Cannabis Use and Risk of Prescription Opioid Use Disorder in the United States
Abstract
Objective:
The authors sought to determine whether cannabis use is associated with a change in the risk of incident nonmedical prescription opioid use and opioid use disorder at 3-year follow-up.
Method:
The authors used logistic regression models to assess prospective associations between cannabis use at wave 1 (2001–2002) and nonmedical prescription opioid use and prescription opioid use disorder at wave 2 (2004–2005) of the National Epidemiologic Survey on Alcohol and Related Conditions. Corresponding analyses were performed among adults with moderate or more severe pain and with nonmedical opioid use at wave 1. Cannabis and prescription opioid use were measured with a structured interview (the Alcohol Use Disorder and Associated Disabilities Interview Schedule–DSM-IV version). Other covariates included age, sex, race/ethnicity, anxiety or mood disorders, family history of drug, alcohol, and behavioral problems, and, in opioid use disorder analyses, nonmedical opioid use.
Results:
In logistic regression models, cannabis use at wave 1 was associated with increased incident nonmedical prescription opioid use (odds ratio=5.78, 95% CI=4.23–7.90) and opioid use disorder (odds ratio=7.76, 95% CI=4.95–12.16) at wave 2. These associations remained significant after adjustment for background characteristics (nonmedical opioid use: adjusted odds ratio=2.62, 95% CI=1.86–3.69; opioid use disorder: adjusted odds ratio=2.18, 95% CI=1.14–4.14). Among adults with pain at wave 1, cannabis use was also associated with increased incident nonmedical opioid use (adjusted odds ratio=2.99, 95% CI=1.63–5.47) at wave 2; it was also associated with increased incident prescription opioid use disorder, although the association fell short of significance (adjusted odds ratio=2.14, 95% CI=0.95–4.83). Among adults with nonmedical opioid use at wave 1, cannabis use was also associated with an increase in nonmedical opioid use (adjusted odds ratio=3.13, 95% CI=1.19–8.23).
Conclusions:
Cannabis use appears to increase rather than decrease the risk of developing nonmedical prescription opioid use and opioid use disorder.
After more than two decades of increasing prevalence of prescription opioid use disorder in the United States (1, 2), the number of people in the U.S. population with prescription opioid use disorders reached 2 million in 2015 (3). Rising rates of prescription opioid use disorder have coincided with the largest epidemic of opioid overdose deaths in U.S. history. In 2015, unintentional drug overdose deaths, most of which involved opioids, claimed over 47,000 lives (4). The crisis in nonmedical use of prescription opioids, which has exacted a heavy burden not only on individuals but also on their families and communities, has prompted federal policy makers to consider prescription opioid use disorder a threat to public health (5).
In the wake of rising rates of nonmedical prescription opioid use, there has been increased public (6) and professional (7) interest in the possibility that cannabis might help to curb or prevent opioid use disorder. Support comes from two widely publicized ecological analyses indicating that compared with states that do not permit medical marijuana, annual death rates due to opioid overdoses were nearly one-quarter lower in states that do permit medical marijuana (8, 9). Significant reductions in opioid prescribing have also been reported following passage of medical marijuana laws (10). Such ecologic analyses, however, provide no information on whether individual patients who use cannabis have a lower or higher risk of developing opioid use disorders (11).
The possibility that cannabis lowers the risk of opioid-related morbidity has fueled speculation concerning potential mechanisms. A leading hypothesis is that cannabis use tends to lower opioid use and risk of opioid use disorder through increased control of pain (8, 12). A recent meta-analysis of randomized controlled trials provides a moderate level of evidence that cannabinoids improve some forms of chronic pain (13). A large Dutch study reported that just over half of adults in registered cannabis programs also received prescriptions for pain medications, suggesting that medical marijuana is frequently used for pain control (14). In a small, uncontrolled cross-sectional survey of medical marijuana users with chronic pain recruited from a cannabis dispensary, cannabis use was associated with a 64% decline in opioid use (N=118) (12). Cannabis exposure has also been associated with increased analgesia among opioid-treated patients with chronic pain (15), suggesting that cannabis may potentiate antinociceptive effects of opioids, permitting lower and presumably safer opioid dosing to achieve comparable analgesia.
