Age-Associated Deviations of Amygdala Functional Connectivity in Youths With Psychosis Spectrum Disorders: Relevance to Psychotic Symptoms
Abstract
Objective:
The authors created normative growth charts of amygdala functional connectivity in typically developing youths, assessed age-associated deviations of these trajectories in youths with psychosis spectrum disorders, and explored how these disruptions are related to clinical symptomatology.
Methods:
Resting-state functional neuroimaging data from four samples (three cross-sectional, one longitudinal) were collected for 1,062 participants 10–25 years of age (622 typically developing control youths, 194 youths with psychosis spectrum disorders, and 246 youths with other psychopathology). The authors assessed deviations in the psychosis spectrum and other psychopathology groups in age-related changes in resting-state functional MRI amygdala-to-whole brain connectivity from a normative range derived from the control youths. The authors explored relationships between age-associated deviations in amygdala connectivity and positive symptoms in the psychosis spectrum group.
Results:
Normative trajectories demonstrated significant age-related decreases in centromedial amygdala connectivity with distinct regions of the brain. In contrast, the psychosis spectrum group failed to exhibit any significant age-associated changes between the centromedial amygdala and the prefrontal cortices, striatum, occipital cortex, and thalamus (all q values <0.1). Age-associated deviations in centromedial amygdala–striatum and centromedial amygdala–occipital connectivity were unique to the psychosis spectrum group and were not observed in the other psychopathology group. Exploratory analyses revealed that greater age-related deviation in centromedial amygdala–thalamus connectivity was significantly associated with increased severity of positive symptoms (r=0.19; q=0.05) in the psychosis spectrum group.
Conclusions:
Using neurodevelopmental growth charts to identify a lack of normative development of amygdala connectivity in youths with psychosis spectrum disorders may help us better understand the neural basis of affective impairments in psychosis, informing prediction models and interventions.
Affective dysfunction is a prominent feature of psychosis. Affective deficits are present before the onset of the full-blown illness (1, 2), and their severity contributes to improved prediction of psychosis in high-risk samples (3, 4). Psychosis often develops during the transition from adolescence to adulthood, a time when significant specialization and strengthening in cognitive control of affective processes occurs (5). Additionally, adults with psychosis consistently exhibit structural and functional alterations in the amygdala (6–12), a brain structure that plays a key role in affective processes. Connectivity between the amygdala and brain regions supporting multiple cognitive and emotional functions undergo significant changes through adolescence (13–15). Thus, how amygdala connectivity is neurodevelopmentally affected in psychosis is critical to understanding the neural basis of affective impairment in psychosis.
We recently reported (13) that two nuclei in the amygdala, the centromedial and basolateral amygdalae, exhibit differential developmental resting-state connectivity trajectories. The majority of typical developmental decreases occurred in connectivity between the centromedial amygdala and other brain regions (13). Here, we extend these findings by combining different developmental data sources to form a large data set and construct a normative template of age-related changes to be used as a growth chart for the development of amygdala connectivity. The use of growth charts, typically as references for early identification of atypical development for metrics such as weight and head circumference (16), has recently been extended to assess how psychiatric disorders are related to deviations from normative development (17, 18). Multisite sample characterization of typical development of amygdala connectivity will provide a template that can then be used to assess abnormal development of brain function in young people with psychosis spectrum disorders. A growing body of literature has used resting-state functional MRI (fMRI) to identify amygdala connectivity disruptions in adults with psychosis (7, 19–21), and individuals with psychosis are impaired in social-cognitive processes that continue to develop during adolescence (18, 22–24). Thus, characterizing age-associated deviations in amygdala connectivity can inform us about affective dysregulation in psychosis. These age-associated alterations may underlie the development of psychotic symptoms and disruptions in social cognitive processes.
Unique neurodevelopmental trajectories of amygdala connectivity may distinguish psychosis spectrum disorders from other forms of psychopathology. Multiple social cognitive processes that involve the amygdala, including facial affect recognition, emotion regulation, and theory of mind, are more impaired in schizophrenia compared with other psychiatric disorders, providing behavioral support for a differential deficit (25–27). Distinct patterns of amygdala-prefrontal connectivity differentiate individuals with psychosis from those without a psychosis history (19, 21). However, disruption of age-associated amygdala trajectories and their specificity to psychosis spectrum disorders has not been examined. Understanding the timing of disruption in psychosis in comparison to other psychopathology may help us identify individuals who are at greater risk for developing psychosis spectrum disorders.
Our goals in this study were 1) to identify the strongest age-associated changes in centromedial and basolateral amygdala connectivity in typically developing youths across multiple samples, 2) to characterize deviations from normative amygdala connectivity trajectories in psychosis, 3) to determine the specificity of these abnormalities by comparing age-associated amygdala connectivity in youths with other forms of psychopathology, and 4) to test whether age-associated deviations in amygdala connectivity are related to psychotic symptoms. We hypothesized that 1) consistent with our previous research, the strongest age-associated decreases would be observed in centromedial amygdala connectivity in typically developing youths (13); 2) youths with psychosis spectrum disorders would fail to show age-associated decreases in centromedial amygdala–prefrontal connectivity, given that increased amygdala-prefrontal connectivity has previously been associated with psychosis (19, 21); and 3) distinct patterns of age-associated amygdala-prefrontal connectivity would differentiate youths with psychosis spectrum disorders from those with other psychopathologies. Finally, we tested the exploratory hypothesis that age-associated deviations in amygdala connectivity would be associated with positive symptoms.
