Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH?
Abstract
Objective:
The authors sought to assess associations among early-life exposure to adversity, the development and maturation of neurons and brain circuits, and neurodevelopmental outcomes. Specifically, they examined whether fetal exposure to placental corticotropin-releasing hormone (CRH), a molecule conveying maternal signals to the fetus, predicts brain growth and neuropsychiatric outcomes in school-age children.
Method:
In a large, well-characterized prospective cohort, concentrations of placental CRH (pCRH) in maternal plasma were determined during five intervals during gestation. When the children reached school age, their brain structures were examined using MRI, and emotional and cognitive tests assessing internalizing and externalizing behaviors and attention were administered (N=97, 49 of them girls).
Results:
Levels of pCRH during gestation predicted structural and functional brain outcomes in children. Specifically, fetal exposure to elevated pCRH levels was associated with thinning of selective cortical regions and with commensurate cognitive and emotional deficits. The relations among fetal exposure to pCRH, cortical thinning, and childhood function were sex specific.
Conclusions:
In view of the established effects of CRH on maturation and arborization of cortical neurons, and the major contribution of dendrites to cortical volume, these findings position pCRH as an important mediator of the consequences of early-life adversity on neuropsychiatric outcomes.
Exposure to early-life adversity disrupts brain development, resulting in altered brain networks and structure (1–3). These structural changes are associated with functional alterations that are often maintained throughout the lifespan, mediated by enduring epigenetic modifications of gene expression (2–4). Indeed, enduring alterations of brain structure and function at molecular, cellular, and circuit levels (5, 6) are considered fundamental mechanisms of how early-life experiences influence health and disease (1–5). In the aggregate, exposures to intrauterine and neonatal insults (7) and consequent altered brain anatomy (8, 9) and connectivity (10) contribute significantly to the global burden of mental illness.
Specifically, early-life exposure to maternal stress and trauma is linked to subsequent depression (11–13), posttraumatic stress disorder, panic disorder, substance abuse, abnormal stress response (13, 14), and other serious disorders (2, 3, 11). Emerging evidence suggests that exposure to early-life adversity may be causally related to brain changes underlying the risk for psychopathology (7, 15, 16). Because the fetal period is unmatched by any other in growth and development, this stage in the human lifespan is the most vulnerable to both organizing and disrupting maternal signals.
Among the most salient signals shaping the human fetus is the stress hormone corticotropin-releasing hormone (CRH). CRH, a 41-amino-acid neuropeptide that is normally synthesized primarily in the paraventricular nucleus of the hypothalamus, has a major role in regulating pituitary-adrenal function and the physiological response to stress (17, 18). CRH synthesized and released in other brain regions, such as the hippocampus (19) and the cortex (20, 21), contributes to the sculpting of neuronal dendritic development and maturation via its actions on specific receptors that are located on dendritic spines. Indeed, nanomolar concentrations of CRH have been shown to cause excessive pruning of dendritic trees of developing rodent cortical neurons (22). CRH of brain origin is not detectable in the circulation (23). However, during human pregnancy, the CRH gene, located on the long arm of chromosome 8 (24), is expressed in the human placenta and amniotic membrane (25, 26). Placental CRH (pCRH) is released into the maternal and fetal compartments as early as the eighth week of gestation and increases exponentially across gestation to regulate fetal maturation (27), metabolic functions (28, 29), and the timing of birth (30).
pCRH expression is responsive to a range of maternal stress signals, including increased cortisol, norepinephrine, and epinephrine; reduced uterine blood flow; and infection (31–33). Thus, pCRH represents an integrative pathway through which diverse prenatal stressors inform the fetus of the state of its environment and shape fetal developmental trajectories in preparation for life after birth (34, 35). There are several reports linking elevated human fetal exposure to pCRH, including links to decreased fetal startle and habituation (27, 36); delayed neonatal neuromotor development (37); increased infant fear and distress (38); and prodromal markers of increased risk for affective disorders in young children (39). Here, we demonstrate the potential consequences of fetal exposure to elevated levels of CRH and identify putative mechanisms. Specifically, we find that higher levels of pCRH are associated with cortical thinning in selective brain regions and with decreased cognitive and emotional function in preadolescents.