Much remains to be learned about the association between cannabis use and nonmedical prescription opioid use or opioid use disorders. No prospective epidemiological or clinical studies have demonstrated that cannabis use reduces use of opioids. Moreover, epidemiologic research suggests that cannabis may actually increase the risk of other drug use disorders, including opioids. A retrospective Australian twin study reported that early initiation of cannabis use was associated with increased risks of other drug use and abuse/dependence, including opioid use and opioid abuse/dependence (16). Prospective epidemiological research further suggests that cannabis use is a risk factor for other drug use disorders (17). However, prospective epidemiological research has not previously examined the specific association between cannabis use and nonmedical prescription opioid use or opioid use disorder to inform clinical practice and policy.
We sought to address this critical gap in knowledge with prospective data from the National Epidemiological Survey on Alcohol and Related Conditions (NESARC), a large, nationally representative sample. We examined the association between cannabis use and incident nonmedical prescription opioid use and disorder 3 years later, after adjusting for several relevant demographic and clinical covariates. We also evaluated whether cannabis use among adults with nonmedical prescription opioid use was associated with a subsequent decrease in nonmedical opioid use.
Method
Sample
The 2001–2002 NESARC (wave 1), and the 2004–2005 follow-up (wave 2) is a nationally representative sample of the noninstitutionalized adult U.S. population conducted by the U.S. Census Bureau under the direction of the National Institute on Alcoholism and Alcohol Abuse (18, 19). The response rate for wave 1 was 81.0%. Excluding ineligible respondents (e.g., those who were deceased), the wave 2 response rate was 86.7%, resulting in a cumulative response rate of 70.2% (N=34,653). Wave 2 NESARC weights include adjustments for nonresponse, demographic factors, and psychiatric diagnoses to ensure that the wave 2 sample approximated the target population, which was the original sample minus attrition between the two waves (18).
Assessment
All diagnoses were made according to DSM-IV criteria, using the Alcohol Use Disorder and Associated Disabilities Interview Schedule–DSM-IV version (AUDADIS-IV) for waves 1 and 2 (20). Consistent with previous reports, nonmedical use of a prescription opioid was defined as using a prescription analgesic “without a prescription, in greater amounts, more often, or longer than prescribed, or for a reason other than a doctor said you should use them” during the 12 months preceding the interview. More than 30 symptom items were used by the AUDADIS-IV to define 12-month prescription opioid use disorder according to DSM-IV criteria. The NESARC also collected information for other substance use disorders (nicotine dependence, alcohol use disorder, and drug use disorders, including other prescription drug use disorders). The reliability of the AUDADIS-IV prescription opioid use questions (kappa=0.66) and associated substance use disorder diagnoses (kappa=0.53–0.84) are well documented in several psychometric studies, including in clinical (20) and general population (21) samples. Further concurrent and predictive validity of the prescription opioid use disorder diagnosis has been documented by increased risk of related psychopathology, impairment, and probability of seeking treatment (22).
The frequency of past-year cannabis use was assessed with an 11-level item ranging from no use in the past 12 months to use every day in the past 12 months. Cannabis use was collapsed into a four-level variable including no use in the past last 12 months, occasional use (at least once a year but less than once a month), frequent use (from once a month or more to twice a week), and very frequent use (from three times a week to every day) (23). A similar four-level scale was developed for past-year prescription opioid use.
