Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample
Abstract
Objective:
Previous studies have implicated aberrant reward processing in the pathogenesis of adolescent depression. However, no study has used functional connectivity within a distributed reward network, assessed using resting-state functional MRI (fMRI), to predict the onset of depression in adolescents. This study used reward network-based functional connectivity at baseline to predict depressive disorder at follow-up in a community sample of adolescents.
Method:
A total of 637 children 6–12 years old underwent resting-state fMRI. Discovery and replication analyses tested intrinsic functional connectivity (iFC) among nodes of a putative reward network. Logistic regression tested whether striatal node strength, a measure of reward-related iFC, predicted onset of a depressive disorder at 3-year follow-up. Further analyses investigated the specificity of this prediction.
Results:
Increased left ventral striatum node strength predicted increased risk for future depressive disorder (odds ratio=1.54, 95% CI=1.09–2.18), even after excluding participants who had depressive disorders at baseline (odds ratio=1.52, 95% CI=1.05–2.20). Among 11 reward-network nodes, only the left ventral striatum significantly predicted depression. Striatal node strength did not predict other common adolescent psychopathology, such as anxiety, attention deficit hyperactivity disorder, and substance use.
Conclusions:
Aberrant ventral striatum functional connectivity specifically predicts future risk for depressive disorder. This finding further emphasizes the need to understand how brain reward networks contribute to youth depression.
Major depressive disorder, a leading cause of disease burden (1), commonly begins in adolescence (2). Expanding knowledge on reward-system function in depression could inform attempts to identify at-risk adolescents. Here, we use a longitudinal design in a community-based sample to test the hypothesis that aberrant intrinsic reward-system connectivity in early adolescence predicts risk for depressive disorder 3 years later.
The incidence of depression rises markedly in adolescence (2), potentially because of maturing reward-system function (3). Most evidence linking aberrant reward processing to adolescent depression derives from task-based functional MRI (fMRI) studies targeting reward-related areas, such as the striatum (4, 5). However, few studies have adopted network-based approaches, and most are cross-sectional (5). Given the distributed nature of neural perturbations in depression, research is needed applying network-based approaches to intrinsic functional connectivity (iFC) data (6–8). Such work quantifies the degree to which brain “nodes” (9) facilitate signal integration among network components. Applying this approach to longitudinal data could support inferences about causality (5). While iFC studies implicate reward-network function in depression (10, 11), most studies have examined small, clinically referred samples of adults on medication. There is a particular need for longitudinal studies of iFC in adolescent depression, to extend promising cross-sectional results (12).
We used a longitudinal design to link reward-network iFC to later risk for a depressive disorder in a community-based adolescent sample. Based on previous findings, we hypothesized that aberrant ventral striatal iFC in early adolescence increases risk for future depressive disorder at 3-year follow-up (4). Previous work suggests that such aberrancies reflect perturbed striatal integration of coalescing signals from a distributed reward network encompassing the ventral tegmental area, the anterior cingulate cortex, and the ventromedial prefrontal cortex (13–15). We quantified this integrative function through a measure of ventral striatum “node strength” (i.e., degree centrality), assessed as the region’s weighted sum of connection with other reward-network regions. We stringently tested this hypothesis by probing the existence of a putative reward network (10) in separate discovery and replication samples, both assessed with relatively conservative statistical thresholds. We then assessed specificity by 1) evaluating other reward-system-related brain areas in the prediction of depression and 2) testing striatal node strength as a predictor of other psychiatric outcomes, including anxiety disorders, attention deficit hyperactivity disorder (ADHD), and substance abuse.
Method
Study Design
Baseline.