Method
All methods, human procedures, and protocols were approved by the Institutional Review Boards of the University of California, Irvine, the University of California, Los Angeles, and the Cedars-Sinai Medical Center, Los Angeles. Mothers gave written informed consent for all aspects of the protocol, and children (ages 6–9) provided assent.
Participants
Ninety-seven mother-child dyads participated in a longitudinal study of the effect of fetal exposure to maternal stress hormones on child MRI measures (see the CONSORT diagram in Figure S1 in the data supplement that accompanies the online edition of this article; see also Tables S1A–C in the data supplement). Women provided informed consent to provide blood samples at five intervals during gestation: 13.5–16.6 weeks (mean=15.3 weeks), 17.8–20.5 weeks (mean=19.2 weeks), 23.7–26.5 weeks (mean=24.9 weeks), 29.9–32.3 weeks (mean=30.9 weeks), and 34.6–38.1 weeks (mean=35.9 weeks). All women were English-speaking healthy adult (>18 years of age) pregnant women with singleton intrauterine pregnancies. Women were excluded if they had multiple births; used tobacco, alcohol, or others drugs during pregnancy; had uterine or cervical abnormalities; or had any conditions associated with dysregulated neuroendocrine function. Their children (48 boys, 49 girls) were enrolled in the study at 6–9 years of age (mean=7.3 years [SD=0.91]).
Assessments of pCRH in Pregnant Women
Gestational age at testing was determined by last menstrual period and was confirmed by obstetric ultrasonographic biometry before 20 weeks. Maternal blood samples (20 mL) were collected serially at five intervals throughout pregnancy by antecubital venipuncture into siliconized EDTA (purple top) Vacutainers and then immediately chilled to 60°C. Samples were centrifuged at 2,000 g for 15 minutes, decanted into polypropylene tubes prepared with aprotinin (Sigma Chemical, St. Louis; 500 KIU/mL blood), and stored at −80°C until assayed.
pCRH Determination
Following previously reported methods (40), the concentration of total maternal pCRH was determined by radioimmunoassay (Bachem Peninsula Laboratories, San Carlos, Calif.). The CRH assay had less than 0.01% cross-reactivity with ovine CRH, 36% cross-reactivity with bovine CRH, and nondetectable reactivity with human ACTH. The intra- and interassay coefficients of variance ranged from 5% to 15%. The minimum detectable dose of the assay is 2.04 pg/mL (see the online data supplement for additional assay details).
Data reduction for the radioimmunoassay was conducted with a computer-assisted four-parameter logistics program (41). Values exhibiting greater than 25% error (deviation from the standard curve) were not included in the analyses. A subset of samples (N=60) was sent to a clinical laboratory (Quest Diagnostics) for further validation. The correlation between the two sets of data was 0.87 (p<0.01). Shared variance between 19- and 31-week CRH samples was a modest 14% (r=0.38). Mother-child dyads were recruited from two separate studies with identical prenatal assessments. To ensure comparability between the cohorts for analysis, the pCRH values were standardized within the two groups and then combined for statistical analysis (see the data supplement for details). As expected, pCRH levels increased geometrically as gestation advanced (see Figure S4 and Table S9 in the data supplement).
Assessments in Children
All children (mean age, 87.3 months [SD=10.9]) had a stable neonatal course (median Apgar score, 9 [range, 8–10]; gestational age at birth, 39.2 weeks [SD=1.5]) and were without known neonatal illness or congenital, chromosomal, or genetic anomalies. Participants had no evidence of neurological abnormalities in the newborn period. Children’s structural MR images were assessed for normal anatomical appearance. Seven children with motion artifacts and three with abnormal scans were not included in the final sample (N=97). These 10 children did not differ significantly on any measure from those whose scans were usable (see Table S8 in the data supplement). At 6–9 years of age, no physical conditions were reported by the parents in a structured interview format of the MacArthur Health and Behavior Questionnaire (42). The majority of children (88%) were right hand dominant (Edinburgh Handedness Inventory) (43).