Mood disorders included DSM-IV major depressive disorder, dysthymia, bipolar I disorder, and bipolar II disorder. Anxiety disorders included DSM-IV panic disorder, social anxiety disorder, specific phobia, and generalized anxiety disorder. Test-retest reliabilities for AUDADIS-IV mood, anxiety, and personality disorders in the noninstitutionalized population and clinical settings have been found to be fair to good. The criterion validity of mood and substance use disorders with psychiatrist reappraisal has also been found to be good to excellent (kappa values, 0.64–0.83) (24). Family histories of alcohol use disorder, drug use disorders, depression, and antisocial personality disorder referred to first-degree relatives. The test-retest reliability of AUDADIS family history variables has been shown to be very good to excellent (25).
Pain was assessed using the pain item of the Medical Outcomes Study 12-Item Short Form Health Survey, Version 2 (SF-12) (26), a valid measure that is commonly used in population surveys (27). The pain item uses a 5-point scale (not at all, a little bit, moderately, quite a bit, and extremely) to measure the degree to which pain interferes with daily activities during the past 4 weeks (28). The pain measure was collapsed into two levels depending on whether pain was associated with no or little interference (“no pain”), or with moderate to extreme interference (“pain”) (29).
Statistical Analysis
Wave 1 descriptive demographic and clinical characteristics were compared between individuals with and without any cannabis use in the year before the wave 1 interview. Group differences were evaluated with chi-square or t tests. Unadjusted percentages of respondents with wave 2 incident opioid use disorders were determined by frequency of wave 1 cannabis use.
Separate logistic regression models were fitted with nonmedical opioid use and disorder outcomes at wave 2 predicted by past-year cannabis use at wave 1. To differentiate between prevalent and incident opioid outcomes at wave 2, we defined four outcomes: 1) prevalent nonmedical opioid use, defined as any nonmedical opioid use since the wave 1 interview; 2) incident nonmedical opioid use, defined as any nonmedical opioid use since wave 1, restricted to respondents with no lifetime nonmedical opioid use at wave 1; 3) prevalent prescription opioid use disorder, defined as meeting opioid use disorder criteria since wave 1; and 4) incident prescription opioid use disorder, defined as meeting opioid use disorder criteria since wave 1, restricted to respondents with no lifetime opioid use disorder at wave 1. Results are presented as unadjusted odds ratios and adjusted odds ratios controlling for age, sex, race/ethnicity, family history variables, antisocial personality disorder, and other substance use disorders and mood or anxiety disorders at wave 1 (29). Adjusted models of wave 2 opioid use disorder also controlled for wave 1 past-year nonmedical opioid use. Regressions were fitted among the overall population of NESARC wave 1 and 2 respondents and then repeated, as a sensitivity analysis, among respondents without wave 1 past-year cannabis use disorders and among respondents with moderate or more severe pain impairment.
We further examined whether, among respondents with wave 1 past-year nonmedical opioid use, cannabis use was associated with an increase or decrease in the level of opioid use at wave 2. A separate logistic regression was fitted for respondents with wave 1 opioid use and moderate or more severe pain. All analyses were performed using SUDAAN to take into account the complex design features of the NESARC.
Results
Background Characteristics of Adults Who Use Cannabis
At wave 1, individuals with any past-year cannabis use were younger on average than those without cannabis use, more likely to be male, and more likely to have past-year opioid use disorder, cannabis use disorder, other substance use disorders, or any past-year mood or anxiety disorder. They were also significantly more likely to have a family history of alcohol use disorders, drug use disorders, depression, and antisocial personality disorder. The two groups did not differ significantly with respect to the proportion who reported moderate or more severe pain during the month before the wave 1 interview (Table 1).