This study is part of an ongoing cohort study, the High Risk Cohort (HRC). All parents signed informed consent and children provided verbal assent. The ethics committees of all universities involved in the cohort approved the project. For a detailed description of HRC sampling, see Salum et al. (16). Fifty-seven schools from two Brazilian cities, São Paulo and Porto Alegre, participated. In Brazil, parents are required to register their children in a local school. On school registry day, we invited biological parents of 6- to 12-year-old children at these schools to participate in the study. Biological parents (the mother in 87.3% of cases) answered the Family History Screen (17) for 8,012 families representing 9,937 children. From this pool, we created the HRC by combining two strata (N=2,511). The first stratum, “the random group” (N=958), included a sample of randomly selected individuals; the second stratum, “the high-risk group” (N=1,553), included children at risk for psychopathology, selected using a validated prioritization algorithm (16). Only one child per family was included. The baseline evaluation included a household lay interview with a biological parent (the mother in 94.5% of cases), including extensive risk factor evaluation and structured psychiatric interview using the Developmental and Well-Being Assessment, Brazilian Portuguese version (18, 19).
Follow-up.
Three years later, we contacted the parents to ask them to participate in the HRC follow-up. The first follow-up evaluation included a household visit by a lay interviewer, who interviewed the parents or main caregivers of study subjects. In a second household visit, certified psychologists interviewed the adolescents, using the self-report version of the Developmental and Well-Being Assessment. In 10.2% (N=255) of cases, we were unable to contact any family member using all available information. Strategies to contact these individuals included telephoning family members, calling at several different times of day, searching school registries, attempting contact by mail, and visiting the address where the baseline evaluation occurred. Another 9.8% (N=246) of families declined to participate in the follow-up evaluation. The remaining sample consisted of 2,010 participants, comprising 80.05% of the baseline sample. Higher maternal education (χ2=14.07, p<0.001) and socioeconomic status (χ2=6.24, p<0.05), living in Porto Alegre (χ2=4.57, p<0.05), and having a child who met criteria for an anxiety disorder at baseline (χ2=9.75 p<0.01) were associated with a higher likelihood of successful follow-up.
Measures
Psychopathology.
Data on psychopathology were obtained only from parents at baseline, through the Developmental and Well-Being Assessment interview. Data suggest that youth report before age 11 is relatively unreliable (20). Hence, we collected only parent-reported symptoms of depression in the child at baseline (18). For the follow-up, we used both parental and adolescents’ self-report. At the follow-up wave, trained psychiatrist raters evaluated parent- and self-reported information using a digital platform (youthinmind.com) that integrates verbatim responses to open-ended questions, thus supplementing information from the structured questions. Inconclusive cases were discussed in research group meetings with senior psychiatrists. Raters were blind to study site at both time points. At follow-up, they were blind to baseline psychiatric disorders but were allowed to integrate parent- and self-report sources of information to arrive at a clinical diagnosis.
Depressive disorder.
We computed the depressive disorder category by merging Developmental and Well-Being Assessment clinical diagnoses of major depressive disorder with the “other depression” category, which encompasses DSM’s other specified depressive disorder and unspecified depressive disorder. Briefly, the “other depression” category included individuals who met the impairment criterion for major depressive disorder but failed to meet specific symptomatic or duration criteria, as assessed by the clinician rating. We also used the “loss of interest” question from the Developmental and Well-Being Assessment’s depression section to evaluate anhedonia at baseline. We specifically investigated anhedonia because of evidence on its association with reward aberrations in depressive disorder.
Substance use.
We investigated substance use by parent- and self-report, merging any lifetime use of alcohol, tobacco, or any drug into a dichotomized variable called “any substance use.”
Use of medication.
We excluded children whose parents reported regular use of a psychotropic medication within the 30 days before the MRI scan (N=18). Among those excluded children, eight were taking antidepressants but did not meet criteria for a depressive disorder.
Neuroimaging
Data acquisition.
The HRC study’s goal was to perform MRI scans on a subsample at baseline. Children who completed household and school evaluation were eligible to participate, following the same procedure of the screening phase. From the pool of 2,511 children, MRIs were successfully acquired from 741 (for a full description of procedures, see the data supplement that accompanies the online edition of this article). We used 1.5-T MRI scanners (GE Signa HDX and GE Signa HD; GE, USA) at two sites, running identical imaging protocols. fMRI parameters were as follows: TR=2000 ms, TE=30 ms, slice thickness=4 mm, gap=0.5 mm, flip angle=80°, matrix size=80×80, reconstruction matrix=128×128, 1.875×1.875 mm, NEX=1, number of slices=26, and total acquisition time=6 minutes. Total acquisition protocol consisted of 180 echo planar imaging (EPI) dynamic volumes. We asked participants to fixate on a target during resting-state acquisition. T1-weighted scans (three-dimensional fast spoiled gradient sequence) used the following parameters: 160 axial slices for whole brain coverage, TR=10.91 ms, TE=in-phase 4.2 ms, thickness=1.2 mm, flip angle=15°; matrix size=256×192, FOV=24.0×18.0 cm, and NEX=1.
