating Synaptic Parameters of FSIs in the Model Network
Previous studies demonstrated that 1) excitatory postsynaptic currents in PVIs are predominantly mediated by AMPA receptors (34, 37–40); 2) loss of excitatory synaptic inputs to PVIs primarily reduces AMPA receptor–mediated excitatory postsynaptic currents (15); 3) knockout of AMPA receptor subunit in PVIs reduces gamma power (17); and 4) NMDA receptors in PVIs regulate PVI-mediated inhibition via presynaptic mechanisms (41) but minimally contribute to excitatory postsynaptic currents in these neurons (34, 37–40). Thus, excitatory synaptic strength across PVIs was modeled by AMPA conductance onto individual FSIs from their presynaptic RSEs (gei). To simulate variability in excitatory synaptic strength across PVIs, we computed the CV of gei, which we termed CVg, by assigning gei randomly drawn from a normal distribution with a mean of ḡei=2 and varying standard deviations. The normal distribution had a lower limit of 0 to avoid assigning negative values to gei. To simulate the effect of fewer excitatory inputs to PVIs on gamma power, the probability of excitatory synapse connectivity on FSIs (Connei) was lowered from 1. Finally, to simulate the effect of lower strength of inhibitory outputs from PVIs, the GABA conductance from FSIs to RSEs (ḡie) was reduced from 0.8. For all experiments, the number of FSIs in the model network was kept constant based on findings that the relative density of PVIs (as assessed by both mRNA and protein measures) is not altered in the PFC of subjects with schizophrenia (28, 42, 43).
Results
Greater Variability of Excitatory Input Strength Across PVIs in Schizophrenia
The CV of VGlut1 or PSD95 levels across PVIs was significantly higher by 20% (Figure 2A) or 28% (Figure 2B), respectively, in the PFC of schizophrenia relative to unaffected comparison subjects. The greater CV in schizophrenia was due to a higher standard deviation of VGlut1 and PSD95 levels (Figure 2C,D), and not to differences in their mean values (Figure 2E,F). These findings support the idea that schizophrenia is associated with greater variability in excitatory synaptic strength across PVIs in the PFC.
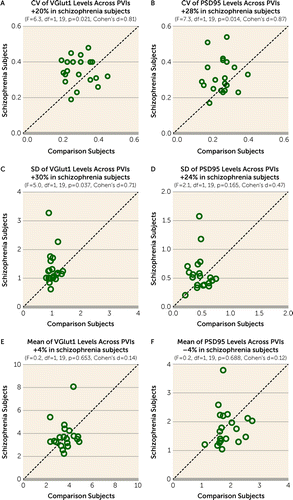
FIGURE 2. Greater variability in excitatory synaptic strength across parvalbumin interneurons (PVIs) in the PFC of schizophrenia subjectsa
a Presented here are scatterplots for coefficient of variation (CV) (panels A and B), standard deviation (panels C and D), and mean (panels E and F) of VGlut1 and PSD95 levels within excitatory inputs across PVIs for each unaffected comparison subject (x-axis) and schizophrenia subject (y-axis) in a pair. Data points above the diagonal unity line indicate a higher level in the schizophrenia subject relative to the matched unaffected comparison subject. The greater CV in schizophrenia subjects was evident in both VGlut1 and PSD95 levels, and this difference was due to higher standard deviations and not differences in mean values.
Next, we investigated whether greater synaptic variability across PVIs in schizophrenia might be due to other factors commonly associated with the illness. Neither diagnosis of schizoaffective disorder; nor history of substance abuse or nicotine use at the time of death; nor use of antidepressants, benzodiazepines, or valproic acid at the time of death; nor death by suicide had a significant effect on the CV of VGlut1 or PSD95 levels across PVIs among schizophrenia subjects (see Figure S2 in the online supplement). Moreover, the CV of VGlut1 or PSD95 levels across PVIs did not differ between monkeys chronically exposed to olanzapine or sham treatment, although the CV of VGlut1 appeared to be lower in monkeys exposed to haloperidol, perhaps suggesting a normalizing effect of this medication on the variability of excitatory synaptic strength onto PVIs (see Figure S3 in the online supplement). These findings suggest the absence of effects from comorbid factors or antipsychotic medications on greater synaptic variability across PVIs in schizophrenia.