Methods
Participants
The final neuroimaging data set consisted of 1,062 participants 10–25 years old (typically developing control subjects, N=622; individuals with psychosis spectrum disorders, N=194; individuals with other psychopathology, N=246) from four different samples. Three data sets were acquired at the University of Pittsburgh and one at the University of Pennsylvania (28, 29). Information on the study participants is presented in Table 1. One data set was from a longitudinal sample, and the other three were cross-sectional. (Details on participant recruitment and inclusion and exclusion criteria are provided in the online supplement; see the Supplemental Methods section and Figure S1.)
| Typically Developing Group | Psychosis Spectrum Group | Other Psychopathology Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Range (years) | N | Age (years) | Male/ Female (N/N) | Age (years) | Male/ Female (N/N) | Age (years) | Male/ Female (N/N) | |||||||
| Cohorta | Study Design | Mean | SD | N | Mean | SD | N | Mean | SD | |||||
| Luna 1 | Longitudinal (1–3 visits) | 10–25 | 213 | 16.7 | 3.0 | 113/100 | 0 | 0 | ||||||
| Luna 2 | Cross-sectional | 14–25 | 88 | 19.5 | 3.6 | 45/43 | 0 | 0 | ||||||
| Philadelphia Neurodevelopmental Cohort | Cross-sectional | 10–22 | 292 | 16.2 | 3.4 | 151/141 | 162 | 16.0 | 2.9 | 75/87 | 246 | 16.4 | 3.3 | 96/150 |
| Pitt | Cross-sectional | 12–25 | 29 | 21.0 | 2.7 | 18/11 | 32 | 20.8 | 3.1 | 19/13 | 0 | |||
TABLE 1. Demographic information for all samples in a study of age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders
Clinical Measures
Positive and negative symptoms were measured by summing the relevant Structured Interview for Prodromal Syndromes/PRIME Screen–Revised responses (0=definitely disagree, 1=somewhat disagree, 2=slightly disagree, 3=not sure, 4=slightly agree, 5=somewhat agree, 6=definitely agree). Table S1 in the online supplement lists the included questions.
MR Data Acquisition
For all samples, scanning data were acquired using Siemens 3-T Tim Trio scanners. Resting-state data were collected using an echo-planar sequence sensitive to blood-oxygen-level-dependent contrast (T2*). A magnetization-prepared rapid gradient-echo sequence (MPRAGE) was acquired to measure brain structure and for alignment of the resting-state functional MR images. Table S2 in the online supplement includes scan instructions and parameters; details of resting-state fMRI data processing are provided in the Supplemental Methods section.
Statistical Analyses
Resting-state fMRI first-level statistical analyses.
We conducted voxelwise regressions on processed data using AFNI’s 3dDeconvolve with the average of each amygdala subregion region of interest (centromedial, basolateral) time series as the seed. AFNI’s 3dREMLfit program was applied to correct for temporal autocorrelation between voxels. These analyses resulted in voxelwise subject-level maps of Pearson correlations (r) between the average amygdala subregion region of interest time course and each voxel’s time course. R values were then normalized using the Fisher r-to-z transformation.
Voxelwise developmental changes in amygdala subregion connectivity.
We used the 3dLME program in AFNI to examine voxelwise developmental effects of age for each amygdala subregion in typically developing control youths. 3dLME is a group analysis program that computes linear mixed models (30). Subject was included as a random effect, which allows us to model and account for the nonindependence of data (multiple visits) in the longitudinal cohort (31). Age, sex, and site were included as fixed effects. Linear, inverse, and quadratic forms of age were examined. Results were corrected for multiple comparisons using a combination of cluster size and voxel probability, with parameters determined through a Monte Carlo simulation using AFNI’s 3dClustSim program (see the online supplement for details). This implementation is the most current, most stringent procedure recommended by the AFNI developers to prevent obtaining false positive clusters of connectivity (32, 33).
For clusters for which significant results were obtained across multiple forms of age (linear, inverse, quadratic), we determined a conjunction cluster and extracted the cluster’s mean region of interest for each individual. We reran the mixed-effects models described above with linear, inverse, and quadratic forms of age on these data. The model with the lowest combination of the Akaike information criterion and the Bayesian information criterion was considered to be the best-fitting one.
To ensure that motion artifacts did not drive our results, we extracted the mean region of interest from the significant clusters and reran the linear mixed-effects models, including average framewise displacement as an additional fixed effect. We also reran developmental analyses on subsets of “low-motion” subjects, removing participants with average framewise displacement in the upper 25th percentile (framewise displacement >0.17 mm).
Disruption of age-associated amygdala connectivity in psychosis.
To determine age-associated disruption of amygdala connectivity in youths with psychosis spectrum disorders, we extracted mean regions of interest for clusters that exhibited significant developmental changes in typically developing control youths and ran a linear mixed model with each connectivity measure as the dependent variable. Fixed effects included an interaction term between age (inverse form, and group [typically developing control, psychosis spectrum]), as well as the main effects of both variables. Subject was included as a random effect. Site, sex, and framewise displacement were included as fixed effects. The false discovery rate method was used to correct for multiple comparisons (34). To determine the specificity of age-related deviations in youths with psychosis spectrum disorders in any clusters that exhibited significant age-by-group deviations, we added the other psychopathology group to the model and reran it. All significant interaction terms were further examined with the simple slopes of each group using least-squares means (using the lsmeans package [35]).
To explore whether there were any age-related alterations in the psychosis spectrum or other psychopathology groups that were not observed in the above analysis, we used 3dLME to run the above interaction model using a voxelwise approach (p<0.001, cluster-wise p=0.05, 30 continuous voxels).
Relationships between age-associated deviations in amygdala connectivity and positive symptoms.
We took a two-step, developmentally informed approach to examine relationships between amygdala connectivity and psychotic symptoms. In regions where we observed significant age-related alterations in psychosis, we first conducted analyses to characterize the extent to which age-associated deviations from normal development—independent of the directionality of the connectivity differences—were associated with psychotic symptoms. Using the model of best fit for developmental changes observed for amygdala connectivity in the typically developing control group, we predicted what the expected “normative” amygdala connectivity value would be for the psychosis spectrum group. We then subtracted the predicted value from the actual amygdala connectivity value for each individual and took the absolute value of this score. This created an amygdala connectivity maturation deviation score, a method that has been used previously to identify deviations from normative growth in brain connectivity metrics (17). We computed Pearson correlations between the amygdala brain maturation deviation score and positive symptoms in the psychosis spectrum group. The false discovery rate method was used to correct for multiple comparisons (q<0.1).
In a post hoc analysis, we wanted to determine the direction of any identified relationships and focus our analyses on the discrete developmental periods in which there were significant differences in amygdala connectivity between the psychosis spectrum group and the typically developing control group (see Table S5 in the online supplement). After regressing out the effects of age, sex, and motion covariates on the connectivity measure of interest, we conducted Pearson correlation analyses with the connectivity value and positive symptoms during the developmental period that were significantly different between the psychosis spectrum group and the typically developing control group. We also computed Pearson correlations between the connectivity value and positive symptoms in the developmental period in which amygdala connectivity values were not statistically different from each other.