| Characteristic | Cannabis Use (N=1,267) | No Cannabis Use (N=33,352) | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 29.91 | 10.66 | 45.72 | 17.27 | <0.001 |
| N | % | N | % | ||
| Sex | |||||
| Male | 766 | 66.49 | 13,780 | 47.14 | <0.001 |
| Female | 501 | 33.51 | 19,572 | 52.86 | <0.001 |
| Race/ethnicity | 0.96 | ||||
| White, non-Hispanic | 789 | 70.82 | 19,360 | 70.91 | |
| Other | 478 | 29.18 | 13,992 | 29.09 | |
| Family history | |||||
| Alcohol use disorders | 598 | 46.51 | 11,636 | 33.96 | <0.001 |
| Drug use disorders | 427 | 33.08 | 5,537 | 15.97 | <0.001 |
| Depression | 614 | 48.39 | 10,503 | 31.98 | <0.001 |
| Antisocial personality disorder | 248 | 21.42 | 902 | 2.89 | <0.001 |
| Painb | 254 | 20.06 | 6,680 | 18.60 | 0.29 |
| Nonmedical prescription opioid use, past 12 months | <0.001 | ||||
| None | 1,064 | 81.92 | 33,020 | 98.94 | |
| Occasional use | 108 | 10.11 | 150 | 0.54 | |
| Frequent use | 56 | 4.37 | 99 | 0.32 | |
| Very frequent use | 39 | 3.61 | 83 | 0.21 | |
| Mental disorders, past 12 months | |||||
| Opioid use disorder | 45 | 4.05 | 58 | 0.18 | <0.001 |
| Cannabis use disorder | 443 | 36.21 | 0 | 0.00 | <0.001 |
| Other substance use disorder | 591 | 48.89 | 2,160 | 6.78 | <0.001 |
| Mood or anxiety disorder | 440 | 33.00 | 5,478 | 15.53 | <0.001 |
TABLE 1. Background Characteristics of NESARC Respondents, by Any Past-Year Cannabis Use at Wave 1a
Prospective Associations Between Cannabis Use and Nonmedical Prescription Opioid Use
Within the overall survey population, cannabis use at wave 1 was associated with a significant increase in the odds of prevalent nonmedical prescription opioid use during the follow-up period (Table 2). After adjustment for the background demographic and clinical characteristics, a strong association persisted between wave 1 cannabis use and wave 2 prevalent nonmedical opioid use. These associations were also observed among adults without past-year cannabis use disorder and among adults with moderate or more severe pain at wave 1. Among individuals without nonmedical opioid use during the 12 months before the wave 1 interview, there was a significant association between cannabis use at wave 1 and incident nonmedical opioid use during the follow-up period. This association was also observed among adults without cannabis use disorder at wave 1 and among adults with moderate or more severe pain at wave 1.
| Wave 1 Past-Year Cannabis Use Predicting: | N in Analysis | Odds Ratio | 95% CI | Adjusted Odds Ratiob | 95% CI |
|---|---|---|---|---|---|
| Overall population | |||||
| Wave 2 prevalent nonmedical opioid use | 34,534 | 8.74 | 6.98–10.93 | 3.54 | 2.74–4.57 |
| Wave 2 incident nonmedical opioid use | 32,888 | 5.78 | 4.23–7.90 | 2.62 | 1.86–3.69 |
| Population without wave 1 cannabis use disorders | |||||
| Wave 2 prevalent nonmedical opioid use | 34,091 | 7.43 | 5.59–9.87 | 3.35 | 2.48–4.52 |
| Wave 2 incident nonmedical opioid use | 32,616 | 5.67 | 3.97–8.09 | 2.78 | 1.91–4.04 |
| Population with painc | |||||
| Wave 2 prevalent nonmedical opioid use | 6,920 | 10.30 | 6.89–15.39 | 3.97 | 2.44–6.46 |
| Wave 2 incident nonmedical opioid use | 6,518 | 6.74 | 4.09–11.10 | 2.99 | 1.63–5.47 |
TABLE 2. Prospective Associations of Wave 1 Cannabis Use and Wave 2 Prevalent and Incident Nonmedical Prescription Opioid Use in the NESARCa
In analyses restricted to individuals with past-year nonmedical opioid use, in unadjusted and adjusted regressions, wave 1 cannabis use was significantly associated with an increase in the level of opioid use during the year before the wave 2 interview. Cannabis use was also associated with lower odds of decreasing the level of opioid use. When the sample was further restricted to adults with wave 1 nonmedical opioid use and moderate or more severe pain, wave 1 cannabis use was associated with lower unadjusted odds of decreasing opioid use, although the other regressions did not yield significant associations. Among individuals with nonmedical opioid use at wave 1 who either used or did not use cannabis, however, decreases in opioid use at wave 2 were markedly more common than increases in opioid use (Table 3).