Data preprocessing.
Data were preprocessed using AFNI, version 2011_12_21_1014, and the FMRIB Software Library, version 5.0. We used the following stepwise procedure (http://www.nitrc.org/projects/fcon_1000/) (21): discard the first four volumes of EPI; skull stripping; head motion correction; despiking; rescale to a grand mean of 10,000; band-pass filtering using classical resting-state band (0.01 and 0.1 Hz); detrending; spatial smoothing (full width at half maximum=8 mm); linear registration to the subject’s structural scan; structural image nonlinear registration to the Montreal Neurological Institute (MNI) template (MNI152); nonlinear registration of functional scans; and regression out of nuisance covariates (CSF, white matter, global signal, and six linear motion parameters).
Head movement.
To minimize bias from head motion (22), we excluded subjects whose data did not pass quality control thresholds (see the online data supplement). We then applied the Power et al. (23) scrubbing method by discarding scans in which the frame-wise displacement exceeded 0.5 mm (see equation 9 in Yan et al. [24], and reference 25). We also entered the number of discarded volumes per subject from this scrubbing procedure as a covariate in all adjusted models. Finally, we performed a sensitivity analysis excluding subjects with more than 30 scrubbed volumes. No subject had a mean frame displacement >0.3 mm after scrubbing.
Statistical Analysis
Analyses were conducted using SPSS, version 22 (IBM, Armonk, N.Y.), and R, version 2.15.3 (www.r-project.org); figure templates were created in the MRIcron software program (www.nitrc.org/projects/mricron). All analyses were two-tailed, with significance threshold set at 5%.
Reward network.
First, we selected spheres centered at coordinates (in MNI space) reported in the meta-analysis by Bartra et al. (26) of the valuation system (see Table S2 in the online data supplement). We defined the 11 regions of interest in Satterthwaite et al. (10). The sphere radius for the regions of interest was set at 5 mm. We used the Spearman coefficient to evaluate the correlation of the preprocessed (and scrubbed) BOLD signal between each pair of regions of interest. This procedure created a matrix for each subject with 55 correlations between regions of interest. We applied Fisher’s z transformation to the correlation coefficients. Then we divided the sample by scanning site, creating two subsamples, a discovery sample (site 1; N=328) and a replication sample (site 2; N=309). At site 1, we performed 55 one-sample t tests to identify correlations connecting each pair of the 11 regions of interest that were statistically different from zero. We used Bonferroni correction (p<0.05/55=0.00091) to account for multiple comparisons. Then we confirmed the Bonferroni-corrected significant findings in the independent replication sample (site 2) with an uncorrected threshold (p<0.05). Following convention (27), we termed these region-of-interest correlations the edges of the reward network (43 edges; see Table S3 in the data supplement). We then computed reward-network connectivity measures among these 11 nodes by summing the absolute values of edges that survived the discovery and replication procedure connected to every given node. This measure is classically referred to as the weighted node degree centrality or the node strength (9), and it reflects the importance of a specific node within the network.
Reward network and depressive disorder.
After probing reward network edges and nodes in discovery and replication samples, we proceeded to investigate the role of the reward network in adolescent depressive disorder using the entire sample, while also controlling for scanning site. We used logistic regression to test the effect of left and right ventral striatum node strength (independent variable) as predictor for depressive disorder (dependent variable) using the DSM-based clinician rating. We controlled for the following nuisance independent variables: number of scrubbed volumes, site, sex, age, and any anxiety disorder, ADHD, and depressive disorder at baseline. The analysis survived Bonferroni correction for laterality (p<0.05/2). Then we restricted our analysis to new-onset depressive disorder by running the same model while excluding participants who had depressive disorder at baseline (N=22).