Results
Typical Age-Associated Development of Amygdala Subregion Connectivity
Developmental effects were observed for functional connectivity between the centromedial amygdala and 19 clusters (Table 2; see also Figure S2 in the online supplement). These clusters included the following bilateral brain regions: posterior cingulate, insula, parahippocampal cortex, and precentral gyrus/frontal eye fields. Significant clusters were also observed for the left ventrolateral prefrontal cortex, left caudate, left dorsolateral prefrontal cortex, right thalamus, and right postcentral gyrus. We observed age-related decreases in connectivity strength between the centromedial amygdala and all clusters, with children exhibiting positive centromedial amygdala connectivity (mean=0.19 at age 10) and adults exhibiting near-zero levels of connectivity (mean=0.04 at age 25).
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cluster | Brain Region | Voxels | x | y | z | χ2 | p | q |
| Centromedial amygdala connectivity | ||||||||
| 1 | Left posterior cingulate/precuneus | 206 | –13 | –61 | 15 | 0.2 | 0.63 | 0.81 |
| 2 | Right posterior cingulate/precuneus | 191 | 24 | –61 | 19 | 0.5 | 0.48 | 0.64 |
| 3 | Left frontal eye fields/BA 6 and precentral gyrus | 186 | –61 | –3 | 35 | 0.1 | 0.75 | 0.83 |
| 4 | Right frontal eye fields/BA 6 and precentral gyrus | 162 | 52 | –10 | 38 | 0.1 | 0.75 | 0.83 |
| 5 | Right insula/claustrum | 147 | 36 | –10 | 10 | 1.4 | 0.24 | 0.37 |
| 6 | Left insula/claustrum | 121 | –33 | –15 | –2 | 1.0 | 0.33 | 0.35 |
| 7 | Left parietal cortex/middle temporal gyrus | 101 | –52 | –70 | 24 | 0.0 | 0.92 | 0.64 |
| 8 | Right parahippocampal gyrus | 99 | 26 | –33 | –15 | 0.0 | 0.83 | 0.98 |
| 9 | Left parahippocampal gyrus | 97 | –20 | –47 | –8 | 0.0 | 0.83 | 0.89 |
| 10 | Right precentral/postcentral gyrus | 85 | 45 | –21 | 58 | 0.0 | 0.95 | 0.98 |
| 11 | Left ventrolateral prefrontal cortex | 70 | –31 | 29 | –2 | 7.5 | 0.006 | 0.04 |
| 12 | Left putamen | 61 | –22 | 2 | 8 | 8.2 | 0.004 | 0.04 |
| 13 | Left BA 10/superior frontal gyrus | 57 | –13 | 71 | 1 | 2.6 | 0.11 | 0.23 |
| 14 | Right thalamus | 57 | 20 | –33 | 10 | 6.7 | 0.009 | 0.05 |
| 15 | Right insula | 51 | 38 | –28 | 15 | 3.6 | 0.05 | 0.15 |
| 16 | Left caudate | 43 | –15 | 18 | 3 | 8.5 | 0.004 | 0.04 |
| 17 | Left dorsolateral prefrontal cortex/BA 9 | 40 | –20 | 41 | 38 | 4.1 | 0.04 | 0.09 |
| 18 | Left parahippocampal gyrus | 39 | –29 | –38 | –11 | 3.1 | 0.08 | 0.20 |
| 19 | Right middle occipital cortex | 36 | 47 | –77 | 1 | 5.4 | 0.02 | 0.05 |
| Basolateral amygdala connectivity | ||||||||
| 1 | Left uncus | 33 | –24 | –3 | –34 | 0.9 | 0.340 | 0.35 |
TABLE 2. Clusters and associated brain regions that exhibited significant age-associated changes in typically developing youthsa
Developmental effects were also observed for functional connectivity between the basolateral amygdala and one cluster, which encompassed the left uncus (Table 2). In this case, children exhibited positive centromedial amygdala connectivity (mean=0.27 at age 10), and adults exhibited positive connectivity as well, albeit to a lesser extent (mean=0.17 at age 25).
For all significant clusters, the inverse form of age was the best fit. All developmental effects remained significant when motion covariates (average framewise displacement) and MRI software version were included in the model and when high-motion subjects were excluded from the analysis (see Tables S3–S4 in the online supplement). Strikingly, age-associated changes were consistent across sites (see Figure S3 in the online supplement).
We also confirmed that site effects were appropriately accounted for by including the measure as a covariate. After regressing out the effects of site in each region, we then plotted the residuals (see Figure S4 in the online supplement). The residuals all clustered around zero, which provides evidence that we were able to effectively account for site in our model. Furthermore, when we conducted a mixed-model linear regression to compare residuals between sites, there were no significant differences between sites for any of the regions of interest (all χ2=0, p=1), further solidifying evidence that region-of-interest values were similar across sites once site was included in the model.
Age-Associated Disruptions in Amygdala Connectivity in Youths With Psychosis Spectrum Disorders
After correcting for multiple comparisons, significant age-associated deviations (inverse age-by-group interactions) were observed in the psychosis spectrum group for connectivity between the centromedial amygdala and six clusters in the following brain regions: left ventrolateral prefrontal cortex, right thalamus, left dorsolateral prefrontal cortex, left caudate, left putamen, and right middle occipital gyrus (Figure 1A). In five clusters (ventrolateral prefrontal cortex, thalamus, caudate, putamen, and middle occipital gyrus), slope comparison analyses revealed that the typically developing control group exhibited significant age-related decreases with increasing age, while the psychosis spectrum group failed to exhibit significant age-associated changes (see Table S5 in the online supplement). Contrasts revealed that during late childhood the psychosis spectrum group, in comparison to the typically developing control group, exhibited significantly lower connectivity in the following pairs: centromedial amygdala–ventrolateral prefrontal, centromedial amygdala–putamen, and centromedial amygdala–caudate. In adulthood, the psychosis spectrum group exhibited higher connectivity in comparison to the typically developing control group in centromedial amygdala–ventrolateral prefrontal cortex connectivity, centromedial amygdala–putamen connectivity, and centromedial amygdala–occipital cortex connectivity. These results, illustrated in Figure 1A, reflect a lack of developmental decreases in psychosis emerging from underconnectivity in childhood. For connectivity between the centromedial amygdala and dorsolateral prefrontal cortex, the psychosis spectrum group exhibited higher connectivity during late childhood and early adolescence in comparison to the control group. This significant difference was no longer observed in adulthood. Specific time periods in which group differences were observed are presented in Table S5. All significant age-by-group interactions remained when psychiatric medication status was included as a covariate. Amount of variance explained by the full model, the inverse age-by-group interactions, and the main effects of inverse age and group are reported in Table S7 in the online supplement.