Finally, we assessed variability in excitatory synaptic strength across calretinin neurons, a subclass of inhibitory neurons that do not share local excitatory inputs with PVIs (11, 44) and do not directly contribute to the generation of gamma oscillations (31, 32). The CV of VGlut1 or PSD95 levels across calretinin neurons did not significantly differ between subject groups (see Figure S4A,B in the online supplement). These findings suggest that variability in excitatory synaptic strength is not altered across calretinin neurons in schizophrenia, consistent with previous studies demonstrating that these neurons are relatively unaffected in the illness (28, 42, 45–47).
Simulated Effect of Greater Synaptic Variability Across PVIs on Prefrontal Gamma Power
Based on these findings of greater variability in excitatory synaptic strength across PVIs in schizophrenia, we explored how synaptic variability affects the generation of gamma oscillations in a computational model network of regular-spiking excitatory (RSEs) and fast-spiking inhibitory (FSIs) neurons that can robustly generate network gamma oscillations (Figure 1A–D). We first characterized how changes in mean excitatory synaptic strength from RSEs to FSIs (ḡei) influence gamma power in our model network when variability in excitatory synaptic strength across individual FSIs (CVg) is 0. Gamma power sharply increased as ḡei increased from 0 and reached a peak at ḡei=2, which was maintained as ḡei was further increased from 2 to 4 (Figure 1E). Gamma power sharply decreased with ḡei>4 and reached a stable nadir at ḡei≥7. Thus, our model network replicated the inverted U relationship between ḡei and gamma power observed in a previous study (48).
Next, we assessed how shifts in CVg regulate gamma power in the model network. To simulate biologically relevant shifts in CVg, we utilized the VGlut1 and PSD95 levels within excitatory inputs to PVIs in our 20-pair human cohort. To obtain a single molecular index to model excitatory synaptic strength onto each FSI (gei), the mean VGlut1 and the mean PSD95 levels, measures of synaptic strength in pre- and postsynaptic compartments (29, 30), respectively, at excitatory inputs onto each PVI were summed (VGlut1 + PSD95 levels). The mean VGlut1 levels and the mean PSD95 levels within excitatory inputs onto PVIs (N=723) sampled from all 40 subjects were significantly positively correlated (Figure 3A), supporting the use of a single index to simulate gei. Further analyses showed that the VGlut1 + PSD95 levels onto each PVI sampled from comparison subjects conformed to a normal distribution (Figure 3B) (Shapiro-Wilk test: W=4.7, p=0.062), whereas those sampled from schizophrenia subjects had a distribution with skewness of 1.58 and kurtosis of 7.57 (Figure 3C) (Shapiro-Wilk test: W=0.8, p<0.001). Also, the mean for the CV of VGlut1 + PSD95 levels across PVIs was 0.24 (SD=0.05) in unaffected comparison subjects and 0.30 (SD=0.07) in schizophrenia subjects. Finally, the CV of VGlut1 + PSD95 levels across PVIs ranged from 0.1 to 0.5 across all subjects (Figure 3D). Based on these empirical findings, we generated values for gei from either a normal distribution for comparison subjects (Figure 3E) or a skewed distribution for schizophrenia subjects (Figure 3F). In each distribution, we varied the standard deviation without changing the mean value to introduce shifts in CVg from 0.1 to 0.5 in the model network.