We further investigated the specificity of ventral striatum node strength as a predictor of depressive disorder in two ways. First, we broadened our hypothesis-driven focus on the ventral striatum and tested the node strength of all 11 nodes of the reward network as predictors for depressive disorder. We used logistic regression models to test node strength (independent variable) of reward nodes as predictors for depressive disorder (dependent variable) while controlling for number of scrubbed volumes, site, sex, age, and any anxiety disorder, ADHD, and depressive disorder at baseline as nuisance independent variables. Second, we assessed diagnostic specificity by testing whether ventral striatum node strength predicts anxiety disorders, ADHD, or any substance use (by parent- and self-report). We performed logistic regression models testing ventral striatum node strength as a predictor of clinician rating variables for anxiety disorders and ADHD from the Developmental and Well-Being Assessment, as well as any substance use (by parent- and self-report). These models also included number of scrubbed volumes, site, sex, age, and psychopathology at baseline (any anxiety disorder, ADHD, and depressive disorder according to Developmental and Well-Being Assessment clinician rating) as nuisance controlling variables.
Results
The prevalence of depressive disorder was 4.2% at baseline (N=27; major depressive disorder, N=25; other depression, N=2) and 8.8% at follow-up (N=56; major depressive disorder, N=47; other depression, N=9). Predictors for depressive disorder at follow-up were female sex and older age, depressive disorder, or ADHD at baseline. Additionally, baseline anhedonia significantly predicted depressive disorder at follow-up (odds ratio=3.00, 95% CI=1.34–6.60, p=0.01). At follow-up, older age, ADHD, any anxiety disorder, and any substance use (by parent- and self-report) were all associated with depressive disorder (Table 1).
| Measure and Time of Assessment | No Depressive Disorder at Follow-Up (N=529) | Depressive Disorder at Follow-Up (N=56) | p | ||
|---|---|---|---|---|---|
| Baseline | |||||
| Sociodemographic | N | % | N | % | |
| Female | 241 | 45.6 | 37 | 66.1 | 0.003 |
| Site | |||||
| Porto Alegre | 274 | 51.8 | 35 | 62.5 | |
| São Paulo | 255 | 48.2 | 21 | 37.5 | 0.127 |
| Mother completed high schoolb | 226 | 43.1 | 26 | 46.4 | 0.636 |
| Mean | SD | Mean | SD | ||
| Age at MRI scan (years) | 10.6 | 1.9 | 11.6 | 1.8 | <0.001 |
| Socioeconomic scorec | 20.1 | 4.4 | 20.5 | 5.6 | 0.879 |
| Movement parameters | |||||
| Frame displacement (pre-scrubbing) (mm) | 0.16 | 0.23 | 0.21 | 0.34 | 0.623 |
| Number of scrubbed volumes | 17.0 | 27.1 | 22.5 | 32.7 | 0.354 |
| Frame displacement (post-scrubbing) (mm) | 0.08 | 0.04 | 0.09 | 0.04 | 0.647 |
| Clinical features | N | % | N | % | |
| Any anxiety disorder | 75 | 14.2 | 13 | 23.2 | 0.072 |
| ADHD | 55 | 10.4 | 11 | 19.6 | 0.038 |
| Depressive disorder | 12 | 2.3 | 12 | 21.4 | <0.001 |
| 3-year follow-up | |||||
| Any anxiety disorder | 59 | 11.2 | 26 | 46.4 | <0.001 |
| ADHD | 21 | 4.0 | 6 | 10.7 | 0.022 |
| Any substance use, parent-reportb | 79 | 15.3 | 22 | 40.7 | <0.001 |
| Any substance use, self-reportd | 192 | 59.2 | 32 | 65.3 | <0.001 |
| Mean | SD | Mean | SD | ||
| Time from MRI to follow-up (years) | 2.6 | 0.4 | 2.5 | 0.4 | 0.168 |
TABLE 1. Demographic and Clinical Characteristics of Participants in a Study of Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disordera
Reward Network
We first identified (using Bonferroni correction) and replicated 43 significant correlations (i.e., edges) connecting the 11 nodes of the reward network (see Table S3 in the data supplement). We then created reward-network iFC measures of node strength among these 11 nodes by summing edges connected to every given node.