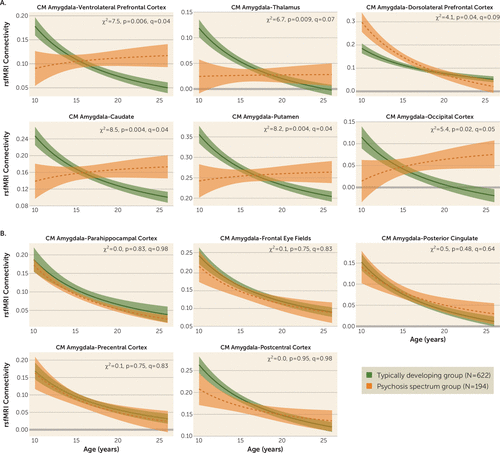
FIGURE 1. Age-associated resting-state fMRI connectivity between the amygdala and multiple brain regions in typically developing youths and youths with psychosis spectrum disordersa
a In panel A, in comparison to typically developing youths, youths with psychosis spectrum disorders exhibited significant deviations from typical centromedial amygdala connectivity development in the following regions: ventrolateral prefrontal cortex, thalamus, dorsolateral prefrontal cortex, caudate, putamen, and middle occipital gyrus. In panel B, youths with psychosis spectrum disorders and typically developing youths exhibited similar patterns of developmental decreases in connectivity between the centromedial amygdala and the parahippocampal cortex, frontal eye fields, and posterior cingulate, precentral, and postcentral cortices. CM=centromedial; rsfMRI=resting-state functional MRI.
Similar to the typically developing control group, the psychosis spectrum group exhibited a decline in centromedial amygdala connectivity with increases in age for clusters in the following regions: parahippocampal cortex, frontal eye fields, posterior cingulate cortex, precentral cortex, and postcentral cortex (Figure 1B).
Specificity of Age-Associated Disruptions in Amygdala Connectivity in Youths With Psychosis Spectrum Disorders
When youths with other psychopathology were added to the model, all five models maintained the significant inverse age-by-group interactions (see Table S6 in the online supplement). Like the typically developing control group, the other psychopathology group showed typical significant age-related decreases with increasing age in connectivity between the centromedial amygdala and three regions: the putamen, caudate, and occipital cortex (see Figures S5 and S6 and Table S7 in the online supplement). However, like the psychosis spectrum group, the other psychopathology group failed to show age-associated changes in centromedial amygdala–ventrolateral prefrontal cortex connectivity and centromedial amygdala–thalamus connectivity, although there were no significant inverse age-by-group interactions between the typically developing control group and the other psychopathology group (p>0.07). Furthermore, the typically developing and other psychopathology groups did not differ in amygdala connectivity levels at any point in development (see Table S7). As seen in Figure 2A–C, the developmental trajectories of centromedial amygdala–ventrolateral prefrontal cortex connectivity, centromedial amygdala–dorsolateral prefrontal cortex connectivity, and centromedial amygdala–thalamus connectivity for the other psychopathology group fell between the trajectories of the typically developing and psychosis spectrum groups during late childhood and early adolescence. In comparison to the other psychopathology group, the psychosis spectrum group exhibited reduced connectivity during late childhood and early adolescence in the following connectivity pairs: centromedial amygdala–putamen (Figure 2D) and centromedial amygdala–occipital cortex (Figure 2F).
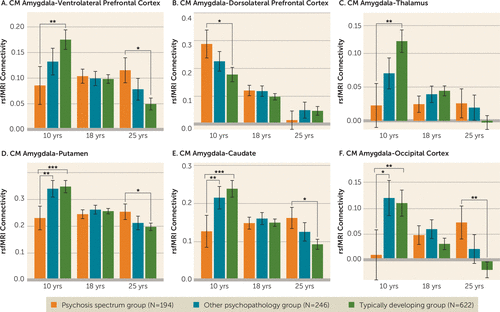
FIGURE 2. Age-associated alterations in centromedial amygdala connectivity in youths with psychosis spectrum disorders and youths with other psychopathology during late childhood, late adolescence, and early adulthooda
a For visualization purposes of inverse age-by-group interactions of centromedial amygdala connectivity, we calculated least-squares means for amygdala connectivity values at ages 10, 18, and 25 for typically developing youths, youths with psychosis spectrum disorders, and youths with other psychopathology. In panel A, in comparison to the typically developing group, the psychosis spectrum group exhibited reduced centromedial amygdala–ventrolateral prefrontal connectivity during late childhood/early adolescence and increased connectivity during adulthood. In panel B, in comparison to the typically developing group, the psychosis spectrum group exhibited increased centromedial amygdala–dorsolateral prefrontal connectivity during late childhood/early adolescence. In panel C, in comparison to the typically developing group, the psychosis spectrum group exhibited reduced centromedial amygdala–thalamus connectivity during late childhood/early adolescence. In panel D, in comparison to the typically developing group and the other psychopathology group, the psychosis spectrum group exhibited reduced centromedial amygdala–putamen connectivity during late childhood/early adolescence. In comparison to typically developing youths, the psychosis spectrum group exhibited greater connectivity between these two regions during adulthood. In panel E, a similar pattern of developmental disruption was observed in centromedial amygdala–caudate connectivity. In panel F, the psychosis spectrum group exhibited reduced centromedial amygdala–occipital cortex connectivity when compared with the typically developing group and with the other psychopathology group in childhood. In adulthood, the psychosis spectrum group exhibited increased amygdala–occipital cortex connectivity in comparison to typically developing youths. CM=centromedial; rsfMRI=resting-state functional MRI.