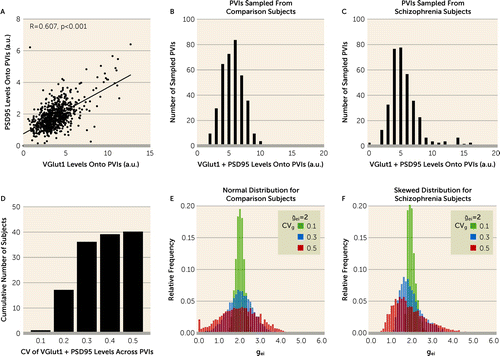
FIGURE 3. Modeling variability in excitatory synaptic strength across FSIs (CVg) using empirical dataa
a Panel A is a correlation graph plotting mean VGlut1 and mean PSD95 levels within excitatory inputs to PVIs. a.u.=arbitrary units; PVIs=parvalbumin interneurons. The regression line represents the significant positive association of these measures across all sampled PVIs (N=723). Given the strength of this correlation, the mean VGlut1 and mean PSD95 levels were summed (VGlut1 + PSD95 levels) to index excitatory synaptic strength for each PVI. Panels B and C show frequency distributions (bars) of VGlut1 + PSD95 levels onto each PVI sampled from comparison subjects (panel B) and schizophrenia subjects (panel C). VGlut1 + PSD95 levels onto each PVI sampled from comparison subjects conformed to a normal distribution, whereas those sampled from schizophrenia subjects conformed to a distribution with skewness of 1.58 and kurtosis of 7.57. Panel D shows the cumulative frequency distribution of the coefficient of variation (CV) of VGlut1 + PSD95 levels across PVIs for all subjects (N=40). Panels E and F show relative frequency distributions of excitatory synaptic strength for individual FSIs (gei) in the model network with CVg=0.1, CVg=0.3, and CVg=0.5 generated from either the normal distribution (panel E) or the skewed distribution (panel F) based on empirical data. ḡei=2 for all conditions.
We first assessed how CVg generated from the normal distribution regulates gamma power and frequency. Shifting CVg from 0.1 to 0.5 progressively reduced peak gamma power (Figure 4A) but had a minimal effect on peak gamma frequency (Figure 4B). The effect of CVg on gamma power was observed over a wide range of background noise levels (see Figure S5 in the online supplement), demonstrating that the effect of CVg on gamma power is robust to noise in the model network. Furthermore, CVg generated from the skewed distribution showed differences of similar magnitudes in peak gamma power and frequency (see Figure S6 in the online supplement), suggesting that the effect of CVg is comparable between the distributions found in the comparison and schizophrenia subject groups. Based on these findings, CVg generated from the normal distribution was used for subsequent analyses.
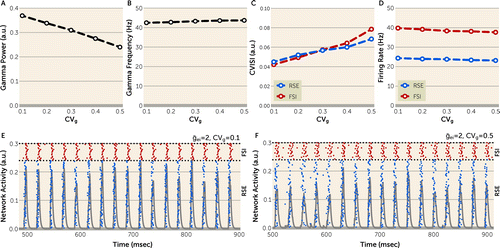
FIGURE 4. Effect of greater CVg on network behavior in pyramidal interneuron gamma (PING) model networka
a In panels A and B, increasing CVg from 0.1 to 0.5 progressively reduces peak gamma power (panel A) without affecting peak gamma frequency (panel B). a.u.=arbitrary units. In panels C and D, increasing CVg from 0.1 to 0.5 increases the coefficient of variation of the interspike interval (CVISI) of regular-spiking excitatory cells (RSEs) and fast-spiking inhibitory cells (FSIs) (panel C) without affecting the firing rates of these cells (panel D), suggesting that greater CVg lowers gamma power by disrupting the synchrony but not the activity of the model network. All data were computed as an average over 200 trials. Panels E and F are representative raster plots (blue dots indicate RSEs, N=80; red dots indicate FSIs, N=20) and network activity (gray) over time for CVg=0.1 (panel E) or 0.5 (panel F). Relative to CVg=0.1, desynchronization (greater horizontal scatter of blue and red dots) is seen at CVg=0.5.
Finally, to investigate the network properties affected by CVg, we assessed the effect of CVg on network synchrony and activity, measured by the coefficient of variation of the interspike interval (CVISI) and the firing rates, respectively, of RSEs and FSIs. Shifting CVg from 0.1 to 0.5 increased the CVISI of RSEs and FSIs (Figure 4C) but had minimal effect on the firing rates of RSEs and FSIs (Figure 4D). Together, these findings demonstrate that CVg regulates gamma power by affecting the synchrony, while minimally affecting the activity, of the model network.