| Wave 1 Past-Year Cannabis Use Predicting: | Respondents With Change in Opioid Use Between Wave 1 and Wave 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cannabis Use | No Cannabis Use | |||||||
| N | % | N | % | Odds Ratio | 95% CI | Adjusted Odds Ratiob | 95% CI | |
| Adults with wave 1 nonmedical opioid use | 203 | 332 | ||||||
| Increase in opioid use at wave 2 | 9 | 5.15 | 4 | 0.97 | 5.57 | 1.47–21.11 | 3.13 | 1.19–8.23 |
| Decrease in opioid use at wave 2 | 169 | 81.27 | 307 | 93.70 | 0.29 | 0.14–0.61 | 0.42 | 0.19–0.91 |
| Adults with wave 1 nonmedical opioid use and painc | 60 | 105 | ||||||
| Increase in opioid use at wave 2 | 2 | 7.78 | 3 | 1.97 | 4.20 | 0.64–27.50 | 2.60 | 0.42–16.05 |
| Decrease in opioid use at wave 2 | 53 | 79.97 | 96 | 93.04 | 0.30 | 0.10–0.86 | 0.60 | 0.18–2.03 |
TABLE 3. Prospective Associations Between Wave 1 Cannabis Use and Increase or Decrease in Nonmedical Prescription Opioid Use at Wave 2 Among Adults With Wave 1 Nonmedical Prescription Opioid Use in the NESARCa
Prospective Associations Between Cannabis Use and Prescription Opioid Use Disorder
In unadjusted analyses, the percentage of adults who developed a new-onset opioid use disorder during the follow-up period was lowest for individuals who did not use cannabis in the year before the wave 1 interview (0.51%), followed by occasional cannabis users (2.86%), frequent cannabis users (4.30%), and very frequent cannabis users (4.43%) (Figure 1).
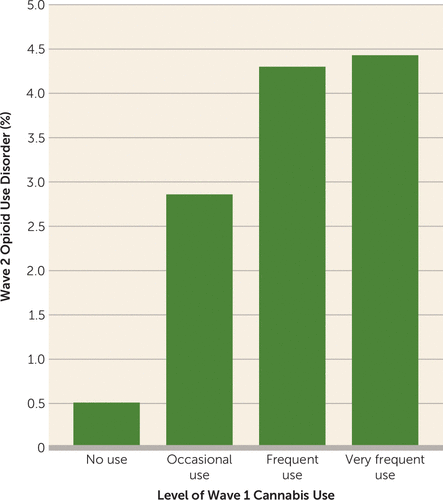
FIGURE 1. Level of Wave 1 Cannabis Use and Incident Wave 2 Prescription Opioid Use Disorder in the NESARCa
a NESARC=National Epidemiological Survey on Alcohol and Related Conditions; wave 1 was conducted in 2001 and 2002, and wave 2 in 2004 and 2005.