*p<0.05. **p<0.01. ***p<0.005.
Confirmatory analyses revealed that there were no significant interactions of inverse age by group by sex, inverse age by group by site, sex by group, or group by site in the psychosis spectrum and typically developing groups in these regions. Exploratory voxelwise analyses of age-by-group interactions failed to find any other significant clusters.
Association of Age-Related Deviation in Centromedial Amygdala–Thalamus Connectivity With Increased Positive Symptoms
After calculating brain maturation deviation scores, we found that greater age-related deviation in centromedial amygdala–thalamus connectivity was associated with greater severity of positive symptoms in the psychosis spectrum group (r=0.19, p=0.01, q=0.05) (Figure 3). Post hoc analyses revealed that increased severity of grandiose ideas (r=0.31, p<0.001) and hallucinations (r=0.23, p=0.003) were related to centromedial amygdala–thalamus age-associated deviation, but not unusual thought content (r=0.08, p=0.32). This relationship was not present in the other psychopathology group (centromedial amygdala–thalamus: r=−0.02, p=0.89). This relationship was not observed between the centromedial amygdala–thalamus brain maturation deviation score and negative symptoms (r=0.02, p=0.8), depressive (r=−0.06, p=0.42), or manic symptoms (r=−0.01, p=0.93).
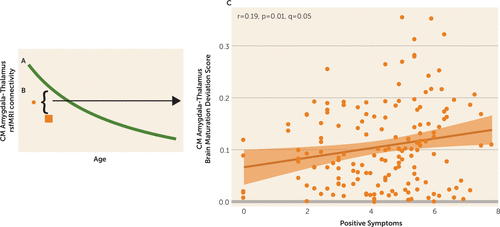
FIGURE 3. Age-associated brain maturation deviation scores in youths with psychosis spectrum disordersa
a Age-associated brain maturation deviation scores were calculated by determining the developmental line of best fit for amygdala connectivity in typically developing youths (letter A in the figure), subtracting the individual score of a participant in the psychosis spectrum group from the line of best fit (letter B in the figure), taking the absolute value of this measure, and calculating Pearson correlations between the brain maturation deviation scores. Greater deviations from centromedial amygdala-thalamus connectivity are associated with increased positive symptoms in the psychosis spectrum group (letter C in the figure). CM=centromedial; rsfMRI=resting-state functional MRI.
We next characterized where in the developmental trajectory amygdala connectivity related to positive symptoms. During late childhood and early adolescence, lower amygdala-thalamus connectivity was associated with greater severity of positive symptoms (r=−0.27, p=0.01, q=0.05).
Discussion
We examined age-associated disruptions in amygdala functional connectivity in youths with psychosis spectrum disorders and explored how alterations in neurodevelopmental connectivity may be related to psychotic symptoms. First, we developed normative amygdala connectivity growth charts in typically developing youths and verified that the strongest age-associated changes occur in connectivity between the centromedial amygdala and multiple brain regions, with decreases in connectivity occurring as age increased, confirming our previous results (13). Next, we showed that the psychosis spectrum group failed to show typical age-associated decreases in connectivity between the centromedial amygdala and these distinct brain regions: the striatum, thalamus, lateral prefrontal cortex, and occipital cortex. Age-associated alterations in centromedial amygdala–putamen connectivity and centromedial amygdala–occipital cortex connectivity were unique to the psychosis spectrum group; youths in the other psychopathology group did not exhibit these age-associated deviations. Exploratory analyses revealed that greater age-related deviations in centromedial amygdala–thalamus functional maturation were associated with greater positive symptoms in the psychosis spectrum group. Our results provide a novel view of developmental alterations in functional connectivity in psychosis spectrum disorders, implicating alterations during discrete developmental windows within neural circuits implicated in a wide array of cognitive and emotional processes.
Developmental Alterations in Centromedial Amygdala Connectivity in Youths With Psychosis Spectrum Disorders
In line with our previous findings, significant typical developmental functional connectivity decreases occurred between the centromedial amygdala and multiple brain regions (13). These results are consistent with previous developmental neuroimaging resting-state fMRI studies reporting decreases in subcortical-cortical connectivity into adulthood (36–39). In comparison to typically developing youths, those with psychosis spectrum disorders exhibited reduced connectivity between the centromedial amygdala and the ventrolateral prefrontal cortex, striatum, thalamus, and occipital cortex during late childhood and early adolescence, with a lack of normative decreases from adolescence to adulthood. These findings suggest either that there is an accelerated developmental decrease in amygdala connectivity in psychosis preceding the normative timetable or that the earlier age at onset reflects deterioration of this circuitry, as is evident later in adulthood. Normatively, decreases in connectivity can be seen as a period of specialization that occurs at a critical time when higher-level systems are becoming established to form adult trajectories. The lack of a marker of specialization through adolescence could reflect impairments in optimal specialization that could contribute to abnormal processing of executive affective information processing in psychosis.
Many of the regions that exhibited disrupted age-associated amygdala connectivity in the psychosis spectrum group are related to perception and salience (e.g., thalamus, striatum, and occipital cortex [40–47]). A primary function of the amygdala is to determine what is salient in one’s environment and to facilitate learning for these items (48–50). Projections of the amygdala to the thalamus are thought to modulate attentional orientation and arousal, broadly speaking (51). Projections of the amygdala to the visual cortex are known to enhance sensitivity, discrimination, and subjective vividness of perceived stimuli (52). Finally, projections of the amygdala to the striatum are thought to provide an interface between the amygdala and dopamine systems, which are known to regulate motivation, learning, and behavioral activation (53). Thus, abnormal connectivity of the amygdala with the thalamus, visual cortex, and striatum could reflect processes that result in the misattribution of salience to stimuli in psychosis, as well as a facilitation of learning associations about threat-related items. Critically, this type of aberrant salience processing may provide the foundation for higher-order features of positive symptoms, such as delusions (54).