Simulated Interaction Between Greater Synaptic Variability and Other Synaptic Alterations in PVIs
These simulations suggested that lower prefrontal gamma power in schizophrenia could be due in part to greater synaptic variability across PVIs. Previous studies have shown alterations in other synaptic parameters of PVIs that may also contribute to lower prefrontal gamma power in schizophrenia. Thus, we utilized the model network to explore the impact of alterations in these synaptic parameters on gamma power and the interaction of these parameters with synaptic variability in the regulation of gamma power.
We first assessed how our model network simulated the effect of two synaptic alterations in PVIs previously reported in the PFC of subjects with schizophrenia. For example, the mean density of excitatory inputs onto PVIs in the PFC was reported to be 18% lower in schizophrenia (28). Also, decreasing excitatory drive to PVIs was shown to lower cortical gamma power in animal models (17, 49). Thus, we assessed how a lower mean probability of excitatory synapse connectivity on FSIs (Connei) affects gamma power in the model network (Figure 5A). At CVg=0, maximal gamma power occurred at Connei=1. Gamma power progressively declined to very low levels as Connei decreased from 1 to 0.4 and reached a stable nadir at Connei≤0.4. Thus, decreasing Connei in the model network provided proof-of-concept evidence that fewer excitatory inputs to PVIs in schizophrenia could result in lower prefrontal gamma power.
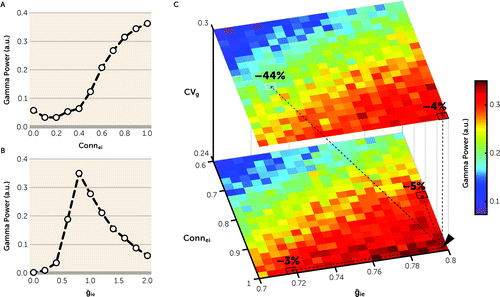
FIGURE 5. Synergistic effect of greater synaptic variability and other synaptic parameters of FSIs on gamma power in the pyramidal interneuron gamma (PING) model networka
a FSIs=fast-spiking inhibitory cells. Panel A is a plot showing the effect of Connei on gamma power. Decreasing Connei from 1 to 0.4 progressively reduces gamma power to very low levels. a.u.=arbitrary units. Panel B is a plot showing the effect of ḡie on gamma power. Peak gamma power is present at ḡie=0.8, with lower and higher levels of ḡie producing lower gamma power. Panel C presents three-dimensional heat maps showing the effects of CVg (z-axis), Connei (y-axis), and ḡie (x-axis) on gamma power. Relative to their initial values at CVg=0.24, Connei=1, and ḡie=0.8 (black arrowhead), increasing CVg to 0.3, decreasing Connei to 0.82, or decreasing ḡie to 0.72 individually (see text for the rationale for each difference) reduces gamma power by 4%, 5%, or 3% (black squares), respectively. Thus, the additive effect of these shifts in these three parameters would be expected to be a 12% reduction in gamma power. However, the model network revealed a 44% reduction in gamma power (white square), demonstrating a synergistic effect of greater CVg, lower Connei, and lower ḡie on gamma power.
Previous studies had reported that mean protein and mRNA levels of the GABA-synthesizing enzyme glutamic acid decarboxylase 67 (GAD67), a marker for GABA conductance, were 10% to 30% lower in the PFC of subjects with schizophrenia (50–53). Also, decreasing GABA conductance to pyramidal neurons was shown to lower cortical gamma power in animal models (54, 55). Thus, we assessed in the model network the effect of lower mean GABA conductance (ḡie) from FSIs to RSEs on gamma power. At CVg=0, maximal gamma power occurred at ḡie=0.8, with ḡie and gamma power forming an inverted U relationship (Figure 5B). Thus, decreasing ḡie from 0.8 (i.e., shift from the peak to the left side of the inverted U) in the model network provided proof-of-concept evidence that lower GABA conductance from PVIs to pyramidal neurons, secondary to less GABA synthesis due to lower GAD67 levels, could result in lower prefrontal gamma power in schizophrenia.