Structural MRI Acquisition
The structural MRI scan was acquired with a 3-T Philips Achieva system. Children received earplugs and watched a movie while in the scanner to increase compliance and minimize movement. A high-resolution T1 anatomical scan was acquired in the sagittal plane with 1 mm3 isotropic voxel dimensions. An inversion-recovery spoiled gradient recalled acquisition sequence with optimal parameters was applied: TR=11 ms, TE=3.3 ms, TI=1,100 ms, turbo field echo factor=192, number of slices: 150, no sensitivity encoding (SENSE) acceleration, flip angle=180°, shot interval (time from inversion pulse to the center of acquisition)=2,200 ms. The images were reviewed by the MRI operator (who was unaware of any of the study parameters) immediately after the scan was completed. If there were visible signs of motion artifacts, the subject was asked to stay for an additional scan. If the subject agreed, a second scan was acquired.
Processing of MRI Data
Cortical surface reconstruction and volumetric segmentation were performed with FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Streamlined image processing procedures included application of intensity normalization prior to segmentation to minimize errors in identifying the boundaries (44); removal of nonbrain tissues (45); and transformation of images into Talairach space. Pial and white matter surfaces were located by finding the highest intensity gradient. Surface inflation was applied to each individual brain (46), and the inflated brains were registered to a spherical atlas. Cortical thickness was the closest distance from the gray matter or white matter surface to the pial surface at each vertex on the tessellated surface (47).
Assessment of Percentage of Cortical Areas Affected by Fetal Exposure to pCRH
Smoothing of images was done prior to regression. The command line interface was used for false discovery rate corrections and to accommodate more than one nuisance variable. Gestational age at birth, birth weight, age of the child at testing, sex, and handedness were included as covariates. A threshold of p<0.05 was used for all statistical tests, and outcomes were corrected for multiple comparisons using false discovery rate.
After false discovery rate corrections and spatial normalization, the number of vertices that were significantly associated with pCRH was determined in each region of the cortex. The numbers of significant vertices were summed for each region and divided by the total number of vertices in that area to provide the percentage of vertices that were significantly associated with pCRH concentrations. The same procedure was used for the number of significant vertices in each lobe. For hemispheric and whole brain percentages, the procedure was the same except that the total number of subcortical vertices was subtracted from the total.
Child Behavioral Analyses
We conducted a battery of tests that interrogate several brain regions and circuits to obtain a relatively broad assessment of cognitive and emotional function.
The Child Behavior Checklist (48) is one of the most widely used parental interviews for identifying affective and conduct disorder problems in children. Structured interviews (rather than a questionnaire) were administered to mothers about their children’s behavior. We focused on two inclusive scales; one that assessed internalizing problems (sum of anxious-depressed, withdrawn-depressed, and somatic-complaints scores) and one that evaluated externalizing problems (sum of rule-breaking and aggressive behavior problem scores). Responses were recorded on a Likert-type scale ranging from 0 (not true) to 2 (very true or often true). Scores were summed, and standardized scores were computed that are age and sex specific.
Reaction Time to Incongruent Stimuli
The flanker task is an executive function task that requires the ability to resolve conflicts when competing information is present (49). Participants view five arrows arrayed horizontally on a screen and are instructed to press a left or right response button based on the direction of the center arrow (target). They are instructed to ignore the surrounding arrows, which are either congruent (all aligned in the same direction) or incongruent with the center arrow. This task consists of 24 congruent trials and 24 incongruent trials. Each set of arrows is presented until the child responds (maximum of 5,000 ms), with a 750-ms intertrial interval. Among the scores, median reaction time to targets with incongruent distractors correlates most highly with other indexes of performance derived from this test (all r values >0.70). The association between externalizing scores on the Child Behavior Checklist and the median reaction time on the flanker task was not significant (r=0.09, p=0.41).