Connections between the centromedial amygdala and lateral prefrontal regions also exhibited a disruption in age-associated changes in the psychosis spectrum group. During late childhood and early adolescence, the psychosis spectrum group exhibited reduced amygdala–ventrolateral prefrontal cortex connectivity; however, during adulthood the psychosis spectrum group exhibited increased connectivity between these two regions. Amygdala–ventrolateral prefrontal cortex connectivity is necessary during the reappraisal phase of regulating one’s emotions (55–59), and how these two structures interact during emotion regulation changes during adolescent development (60). Indeed, impairments in lateral prefrontal–mediated circuitry are related to emotional deficits typically observed in psychosis (61–63) and age-associated amygdala–ventrolateral prefrontal cortex functional connectivity alterations during an affective labeling task have been observed in youths at clinical high risk for developing psychosis (64). Thus, centromedial amygdala–ventrolateral prefrontal cortex age-associated disruptions may underlie the emotional dysregulation that often precedes and predicts increased psychotic symptoms (65–67). Studies using experience sampling have shown that adults with schizophrenia report more intense negative emotions and greater social stress than healthy control subjects (68–70). The heightened amygdala–ventrolateral prefrontal cortex connectivity we observed in adults experiencing psychosis spectrum symptoms may reflect underlying biological vulnerability to psychosis onset, which interacts with these environmental stressors. Studies integrating experience sampling methods (71) with neuroimaging in youths at high risk for developing psychosis are necessary to test this hypothesis.
We also observed a unique, altered age-associated pattern of centromedial amygdala–dorsolateral prefrontal cortex connectivity in the psychosis spectrum group compared with the typically developing control group. During late childhood and early adolescence, the psychosis spectrum group exhibited increased centromedial amygdala–dorsolateral prefrontal cortex coupling compared with the typically developing group. This group difference was no longer present in adulthood, with both the typically developing and the psychosis spectrum groups exhibiting similar levels of connectivity. Previously, in adults with schizophrenia, absent or reduced amygdala–dorsolateral prefrontal cortex functional connectivity has been observed during emotional distraction during a working memory task (72) and at rest (7, 21). Our developmentally sensitive results of increased centromedial amygdala–dorsolateral prefrontal cortex connectivity in the psychosis spectrum group during late childhood and early adolescence contrast with these findings and highlight the importance of examining how developmental stage may affect the directionality and interpretation of brain connectivity (73). Reduced GABA levels are consistently observed in the prefrontal cortex in schizophrenia. While GABA-ergic deficits have not been identified in the amygdala in schizophrenia, the majority of neurons projecting from the centromedial amygdala are GABA-ergic (74). Altered connections may generate down-regulation of GABA interneuron activity in the prefrontal cortex, resulting in a lack of inhibition, which may be responsible for the increased connectivity observed between the amygdala and dorsolateral prefrontal cortex during late childhood and early adolescence in youths with psychosis spectrum disorders.
In summary, we identified developmentally sensitive alterations in cortico-limbic and intralimbic resting-state fMRI connectivity in youths with psychosis spectrum disorders. These neurodevelopmental alterations provide support for multiple theories associated with schizophrenia and complement the growing body of literature that shows progressive maturational disturbances in those who go on to develop psychosis (75–78).
Specificity of Age-Associated Amygdala Connectivity Alterations to Psychosis Spectrum Disorders
Two age-associated amygdala connectivity alterations were distinct to youths with psychosis spectrum disorders. In comparison to both the typically developing and other psychopathology groups, the psychosis spectrum group exhibited reduced connectivity during late childhood and early adolescence in two connectivity pairs: centromedial amygdala–putamen and centromedial amygdala–occipital cortex. Although psychotic symptoms rarely separate clinical samples into discrete groups, our results suggest that these brain abnormalities are unique to youths with psychosis spectrum disorders and, in the future, could potentially differentiate psychotic disorders from other psychiatric disorders. Alternatively, in other amygdala connectivity measures, the developmental trajectories of the other psychopathology group fell in between those of the typically developing and psychosis spectrum groups. These findings suggest that there is a less severe neurobiological impact on the other psychopathology group in these connectivity metrics.
Amygdala-Thalamus Brain Maturation Deviations and Positive Symptoms
The growth charting methods employed in this study establish a novel connection between deviations from amygdala-thalamus connectivity development and increased positive symptom severity. In our exploratory analyses, we found that deviation from the normative trajectory of neurodevelopment is relevant to positive symptoms. Although this relationship was statistically significant after multiple comparisons, it is a small effect and must be replicated in future studies. These findings, along with others (17, 18, 73), highlight the importance of examining the role that (dys)maturation patterns play in the development of psychiatric disorders. While small effect sizes may not indicate a direct intervention, it is important to identify these deviations to fully characterize the pathophysiology of psychosis risk.
Use of “Big Data” to Create Neurodevelopmental Growth Charts
This study also represents a proof-of-principle approach for merging multiple resting-state fMRI data sets to inform normative developmental trajectories and identify aberrant trajectories in youths with psychosis spectrum disorders. Despite the samples having multiple sites, protocols, and recruitment methods, the age-associated changes were remarkably consistent across the different samples of typically developing youths (see Figure S3), and including sample as a covariate effectively removed any site differences (see Figure S4). In conjunction with recent work (10, 17, 79–81), these results support using publicly available data to assess developmental changes in brain function and relevance to psychiatric disorders. Given that age-associated changes can be small but significant, an approach that takes advantage of large sample sizes will be necessary for identifying distinct periods of development in which there are disruptions related to psychiatric disorders or symptoms.
Limitations
Our study was limited by the fact that cross-sectional data were available only for the psychosis spectrum group. Thus, the neurodevelopmental trajectories in psychosis spectrum disorders do not reflect within-person change. Our cross-sectional sample cannot definitively show whether our results are due to altered development or abnormalities due to time of onset of psychosis. Thus, longitudinal studies of youths with psychosis spectrum disorders, with multiple visits per individual, can extend our understanding of psychosis by identifying how the shape and rate of maturation of subject-specific developmental trajectories in youths with psychosis spectrum disorders diverge, converge, or remain stable in comparison to typical development (82–84). Furthermore, many developmental changes that occur during adolescence are nonlinear, and these patterns are most accurately captured with longitudinal analyses (85). Additionally, psychotic symptoms are dynamic and change over time (86, 87), and these changes need to be taken into account when characterizing neurodevelopmental change. Recently, using novel time-varying analytic approaches (88, 89), we found that connectivity measures were differentially related to individual differences in anxiety and depression at different points in adolescent development (13). A similar approach could be applied to longitudinal neuroimaging and psychotic symptom data, to identify particular periods of development in which psychic symptoms are linked to resting-state fMRI connectivity metrics.