Finally, we assessed whether greater CVg, lower Connei, and lower ḡie interact to regulate gamma power in the model network. In this simulation, we utilized empirical findings from the previous and present postmortem studies of schizophrenia to simulate disease-relevant alterations in each parameter as follows: 1) CVg was increased from 0.24 to 0.30 to reflect the observed greater value of CV of VGlut1 + PSD95 levels across PVIs in schizophrenia relative to unaffected comparison subjects (see Results above); 2) Connei was decreased from 1 to 0.82 to reflect the 18% lower mean density of excitatory inputs to PVIs in schizophrenia (28); and 3) ḡie was decreased from 0.8 to 0.72 to reflect a 10% lower mean level of GAD67 in schizophrenia, which represents the smallest mean difference reported in previous studies (50–53). Simulations showed that these changes in CVg, Connei, and ḡie individually reduced gamma power by 4%, 5%, and 3%, respectively (Figure 5C). Consequently, the additive effect of all these parameter changes would be expected to be a 12% reduction in gamma power. However, when combined in the model network, these differences in CVg, Connei, and ḡie reduced gamma power by 44% (Figure 5C), demonstrating a synergistic interaction among these synaptic parameters. Thus, these simulations suggest that modest alterations in multiple synaptic parameters of PVIs might interact synergistically to produce a substantial reduction in prefrontal gamma power in schizophrenia.
Discussion
The neurobiology of schizophrenia has been conventionally studied by assessing the difference in mean values of isolated components of neural circuits. Here, we combined findings from postmortem human brain studies and computational modeling to demonstrate that the disease process of schizophrenia also involves alterations in the variability of synaptic strength, and that these alterations can interact synergistically with other modest alterations in synaptic parameters to produce marked impairments in physiological properties of neural circuits that are critical for working memory.
Greater Variability in Excitatory Synaptic Strength Across PVIs in Schizophrenia: Empirical Evidence
In this study, we report that variability in a molecular index of excitatory synaptic strength across PVIs is greater in the PFC of individuals with schizophrenia relative to unaffected comparison subjects. Greater variability in schizophrenia was evident in both pre- and postsynaptic markers and was solely due to a higher standard deviation without alterations in the mean values of these markers. Furthermore, greater synaptic variability across PVIs was not attributable to factors commonly associated with schizophrenia and was not influenced by long-term exposure to antipsychotic medications in nonhuman primates. Thus, our findings support the idea that the disease process of schizophrenia includes a greater variability in excitatory synaptic strength across PVIs.
In the primate PFC, PVIs are a major target of excitatory synaptic inputs from neighboring pyramidal neurons in layer 3 (11), where gamma oscillations are most prominent during working memory tasks (56). In contrast, calretinin neurons are much less frequent targets of axons from neighboring pyramidal neurons and are thought to receive excitatory inputs primarily from long-range cortico-cortical projections (11, 44). Furthermore, calretinin neurons appear to be unaffected in schizophrenia (28, 42, 45–47). Consistent with these findings, we did not find evidence of altered variability in excitatory synaptic strength across calretinin neurons in subjects with schizophrenia. Thus, greater synaptic variability across PVIs in schizophrenia may be due to disturbances in their inputs from PFC layer 3 pyramidal neurons, which are known to exhibit both transcriptional and morphological alterations in the illness (57).
Variability in the strength of excitatory inputs to PVIs could arise during development via intrinsic and/or experience-dependent mechanisms. For example, in mouse cortex, the strength of excitatory drive to PVIs differs substantially depending on the timing of their neurogenesis (58, 59), demonstrating an intrinsic mechanism that determines synaptic variability across PVIs during prenatal development. The strength of excitatory drive to distinct subsets of PVIs is also differentially refined during critical or sensitive developmental periods in response to learning or environmental enrichment (59–61), suggesting that experience-dependent synaptic plasticity affects variability in excitatory synaptic strength across these neurons. Thus, developmental alterations in the intrinsic and/or experience-dependent mechanisms that regulate excitatory input strength to PVIs could result in an abnormal distribution of synaptic strengths across these neurons in schizophrenia.