Reward Network and Depressive Disorder
Our main aim was to test whether ventral striatum node strength predicted risk for depressive disorder over 3 years. We confirmed this hypothesis for the left ventral striatum node, which is connected to the anterior cingulate cortex, the prefrontal cortex, the thalamus, and the ventral tegmental area, among other reward network regions (Figure 1). This result was significant both in bivariate analyses and in an analysis that controlled for potential baseline confounders (odds ratio=1.54, 95% CI=1.09–2.18, p=0.03, corrected for the left and right striatum) (Table 2), including anxiety, ADHD, and depressive disorder. No association was observed for the right ventral striatum node (Table 3). Elevated node strength of the left ventral striatum node predicted a 50% increase in the odds of a depressive disorder 3 years later. We found similar results when we excluded participants who had depressive disorder at baseline (odds ratio=1.52, 95% CI=1.05–2.20, p=0.027). Ventral striatum node strength was not significantly associated with depressive disorder or anhedonia at baseline (data available upon request). Also, the main results did not change when we conducted sensitivity analyses that excluded participants who had more than 30 volumes eliminated by the scrubbing procedure (see Table S4 in the data supplement).
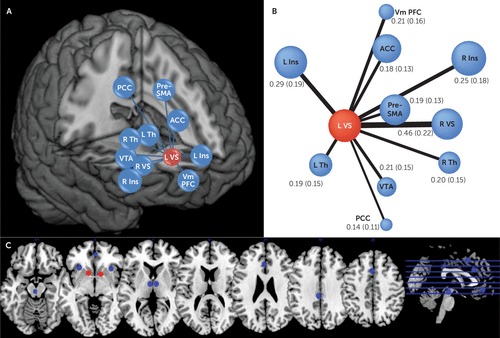
FIGURE 1. Schematic Representation of Edges Connecting Reward Nodes to the Left Ventral Striatum in a Study of Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disordera
a Panel A is a three-dimensional representation of the left ventral striatum node and other nodes of the reward network in MNI space. Panel B shows mean correlation coefficients (with standard deviations) of resting-state time series between reward network nodes and the left ventral striatum node. The width of the lines is proportional to the correlation coefficient (edges), and size of the circles is proportional to node strength within the reward network. Panel C provides an axial view of the actual size and position of the reward network nodes in Montreal Neurological Institute space. L VS=left ventral striatum; R VS=right ventral striatum; Vm PFC=ventromedial prefrontal cortex; L Ins=left anterior insula; R Ins=right anterior insula; PCC=posterior cingulate cortex; VTA=ventral tegmental area; ACC=anterior cingulate cortex; Pre-SMA=pre–supplementary motor area; L Th=left thalamus; R Th=right thalamus.