In the overall survey population, cannabis use at wave 1 was associated with a significant increase in the odds of prevalent and incident prescription opioid use disorder during the follow-up period (Table 4). After adjustment for the background demographic and clinical covariates, including wave 1 nonmedical opioid use, significant associations persisted between wave 1 cannabis use and prevalent as well as incident nonmedical opioid use disorder at wave 2. A similar association was observed among adults without past-year cannabis use disorders and prevalent opioid use disorder, although the association with incident opioid use disorder fell below the level of statistical significance. Among adults with moderate or more severe pain at wave 1, cannabis use was associated with prevalent and incident opioid use disorders in unadjusted analyses and with prevalent opioid use disorder in adjusted analyses (Table 4).
| Wave 1 Past-Year Cannabis Use Predicting: | N in Analysis | Odds Ratio | 95% CI | Adjusted Odds Ratiob | 95% CI |
|---|---|---|---|---|---|
| Overall population | |||||
| Wave 2 prevalent opioid use disorder | 34,619 | 10.63 | 7.09–15.93 | 2.49 | 1.35–4.59 |
| Wave 2 incident opioid use disorder | 34,190 | 7.76 | 4.95–12.16 | 2.18 | 1.14–4.14 |
| Population without wave 1 cannabis use disorders | |||||
| Wave 2 prevalent opioid use disorder | 34,176 | 9.03 | 5.21–15.64 | 2.15 | 1.16–3.99 |
| Wave 2 incident opioid use disorder | 33,798 | 5.42 | 2.99–9.82 | 1.72 | 0.81–3.63 |
| Population with painc | |||||
| Wave 2 prevalent opioid use disorder | 6,934 | 13.21 | 7.03–24.83 | 3.70 | 1.70–8.08 |
| Wave 2 incident opioid use disorder | 6,772 | 6.70 | 3.08–14.55 | 2.14 | 0.95–4.83 |
TABLE 4. Prospective Associations of Wave 1 Cannabis Use and Wave 2 Prevalent and Incident Nonmedical Prescription Opioid Disorder in the NESARCa
Discussion
In a nationally representative sample of adults evaluated at waves 3 years apart, cannabis use was strongly associated with subsequent onset of nonmedical prescription opioid use and opioid use disorder. These results remained robust after controlling for the potentially confounding effects of several demographic and clinical covariates that were strongly associated with cannabis use. The association of cannabis use with the development of nonmedical opioid use was evident among adults without cannabis use disorders and among adults with moderate or more severe pain. Among adults with nonmedical prescription opioid use, cannabis use was associated with an increase in the level of nonmedical prescription opioid use at follow-up.
An independent prospective association between cannabis use and onset of prescription opioid use disorder extends results from previous epidemiological research concerning a link between cannabis use and other forms of problematic drug use (15–17). Previous work in this area has either been retrospective in design (15) or focused on general associations between cannabis use and substance use disorders (17) or problems (16) rather than specifically nonmedical opioid use or opioid use disorder. Because in the present study the association was observed among adults with less than disorder-level of cannabis use and followed a dose-response pattern, it suggests that some increased risk extends to a relatively large population of adult cannabis users. If cannabis use tends to increase opioid use, it is possible that the recent increase in cannabis use (30) may have worsened the opioid crisis.
Several factors may contribute to a tendency for individuals with cannabis use to develop opioid use disorder or increase the frequency of opioid use among opioid users. Heroin and Δ9-tetrahydrocannabinol (Δ9-THC) have similar effects on dopamine transmission through the μ1 opioid receptor (31). As compared with controls, adolescent rats exposed to Δ9-THC have been shown to develop enhanced heroin self-administration as adults (32). Also in relation to controls, rats exposed to Δ9-THC have been found to have a greater behavioral response to morphine challenge (33). These results are consistent with cross-sensitization between cannabis and opioids. In clinical research, cannabis use can lead to behavioral disinhibition, which can increase the risk of using other substances, including opioids (34). Access to cannabis may also provide increased availability and social exposure to other drugs of abuse through peer affiliations (35), although such environmental influences may be less powerful in recent years with increased prevalence of cannabis use and changing public attitudes.