Increasing Variability in Excitatory Synaptic Strength Across PVIs Reduces Gamma Power: Computational Evidence
Based on our findings showing greater variability in excitatory synaptic strength across PVIs in schizophrenia, we used a PING model network to explore whether synaptic variability regulates gamma power. Increasing variability (CVg) progressively lowered gamma power, simulating lower prefrontal gamma power observed during cognitive tasks in persons with schizophrenia (5–7). In addition, greater CVg increased the CVISI of network neurons without affecting their firing rates, similar to previous findings that demonstrated a desynchronizing effect of variability in model networks (20, 25–27). These simulations suggest that greater synaptic variability across PVIs disrupts synchronous neuronal firing in the PFC and could contribute to the lower prefrontal gamma power reported in schizophrenia.
Lower mean density of excitatory inputs to PVIs and lower mean levels of inhibitory strength from PVIs have also been proposed to reduce prefrontal gamma power in schizophrenia (10, 28). Simulating these synaptic alterations (lower Connei and lower ḡie, respectively) resulted in lower gamma power in our model network, similar to previous findings that showed a desynchronizing effect of these alterations (26, 48, 62). Finally, shifts in CVg, Connei, and ḡie comparable to those reported in empirical studies of schizophrenia each resulted in a small reduction in gamma power, but in combination these shifts markedly reduced gamma power. Thus, these simulations provide proof-of-concept evidence that modest alterations in individual synaptic parameters of PVIs reported in postmortem studies could synergistically interact to produce a substantial reduction in prefrontal gamma power in schizophrenia.
Several limitations are important to consider in interpreting the findings of this study. First, previous studies have shown that the effect of variability on network synchrony can be regulated by the strength of gap junctions and shunting inhibition among FSIs (12, 63). Our current model network does not permit the inclusion of parameters for gap junction or shunting inhibition, and thus the effect of synaptic variability reported in our findings might differ in models that include these parameters. Second, our model does not simulate the effect of lower PV levels in the axon terminals of PVIs, which have been reported in the PFC of subjects with schizophrenia (50, 64). PV is a calcium-binding protein that is thought to buffer calcium ions, which regulate the synaptic release of GABA (48). However, because previous studies provided mixed evidence for the effect of lower PV levels on the strength of inhibition from PVIs (65, 66), it is not possible to simulate the effect of lower PV levels at this time. Finally, our model does not simulate the sparsity of excitatory inputs onto pyramidal neurons found in the neocortex (67), but uses all-to-all connectivity with a reduced number of total neurons in the network to decrease the substantial computational demands associated with exploring multiple parameter combinations. However, our previous study showed a comparable effect of network inhibition in the generation of gamma power in networks with sparse or all-to-all connectivity (35).
Prefrontal gamma oscillations are thought to be generated, at least in part, by a local circuit that consists of excitatory pyramidal neurons and inhibitory PVIs in layer 3 (10). In addition to alterations in the PVI component of this circuit, alterations in synaptic inputs to PFC layer 3 pyramidal neurons have also been reported in schizophrenia, which could contribute to lower prefrontal gamma power (68). Given that interactions among synaptic parameters in excitatory and inhibitory neurons can shape the dynamics of neural networks in computational models (69, 70), future studies investigating the interplay of synaptic alterations in pyramidal neurons and PVIs may further inform the nature of the disease process that contributes to altered network properties of PFC circuitry and impaired working memory in schizophrenia.
Conclusions
The present findings suggest several important perspectives on the disease process underlying PFC dysfunction in schizophrenia. First, our empirical findings suggest that schizophrenia is associated with alterations in the variability, even in the absence of differences in the central tendency, of synaptic measures in the PFC. Second, our computational findings suggest that such variability can regulate the physiological properties of cortical circuits. Finally, our computational findings suggest that even modest alterations in different synaptic parameters can, in combination, have a profound effect on gamma power. Thus, our study reveals synaptic variability as an important element of the disease process of schizophrenia and suggests that PFC dysfunction in the illness may emerge from the dynamic interaction of relatively modest alterations in multiple elements of PFC neural circuitry.