| Variables in the Model | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Left ventral striatum node strength | 1.54 | 1.09–2.18 | 0.015 |
| Depressive disorder at baseline | 14.07 | 5.16–38.50 | <0.001 |
| ADHD at baseline | 2.06 | 0.91–4.64 | 0.081 |
| Any anxiety at baseline | 1.21 | 0.54–2.73 | 0.639 |
| Age at MRI | 1.45 | 1.22–1.74 | <0.001 |
| Sex (female) | 2.38 | 1.27–4.45 | 0.007 |
| Site | 1.16 | 0.59–2.29 | 0.667 |
| Number of scrubbed volumes | 1.01 | 0.99–1.02 | 0.342 |
TABLE 2. Depressive Disorder at 3-Year Follow-Up and Left Ventral Striatum Node Strength in a Study of Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disordera
| Node | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Striatal node strength | |||
| Left ventral striatum | 1.54 | 1.09–2.18 | 0.015b |
| Right ventral striatum | 1.23 | 0.83–1.82 | 0.311 |
| Other regions from the reward network | |||
| Ventromedial prefrontal cortex | 0.81 | 0.41–1.59 | 0.534 |
| Left anterior insula | 1.21 | 0.76–1.66 | 0.569 |
| Right anterior insula | 1.33 | 0.90–1.98 | 0.162 |
| Posterior cingulate | 1.16 | 0.48–2.85 | 0.742 |
| Brainstem (ventral tegmental area) | 0.93 | 0.56–1.55 | 0.775 |
| Anterior cingulate | 1.02 | 0.65–1.58 | 0.948 |
| Pre–supplementary motor area | 1.18 | 0.76–1.84 | 0.452 |
| Left thalamus | 1.03 | 0.64–1.65 | 0.904 |
| Right thalamus | 1.16 | 0.72–1.87 | 0.542 |
TABLE 3. Depressive Disorder at 3-Year Follow-Up and Node Strength of all Reward Network Nodes in a Study of Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disordera
Specificity Analyses
We assessed the specificity of the left ventral striatum node as a predictor of depressive disorder in two ways: comparing it with other reward nodes and as a predictor of other common adolescent psychopathology. For the first, we tested all other node strengths of the reward network as predictors of depressive disorder. Only the left ventral striatum node strength significantly predicted depressive disorder (Table 3). For the second, we tested whether left ventral striatum node strength predicted adolescent psychopathology other than depressive disorder. The left ventral striatum was not associated with any anxiety disorder, ADHD, or any substance use (by parent- or self-report) at 3-year follow-up (see Table S5 in the data supplement). Therefore, compared with other common adolescent disorders, the association between left ventral striatum iFC and psychopathology 3 years later was indeed specific to depressive disorder.
Discussion
Using a community-based sample, we found that ventral striatal iFC predicted new-onset depressive disorder 3 years later. We also found evidence of specificity: connectivity of the striatum, but not other regions, predicted depressive disorder, and striatal connectivity predicted depressive disorder, but not other psychopathology. The results of our longitudinal design provide novel evidence for the involvement of the reward network in the pathogenesis of depression.
Several clinical and basic science considerations implicate reward processing in depression. From a clinical perspective, there is the long-standing observation that a subset of depressive behaviors, such as reduced energy and motivation, are related to changes in reinforcement schedules (28, 29). These notions underpin therapeutic approaches, in particular behavioral activation, a key component of cognitive and behavioral therapy in depression (30). These observations converge with basic science findings about reward processing. Several experiments demonstrate the key role that dopaminergic signaling in ventral striatal areas plays in reward valuation and effort expended toward reward (31, 32). In recent years, fMRI has enabled scientists to probe activity in deep brain areas such as the ventral striatum and thereby provide crucial links between long-standing clinical notions and basic science. Indeed, there is mounting evidence from reward task–based fMRI that reduced activity in the striatum is important in the etiology of depression (3, 5, 33). However, there is a need to understand the distributed brain patterns of perturbations in depression (6), and network measures using iFC are well suited to this approach. Unlike task-based fMRI, iFC does not rely on a behavioral paradigm and is therefore less confounded by issues such as ability or motivation to engage with a task (34). This is particularly important when studying developmental effects, where standardizing a task across different age groups can present a daunting challenge. Therefore, since both reward processing and depression prevalence vary with development (2, 35, 36), iFC appears well suited to their study.
Probing the connectivity of a reward network allowed us to show that increased left ventral striatum node strength predicts depressive disorder at follow-up. Our first step was to show resting-state coupling between brain regions typically activated during reward-related behaviors (3, 26, 37). Then, we computed node strength—an important network measure that captures the centrality of a given node within a network (9). The left striatum was the only node whose strength predicted depressive disorder. This suggests that the left ventral striatum is integrating information from various areas of the reward network, including those previously implicated in adolescent depression, such as the anterior cingulate cortex (13, 14). Having rigorously probed ventral striatal iFC within the reward network, we then used it in the prediction of depressive disorder 3 years later. We demonstrate that left ventral striatum node strength predicts new-onset depressive disorder (that is, after excluding depressive disorder cases at baseline).