Finally, while we are fairly confident that we were able to appropriately account for site in our analyses (see Figure S4), we observed a statistically significant effect of site in many of the regions of interest (see Table S4). Despite all scans being performed on the same scanner model, there were still differences in task instructions, MRI resolution, and duration of scan. Site effects may have obscured our ability to identify smaller developmental changes in normative amygdala connectivity developmental trajectories. It is also possible that we failed to identify more subtle age-related deviations in amygdala connectivity between the psychosis spectrum and typically developing groups because of site effects. Recently, methodology from genetics has been used to harmonize structural MRI data across sites (90, 91); modifying and applying this method to resting-state connectivity data is a logical next step. Despite these site differences, we still see a significant interaction between group and age; these findings suggest that multisite neuroimaging data sets will be important for understanding how biomarkers may be sensitive or specific to developmental stage.
Conclusions
Taken together, our results provide compelling novel evidence for developmental disruptions of age-associated trajectories of amygdala connectivity in psychosis, specific to circuitry underlying salience and cognitive control of affect. Notably, these disruptions are present during late childhood and suggest a subsequent lack of normative refinement. We hope to build on these findings and examine how metrics of affective brain dysmaturation may serve as predictors for identification of youths at risk for developing psychosis and other severe psychiatric disorders and impairments in functioning. In addition, our approach adds to the relevance of using “big data” to establish a growth chart to discern impairment and its potential to inform clinical trajectories.
1 : Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry 1993; 150:1654–1660Link, Google Scholar
2 : Childhood and young adult predictors of schizophrenic outcome, in Origins of Psychopathology: Problems in Research and Public Policy. Edited by Ricks D, Dohrenwend B. Cambridge, UK, Cambridge University Press, 1983, pp 129–153Google Scholar
3 : Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med 2015; 45:2959–2973Crossref, Medline, Google Scholar
4 : Emotion recognition as a predictor of transition to a psychotic disorder in ultra-high risk participants. Schizophr Res 2014; 153:25–31Crossref, Medline, Google Scholar
5 : An integrative model of the maturation of cognitive control. Annu Rev Neurosci 2015; 38:151–170Crossref, Medline, Google Scholar
6 : Altered amygdala connectivity within the social brain in schizophrenia. Schizophr Bull 2014; 40:152–160Crossref, Medline, Google Scholar
7 : Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull 2014; 40:1105–1116Crossref, Medline, Google Scholar
8 : Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 2006; 63:139–149Crossref, Medline, Google Scholar
9 : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21:547–553Crossref, Medline, Google Scholar
10 : Functional neuroimaging abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 2015; 72:456–465Crossref, Medline, Google Scholar
11 : A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res 2012; 141:8–14Crossref, Medline, Google Scholar
12 : Disconnection between amygdala and medial prefrontal cortex in psychotic disorders. Schizophr Bull 2016; 42:1056–1067Crossref, Medline, Google Scholar
13 : Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry 2017; 82:511–521Crossref, Medline, Google Scholar
14 : Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci USA 2012; 109:7941–7946Crossref, Medline, Google Scholar
15 : The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 2014; 95:193–207Crossref, Medline, Google Scholar
16 : The development of growth references and growth charts. Ann Hum Biol 2012; 39:382–394Crossref, Medline, Google Scholar
17 : Growth charting of brain connectivity networks and the identification of attention impairment in youth. JAMA Psychiatry 2016; 73:481–489Crossref, Medline, Google Scholar
18 : Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 2014; 71:366–374Crossref, Medline, Google Scholar
19 : Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 2013; 73:565–573Crossref, Medline, Google Scholar
20 : Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 2011; 36:2009–2017Crossref, Medline, Google Scholar
21 : Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr Bull 2014; 40:469–477Crossref, Medline, Google Scholar
22 : Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull 2010; 36:1009–1019Crossref, Medline, Google Scholar
23 : Emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. J Abnorm Psychol 2008; 117:473–478Crossref, Medline, Google Scholar
24 : Empathetic perspective-taking is impaired in schizophrenia: evidence from a study of emotion attribution and theory of mind. Cogn Neuropsychiatry 2006; 11:133–155Crossref, Medline, Google Scholar
25 : Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry 2013; 170:334–341Link, Google Scholar
26 : More pronounced deficits in facial emotion recognition for schizophrenia than bipolar disorder. Compr Psychiatry 2013; 54:388–397Crossref, Medline, Google Scholar
27 : Adaptive associations between social cognition and emotion regulation are absent in schizophrenia and bipolar disorder. Front Psychol 2013; 3:607Crossref, Medline, Google Scholar
28 : The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry 2015; 56:1356–1369Crossref, Medline, Google Scholar
29 : Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 2014; 86:544–553Crossref, Medline, Google Scholar
30 Bates D, Maechler M, Bolker B, et al: lme4: Linear mixed-effects models using Eigen and S4 [Internet]. R package version 2014 (http://keziamanlove.com/wp-content/uploads/2015/04/StatsInRTutorial.pdf)Google Scholar
31 : Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, Oxford University Press, 2003Crossref, Google Scholar
32 : fMRI clustering in AFNI: false-positive rates redux. Brain Connect 2017; 7:152–171Crossref, Medline, Google Scholar
33 : Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113:7900–7905Crossref, Medline, Google Scholar
34 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57:289–300Google Scholar
35 : Least-squares means: the R package lsmeans. J Stat Softw 2016; 69:1–33Crossref, Google Scholar
36 : Development of large-scale functional brain networks in children. PLoS Biol 2009; 7:e1000157Crossref, Medline, Google Scholar
37 : Prediction of individual brain maturity using fMRI. Science 2010; 329:1358–1361Crossref, Medline, Google Scholar
38 : Age related changes in striatal resting state functional connectivity in autism. Front Hum Neurosci 2013; 7:814Crossref, Medline, Google Scholar