1 : Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 2013; 70:1107–1112Crossref, Medline, Google Scholar
2 : Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 2009; 32:209–224Crossref, Medline, Google Scholar
3 : Gamma oscillations correlate with working memory load in humans. Cereb Cortex 2003; 13:1369–1374Crossref, Medline, Google Scholar
4 : Working memory 2.0. Neuron 2018; 100:463–475Crossref, Medline, Google Scholar
5 : Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA 2006; 103:19878–19883Crossref, Medline, Google Scholar
6 : GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin 2014; 4:531–539Crossref, Medline, Google Scholar
7 : Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010; 35:2590–2599Crossref, Medline, Google Scholar
8 : The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull 2011; 37:514–523Crossref, Medline, Google Scholar
9 : Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35:57–67Crossref, Medline, Google Scholar
10 : Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 2015; 77:1031–1040Crossref, Medline, Google Scholar
11 : Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex 2003; 13:452–460Crossref, Medline, Google Scholar
12 : Mechanisms of gamma oscillations. Annu Rev Neurosci 2012; 35:203–225Crossref, Medline, Google Scholar
13 : Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459:663–667Crossref, Medline, Google Scholar
14 : Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009; 459:698–702Crossref, Medline, Google Scholar
15 : Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 2013; 79:1152–1168Crossref, Medline, Google Scholar
16 : NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 2010; 68:557–569Crossref, Medline, Google Scholar
17 : Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 2007; 53:591–604Crossref, Medline, Google Scholar
18 : Functional specificity of local synaptic connections in neocortical networks. Nature 2011; 473:87–91Crossref, Medline, Google Scholar
19 : Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci 2011; 14:1045–1052Crossref, Medline, Google Scholar
20 : The impact of structural heterogeneity on excitation-inhibition balance in cortical networks. Neuron 2016; 92:1106–1121Crossref, Medline, Google Scholar
21 : Robust spatial working memory through homeostatic synaptic scaling in heterogeneous cortical networks. Neuron 2003; 38:473–485Crossref, Medline, Google Scholar
22 : Plasticity of interneuronal species diversity and parameter variance in neurological diseases. Trends Neurosci 2004; 27:504–510Crossref, Medline, Google Scholar
23 : Variability, compensation, and homeostasis in neuron and network function. Nat Rev Neurosci 2006; 7:563–574Crossref, Medline, Google Scholar
24 : Modulation of network behaviour by changes in variance in interneuronal properties. J Physiol 2002; 538:227–251Crossref, Medline, Google Scholar
25 : Effects of noisy drive on rhythms in networks of excitatory and inhibitory neurons. Neural Comput 2005; 17:557–608Crossref, Medline, Google Scholar
26 : Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput 2003; 15:509–538Crossref, Medline, Google Scholar
27 : Minimal size of cell assemblies coordinated by gamma oscillations. PLOS Comput Biol 2012; 8:e1002362Crossref, Medline, Google Scholar
28 : Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am J Psychiatry 2016; 173:1131–1139Link, Google Scholar
29 : Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci 2005; 25:6221–6234Crossref, Medline, Google Scholar
30 : PSD-95 involvement in maturation of excitatory synapses. Science 2000; 290:1364–1368Crossref, Medline, Google Scholar
31 : Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 2013; 16:1068–1076Crossref, Medline, Google Scholar
32 : Cortical interneurons that specialize in disinhibitory control. Nature 2013; 503:521–524Crossref, Medline, Google Scholar
33 : Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Erlbaum Associates, 1988Google Scholar
34 : Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci 2011; 31:142–156Crossref, Medline, Google Scholar
35 : Functional maturation of GABA synapses during postnatal development of the monkey dorsolateral prefrontal cortex. Cereb Cortex 2015; 25:4076–4093Crossref, Medline, Google Scholar
36 : Which model to use for cortical spiking neurons? IEEE Trans Neural Netw 2004; 15:1063–1070Crossref, Medline, Google Scholar