This finding indicates that perturbed connectivity in the reward network is not merely a consequence of experiencing depression, but predates the expression of the disorder. Thus, striatal iFC is a marker of depressive disorder risk and supports its role in the pathogenesis of depression, although our observational study cannot offer conclusive evidence about its causal role (i.e., striatal iFC could still be an early marker but not be itself implicated in the illness). It should be noted that we did not find a significant association between striatal node strength and depressive disorder at baseline. However, the low prevalence of depressive disorder at baseline, which is expected given the young age of participants at that point, may have diminished statistical power to demonstrate this association.
Our study finds that increased rather than decreased iFC predicts depressive disorder. One possible explanation for this finding is that increased iFC is an attempt at compensating for the blunted striatal response to rewards that has been described in depression (6). Coupling resting state connectivity studies with functional imaging probing the ventral striatum could help test this hypothesis. Alternatively, hyperconnectivity within the reward network could reflect a primary pathogenic process in its own right. Resembling hyperconnectivity found within other networks in depression studies, such as the default mode network (7), increased iFC may itself impede adequate reward processing during reward-related tasks, leading to blunted ventral striatum signals, a hypothesis that could also be tested in longitudinal studies that employ serial resting-state and task-based fMRI studies. A previous study found decreased iFC within the reward network (10), yet this discrepancy could be explained by the fact that these were adults who already had depression and, unlike our adolescents, were on medication.
Lastly, since psychiatric disorders in youth are frequently comorbid (38) and ventral striatal dysfunction is implicated in other disorders (39, 40), we examined whether left ventral striatum node strength could predict diagnoses other than depressive disorder. Supporting the specificity of our main result, the node strength of the left ventral striatum did not significantly predict anxiety disorders and ADHD. We also investigated the association of the left ventral striatum node with another reward-related phenotype, any substance use. One previous longitudinal study (40) showed that stronger cortico-striatal iFC (right dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and pre–supplementary motor area) predicted earlier onset of alcohol and substance use. We did not find this association. However, our sample was younger than the expected age for onset of substance use. Further evidence that this is a limitation for detecting substance use findings is that age was positively associated with any substance use (see Table S5 in the data supplement). Future work dissecting the various anatomical and functional components of reward processing may identify differential predictions between major depression and other disorders, such as substance-related and addictive disorders.
Our study has a number of strengths. Our discovery and replication analysis addresses recent concerns regarding the lack of replicability in neuroimaging studies (25). Furthermore, adolescent major depressive disorder studies typically rely on cross-sectional designs and relatively small clinically referred samples, whereas ours was a longitudinal study in a large community-based sample of unmedicated adolescents. However, our study also has limitations. We investigated a specific brain network, despite evidence of several networks being implicated in both major depression and typical development (6, 7). The main advantage of this approach, which narrows brain regions based on previous data, is to avoid spurious associations found in whole-brain investigations. In addition, there was attrition at follow-up, which can introduce bias. However, our loss at follow-up was relatively modest (10% of the imaging sample). Also, adolescence and young adulthood are the age of maximum incidence of depression. This may have led to an underestimation of the strength of our effects, since most participants in our study were in their early adolescence. Moreover, we did not collect child reports of depression at baseline because of the low reliability of youth report in early childhood (18, 20). Finally, iFC data are sensitive to head motion (22, 23). We addressed this issue using distinct techniques and rigorous thresholding. Our main results persisted after we imposed restrictive head movement parameters, which increases confidence in our findings.
In summary, we investigated the iFC of the ventral striatum within the reward network in a community sample of adolescents. Increased ventral striatum node strength was associated with an increase in odds of depressive disorder by approximately 50% after 3 years. This underscores the importance of the brain’s reward network in the pathogenesis of depression and calls for further studies to make clinical use of these findings.