Ecological studies reporting fewer opioid-related deaths (8, 9) and decreased opioid prescribing following passage of medical marijuana laws (10) have been interpreted in the media (6) and scientific literature (7) as supporting cannabis as a means of reducing opioid use disorder. Yet drawing inferences about the behavior of individuals from aggregated data can be misleading. It is possible, for example, that passage of medical marijuana laws increased local clinical awareness of opioid misuse, leading to earlier detection of high-risk patients or more cautious opioid prescribing practices. At the individual level, cannabis use appears to substantially increase the risk of nonmedical opioid use. Moreover, the general association between cannabis use and subsequent use of illicit drugs is not explained by the legal status of cannabis. An association of early cannabis use with increased subsequent risk of other drug abuse has been reported in prospective co-twin studies in Australia (15), which has restrictive cannabis laws, and in the Netherlands, where cannabis is readily available (36).
In accord with previous studies, several demographic and clinical covariates were associated with cannabis use (17). These findings converge to highlight the wide range of factors that may influence initiation of cannabis. However, because cannabis use was not associated with significant pain at baseline, relief from pain does not appear to be a strong determinant of cannabis use in the general U.S. adult population, although we have no means of evaluating the analgesic effects of cannabis with NESARC data.
This study has several limitations. First, the NESARC sampled individuals age 18 and older. The relationship between cannabis and opioid use may differ in younger individuals (16). Second, information on cannabis and opioid use was based on self-report and was not confirmed with urine toxicology, which may have led to underestimates. Third, the analysis was limited to two time points 3 years apart, which may have been too short an interval to observe delayed consequences of cannabis use on later risk of opioid use. Fourth, the data were collected over a decade ago, and the social context of cannabis use may have changed during this period (30). Nevertheless, the NESARC remains the most recent nationally representative prospective cohort of U.S. adults with detailed information on substance use. Fifth, we were unable to distinguish recreational from medical marijuana use. However, typical medical marijuana participants have been reported to be young males with a history of recreational cannabis use (37), and adults often combine medical and nonmedical cannabis use (38). Sixth, some of the associations are based on a small number of individuals and should therefore be interpreted with appropriate caution. Seventh, the NESARC did not assess inmate populations, which may have a high prevalence of substance use disorders (39). Finally, the assessment of nonmedical use of prescription opioids, although extensive, was not exhaustive and included two nonopioid medications (celecoxib and rofecoxib).
A long-standing controversy in drug research and policy concerns the extent to which use of cannabis predisposes to subsequent use of opioids and other drugs of abuse. We report that cannabis use, even among adults with moderate to severe pain, was associated with a substantially increased risk of nonmedical prescription opioid use at 3-year follow-up. Although the great majority of adults who used cannabis did not go on to initiate or increase their nonmedical opioid use, a strong prospective association between cannabis and opioid use disorder should nevertheless sound a note of caution in ongoing policy discussions concerning cannabis and in clinical debate over authorization of medical marijuana to reduce nonmedical use of prescription opioids and fatal opioid overdoses.
1 : Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA 2015; 314:1468–1478Crossref, Medline, Google Scholar
2 : Changes in the prevalence of non-medical prescription drug use and drug use disorders in the United States: 1991–1992 and 2001–2002. Drug Alcohol Depend 2007; 90:252–260Crossref, Medline, Google Scholar
3 Center for Behavioral Health Statistics and Quality: Key Substance Use and Mental Health Indicators in the United States: Results From the 2015 National Survey on Drug Use and Health (HHS Publication No SMA 16-4984, NSDUH Series H-51). Rockville, Md, Substance Abuse and Mental Health Services Administration, 2016Google Scholar
4 : Increases in drug and opioid-involved overdose deaths: United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016; 65:1445–1452Crossref, Medline, Google Scholar
5 : Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC, US Department of Health and Human Services, Office of the Surgeon General, 2016Google Scholar