37 : Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 2009; 34:2028–2040Crossref, Medline, Google Scholar
38 : Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol 2010; 588:2823–2838Crossref, Medline, Google Scholar
39 : Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 2013; 16:1032–1041Crossref, Medline, Google Scholar
40 : The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci 2012; 23:97–109Crossref, Medline, Google Scholar
41 : Presynaptic effects of N-methyl-d-aspartate receptors enhance parvalbumin cell-mediated inhibition of pyramidal cells in mouse prefrontal cortex. Biol Psychiatry 2018; 84:460–470Crossref, Medline, Google Scholar
42 : Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 2003; 23:6315–6326Crossref, Medline, Google Scholar
43 : Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 2016; 41:2206–2214Crossref, Medline, Google Scholar
44 : Synaptic targets of the intrinsic axon collaterals of supragranular pyramidal neurons in monkey prefrontal cortex. J Comp Neurol 2001; 430:209–221Crossref, Medline, Google Scholar
45 : Dysregulated ErbB4 splicing in schizophrenia: selective effects on parvalbumin expression. Am J Psychiatry 2016; 173:60–68Link, Google Scholar
46 : Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry 2010; 167:1479–1488Link, Google Scholar
47 : Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 2002; 52:708–715Crossref, Medline, Google Scholar
48 : Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 2011; 31:18137–18148Crossref, Medline, Google Scholar
49 : Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 2015; 85:1257–1272Crossref, Medline, Google Scholar
50 : Altered parvalbumin basket cell terminals in the cortical visuospatial working memory network in schizophrenia. Biol Psychiatry 2021; 90:47–57Crossref, Medline, Google Scholar
51 : Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 1995; 52:258–266Crossref, Medline, Google Scholar
52 : Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57:237–245Crossref, Medline, Google Scholar
53 : Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 2011; 168:921–929Link, Google Scholar
54 : Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front Neural Circuits 2015; 9:6Crossref, Medline, Google Scholar
55 : Decrease of SYNGAP1 in GABAergic cells impairs inhibitory synapse connectivity, synaptic inhibition, and cognitive function. Nat Commun 2016; 7:13340Crossref, Medline, Google Scholar
56 : Laminar recordings in frontal cortex suggest distinct layers for maintenance and control of working memory. Proc Natl Acad Sci USA 2018; 115:1117–1122Crossref, Medline, Google Scholar
57 : Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022; 47:292–308Crossref, Medline, Google Scholar
58 : Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 2013; 504:272–276Crossref, Medline, Google Scholar
59 : Early- and late-born parvalbumin basket cell subpopulations exhibiting distinct regulation and roles in learning. Neuron 2015; 85:770–786Crossref, Medline, Google Scholar
60 : Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 2015; 349:1216–1220Crossref, Medline, Google Scholar
61 : Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 2017; 95:639–655.e10Crossref, Medline, Google Scholar
62 : The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci 2009; 3:33Crossref, Medline, Google Scholar
63 : Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8:45–56Crossref, Medline, Google Scholar
64 : Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 2014; 19:30–36Crossref, Medline, Google Scholar
65 : Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fast-spiking interneurons. PLoS Biol 2010; 8:e1000492Crossref, Medline, Google Scholar
66 : Downregulation of parvalbumin expression in the prefrontal cortex during adolescence causes enduring prefrontal disinhibition in adulthood. Neuropsychopharmacology 2020; 45:1527–1535Crossref, Medline, Google Scholar
67 : Sparse recurrent excitatory connectivity in the microcircuit of the adult mouse and human cortex. eLife 2018; 7:e37349Crossref, Medline, Google Scholar
68 : Dendritic spine pathology in schizophrenia. Neuroscience 2013; 251:90–107Crossref, Medline, Google Scholar
69 : Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 2000; 10:910–923Crossref, Medline, Google Scholar
70 : Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex 2014; 24:859–872Crossref, Medline, Google Scholar