1 : Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease study 2010. PLoS Med 2013; 10:e1001547Crossref, Medline, Google Scholar
2 : Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord 2009; 11:637–649Crossref, Medline, Google Scholar
3 : Research review: altered reward function in adolescent depression: what, when, and how? J Child Psychol Psychiatry 2012; 53:3–15Crossref, Medline, Google Scholar
4 : The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172:1215–1223Link, Google Scholar
5 : Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin 2013; 4:209–231Crossref, Medline, Google Scholar
6 : Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev 2015; 56:330–344Crossref, Medline, Google Scholar
7 : Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72:603–611Crossref, Medline, Google Scholar
8 : Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23:28–38Crossref, Medline, Google Scholar
9 : Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010; 52:1059–1069Crossref, Medline, Google Scholar
10 : Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology 2015; 40:2258–2268Crossref, Medline, Google Scholar
11 : Common dimensional reward deficits across mood and psychotic disorders: a connectome-wide association study. Am J Psychiatry 2017; 174:657–666Link, Google Scholar
12 : Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry 2013; 52:628–641.e13Crossref, Medline, Google Scholar
13 : Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol Med 2015; 45:1001–1009Crossref, Medline, Google Scholar
14 : Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry 2013; 74:898–907Crossref, Medline, Google Scholar
15 : Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 2014; 71:1138–1147Crossref, Medline, Google Scholar
16 : High risk cohort study for psychiatric disorders in childhood: rationale, design, methods, and preliminary results. Int J Methods Psychiatr Res 2015; 24:58–73Crossref, Medline, Google Scholar
17 : Brief screening for family psychiatric history: the Family History Screen. Arch Gen Psychiatry 2000; 57:675–682Crossref, Medline, Google Scholar
18 : The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–655Crossref, Medline, Google Scholar
19 : Prevalence of child and adolescent psychiatric disorders in southeast Brazil. J Am Acad Child Adolesc Psychiatry 2004; 43:727–734Crossref, Medline, Google Scholar
20 : Reliability of diagnostic reporting for children aged 6–11 years: a test-retest study of the Diagnostic Interview Schedule for Children–Revised. Am J Psychiatry 1994; 151:1048–1054Link, Google Scholar
21 : Making data sharing work: the FCP/INDI experience. Neuroimage 2013; 82:683–691Crossref, Medline, Google Scholar
22 : Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014; 84:320–341Crossref, Medline, Google Scholar
23 : Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59:2142–2154Crossref, Medline, Google Scholar
24 : A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 2013; 76:183–201Crossref, Medline, Google Scholar
25 : Connectome hubs at resting state in children and adolescents: reproducibility and psychopathological correlation. Dev Cogn Neurosci 2016; 20:2–11Crossref, Medline, Google Scholar
26 : The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013; 76:412–427Crossref, Medline, Google Scholar
27 : Age effects on the default mode and control networks in typically developing children. J Psychiatr Res 2014; 58:89–95Crossref, Medline, Google Scholar
28 : Changing reinforcing events: an approach to the treatment of depression. Psychotherapy: Theory, Research, and Practice 1980; 17:322–334Crossref, Google Scholar
29 : Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract 2001; 8:255–270Crossref, Google Scholar
30 : Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74:658–670Crossref, Medline, Google Scholar
31 : Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 2016; 351:aac9698Crossref, Medline, Google Scholar
32 : Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 2012; 32:6170–6176Crossref, Medline, Google Scholar
33 : Annual research review: transdiagnostic neuroscience of child and adolescent mental disorders: differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J Child Psychol Psychiatry 2016; 57:321–349Crossref, Medline, Google Scholar
34 : Validation of a child-friendly version of the monetary incentive delay task. Soc Cogn Affect Neurosci 2013; 8:720–726Crossref, Medline, Google Scholar
35 : Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage 2016; 124(Pt A):409–420Crossref, Medline, Google Scholar
36 : Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J Neurosci 2015; 35:7226–7238Crossref, Medline, Google Scholar
37 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26Crossref, Medline, Google Scholar
38 : Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol Med 2012; 42:1997–2010Crossref, Medline, Google Scholar
39 : Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex 2016; 82:225–236Crossref, Medline, Google Scholar
40 : Investigating distinct and common abnormalities of resting-state functional connectivity in depression, anxiety, and their comorbid states. Eur Neuropsychopharmacol 2015; 25:1933–1942Crossref, Medline, Google Scholar