6 : Patients are ditching opioid pills for weed: can marijuana help solve the opioid epidemic? Atlantic, Feb 2, 2017Google Scholar
7 : Could pot help solve the US opioid epidemic? Science, Nov 3, 2016Crossref, Google Scholar
8 : Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 2014; 174:1668–1673Crossref, Medline, Google Scholar
9 Powell D, Pacula RL, Jacobson M: Do medical marijuana laws reduce addictions and deaths related to pain killers? NBER Working Paper No 21345. Cambridge, Mass, National Bureau of Economic Research, 2015Google Scholar
10 : Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Aff (Millwood) 2016; 35:1230–1236Crossref, Medline, Google Scholar
11 : What ecologic analyses cannot tell us about medical marijuana legalization and opioid pain medication mortality. JAMA Intern Med 2015; 175:655–656Crossref, Medline, Google Scholar
12 : Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 2016; 17:739–744Crossref, Medline, Google Scholar
13 : Cannabinoids for medical use. JAMA 2015; 313:2456–2473Crossref, Medline, Google Scholar
14 : The prevalence and incidence of medicinal cannabis on prescription in the Netherlands. Eur J Clin Pharmacol 2013; 69:1575–1580Crossref, Medline, Google Scholar
15 : Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 2011; 90:844–851Crossref, Medline, Google Scholar
16 : Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA 2003; 289:427–433Crossref, Medline, Google Scholar
17 : Cannabis use and incidence of psychiatric disorders: prospective evidence from a national longitudinal study. JAMA Psychiatry 2016; 73:388–395Crossref, Medline, Google Scholar
18 : Source and Accuracy Statement: The Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Bethesda, Md, National Institute on Alcohol Abuse and Alcoholism, 2007Google Scholar
19 : Source and Accuracy Statement: Wave 1 of the 2001–2002 National Epidemiologic Survey of Alcohol and Related Conditions (NESARC). Bethesda, Md, National Institute on Alcohol Abuse and Alcoholism, 2003Google Scholar
20 : The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability of alcohol and drug modules in a clinical sample. Drug Alcohol Depend 1997; 44:133–141Crossref, Medline, Google Scholar
21 : The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend 1995; 39:37–44Crossref, Medline, Google Scholar
22 : The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol 2015; 50:1609–1640Crossref, Medline, Google Scholar
23 : Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol 2011; 174:929–933Crossref, Medline, Google Scholar
24 : The Spanish Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability and concordance with clinical diagnoses in a Hispanic population. J Stud Alcohol 1999; 60:790–799Crossref, Medline, Google Scholar
25 : The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression, and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend 2003; 71:7–16Crossref, Medline, Google Scholar
26 : A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–233Crossref, Medline, Google Scholar
27 : Quality of life following remission of mental disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2013; 74:e445–e450Crossref, Medline, Google Scholar
28 : SF-36 Health Survey: Manual and Interpretation Guide. Boston, New England Medical Center, Health Institute, 1993Google Scholar
29 : Pain as a predictor of opioid use disorder in a nationally representative sample. Am J Psychiatry 2016; 173:1189–1195Link, Google Scholar
30 : Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015; 72:1235–1242Crossref, Medline, Google Scholar
31 : Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 1997; 276:2048–2050Crossref, Medline, Google Scholar
32 : Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 2007; 32:607–615Crossref, Medline, Google Scholar
33 : Behavioural sensitization after repeated exposure to Δ9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 2001; 158:259–266Crossref, Medline, Google Scholar
34 : Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2011; 115:120–130Crossref, Medline, Google Scholar
35 : Exposure opportunity as a mechanism linking youth marijuana use to hallucinogen use. Drug Alcohol Depend 2002; 66:127–135Crossref, Medline, Google Scholar
36 : Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behav Genet 2006; 36:195–200Crossref, Medline, Google Scholar
37 : Self-reported cannabis use characteristics, patterns, and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014; 40:23–30Crossref, Medline, Google Scholar
38 : Use of marijuana for medical purposes among adults in the United States. JAMA 2017; 317:209–211Crossref, Medline, Google Scholar
39 : The effect of inmate populations on estimates of DSM-IV alcohol and drug use disorders in the United States. Am J Psychiatry 2010; 167:473–474Link, Google Scholar