39 : Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage 2015; 115:235–244Crossref, Medline, Google Scholar
40 : A saliency map in primary visual cortex. Trends Cogn Sci 2002; 6:9–16Crossref, Medline, Google Scholar
41 : Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol 1989; 74:458–462Crossref, Medline, Google Scholar
42 : Valence and salience contribute to nucleus accumbens activation. Neuroimage 2008; 39:538–547Crossref, Medline, Google Scholar
43 : Human striatal responses to monetary reward depend on saliency. Neuron 2004; 42:509–517Crossref, Medline, Google Scholar
44 : Human striatal response to salient nonrewarding stimuli. J Neurosci 2003; 23:8092–8097Crossref, Medline, Google Scholar
45 : Neural responses to salient visual stimuli. Proc Biol Sci 1997; 264:769–775Crossref, Medline, Google Scholar
46 : Gain control in the visual thalamus during perception and cognition. Curr Opin Neurobiol 2009; 19:408–414Crossref, Medline, Google Scholar
47 : The integrative role of the intralaminar system of the thalamus in visual orientation and perception in the cat. Exp Brain Res 1976; 25:231–246Crossref, Medline, Google Scholar
48 : Evidence for a general face salience signal in human amygdala. Neuroimage 2011; 54:3111–3116Crossref, Medline, Google Scholar
49 : Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci 2012; 21:54–59Crossref, Google Scholar
50 : Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 2001; 411:305–309Crossref, Medline, Google Scholar
51 : Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 2014; 37:304–314Crossref, Medline, Google Scholar
52 : Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci 2010; 11:773–783Crossref, Medline, Google Scholar
53 : Beyond the classic VTA: extended amygdala projections to DA-striatal paths in the primate. Neuropsychopharmacology 2017; 42:1563–1576Crossref, Medline, Google Scholar
54 : Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23Link, Google Scholar
55 : Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 2011; 58:275–285Crossref, Medline, Google Scholar
56 : Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 2014; 24:2981–2990Crossref, Medline, Google Scholar
57 : For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004; 23:483–499Crossref, Medline, Google Scholar
58 : Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Soc Cogn Affect Neurosci 2015; 10:172–179Crossref, Medline, Google Scholar
59 : Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 2012; 1251:E1–E24Crossref, Medline, Google Scholar
60 : vlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex 2017; 27:3502–3514Medline, Google Scholar
61 : Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J Abnorm Psychol 2014; 123:190–204Crossref, Medline, Google Scholar
62 : Adults with high social anhedonia have altered neural connectivity with ventral lateral prefrontal cortex when processing positive social signals. Front Hum Neurosci 2015; 9:469Crossref, Medline, Google Scholar
63 : Lateral prefrontal cortex activity during cognitive control of emotion predicts response to social stress in schizophrenia. Neuroimage Clin 2014; 6:43–53Crossref, Medline, Google Scholar
64 : Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res 2012; 134:1–9Crossref, Medline, Google Scholar
65 : Negative cognition, depressed mood, and paranoia: a longitudinal pathway analysis using structural equation modeling. Schizophr Bull 2012; 38:1063–1073Crossref, Medline, Google Scholar
66 : Concomitants of paranoia in the general population. Psychol Med 2011; 41:923–936Crossref, Medline, Google Scholar
67 : The prevalence and clinical associations of mood instability in adults living in England: results from the Adult Psychiatric Morbidity Survey 2007. Psychiatry Res 2013; 205:262–268Crossref, Medline, Google Scholar
68 : Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatr Scand 2003; 107:124–131Crossref, Medline, Google Scholar
69 : Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry 2001; 58:1137–1144Crossref, Medline, Google Scholar
70 : Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophr Bull 2000; 26:847–854Crossref, Medline, Google Scholar
71 : A mobile health platform for clinical monitoring in early psychosis: implementation in community-based outpatient early psychosis care. JMIR Ment Health 2018; 5:e15Crossref, Medline, Google Scholar
72 : Negative and nonemotional interference with visual working memory in schizophrenia. Biol Psychiatry 2011; 70:1159–1168Crossref, Medline, Google Scholar
73 : Unraveling the miswired connectome: a developmental perspective. Neuron 2014; 83:1335–1353Crossref, Medline, Google Scholar
74 : The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83:803–834Crossref, Medline, Google Scholar
75 : Progressive brain structural changes mapped as psychosis develops in “at risk” individuals. Schizophr Res 2009; 108:85–92Crossref, Medline, Google Scholar
76 : Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry 2015; 77:147–157Crossref, Medline, Google Scholar
77 : Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361:281–288Crossref, Medline, Google Scholar
78 : Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage 2005; 25:1023–1030Crossref, Medline, Google Scholar
79 : Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 2016; 73:515–524Crossref, Medline, Google Scholar
80 : Association of heritable cognitive ability and psychopathology with white matter properties in children and adolescents. JAMA Psychiatry 2018; 75:287–295Crossref, Medline, Google Scholar
81 : Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 2018; 17:67–75Crossref, Medline, Google Scholar
82 : Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci 2013; 33:18109–18124Crossref, Medline, Google Scholar
83 : Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage 2014; 92:356–368Crossref, Medline, Google Scholar
84 Montez DF, Calabro FJ, Luna B: The expression of established cognitive brain states stabilizes with working memory development [Internet]. Elife 2017; https://elifesciences.org/articles/25606Google Scholar
85 : Nonlinear growth curves in developmental research. Child Dev 2011; 82:1357–1371Crossref, Medline, Google Scholar
86 : Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two-year follow-up. World Psychiatry 2017; 16:62–76Crossref, Medline, Google Scholar
87 : Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr Bull 2012; 38:1225–1233Crossref, Medline, Google Scholar
88 : A time-varying effect model for intensive longitudinal data. Psychol Methods 2012; 17:61–77Crossref, Medline, Google Scholar
89 : Modeling nonlinear time-dependent treatment effects: an application of the generalized time-varying effect model (TVEM). J Consult Clin Psychol 2014; 82:760–772Crossref, Medline, Google Scholar
90 : Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017; 161:149–170Crossref, Medline, Google Scholar
91 : Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018; 167:104–120Crossref, Medline, Google Scholar