Results
Elevated CRH Levels Throughout Gestation: Association With Cortical Thinning
Significant associations were observed between fetal exposure to pCRH averaged across gestation and areas of cortical thinning in children, as illustrated in the pial maps in Figure 1. Children exposed to high levels of pCRH throughout fetal life exhibited significant thinning in 12% of the whole cortical mantle (see Table S2A in the data supplement). The anatomical distribution of the cortical thinning associated with CRH exposure involved the left and right hemispheres equally (12% and 11% of hemisphere reduced, respectively) (see Table S2A in the data supplement). Regional analyses indicated that cortical thinning associated with pCRH levels was primarily in the temporal (15%) and frontal (16%) regions (Table 1). Panels B–D in Figure 1 are illustrative scatterplots of the associations between total fetal exposure to pCRH across gestation and cortical thinning in the frontal and temporal areas (all significant, p<0.01–0.001). The representative scatterplots, coupled with the data in Table 1, highlight the widespread association between fetal exposures to pCRH and regional cortical thinning, which is most notable in the temporal and frontal regions. Average concentrations of pCRH across gestation or levels at any gestational interval were not associated with increased cortical thickness either globally or in any region (see Table S2B in the data supplement). Focusing on two gestational ages, 19 weeks (early, when pCRH production begins to accelerate) and 31 weeks (late, the time strongly associated with preterm birth), we found that maternal levels of pCRH were significantly associated with cortical thinning.
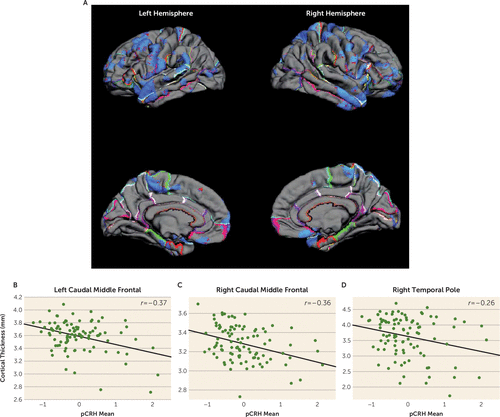
FIGURE 1. Pial Maps of Areas of Cortical Thinning Associated With Mean Levels of pCRH Throughout Gestation and Representative Scatterplots of Significant Associations Between pCRH and Thinning in Cortical Regionsa
a Panel A presents pial maps illustrating statistically significant areas of cortical thinning associated with average levels of placental corticotropin-releasing hormone (pCRH) measured throughout gestation. Panels B–D are representative scatterplots revealing significant associations (p values, <0.01–0.001) between pCRH (plotted as z-scores, with a mean of zero and standard deviation of 1) and cortical thinning in specific cortical regions.
| % of Structure | ||
|---|---|---|
| Structure | Left Hemisphere | Right Hemisphere |
| Medial surface of frontal lobe | ||
| Orbitofrontal gyrus | 11 | 19 |
| Paracentral gyrus | 43 | 14 |
| Lateral surface of frontal lobe | ||
| Superior frontal gyrus | 13 | 44 |
| Rostral middle frontal gyrus | 12 | 14 |
| Frontal pole | 41 | 33 |
| Pars triangularis | 16 | 21 |
| Pars orbitalis | 6 | 5 |
| Lateral orbitofrontal gyrus | 15 | 25 |
| Pars opercularis | 9 | 22 |
| Caudal middle frontal gyrus | 4 | 23 |
| Precentral gyrus | 18 | 20 |
| Insula | 11 | 8 |
| Lateral surface of temporal lobe | ||
| Superior temporal gyrus | 39 | 12 |
| Transverse temporal gyrus | 0 | 0 |
| Middle temporal gyrus | 22 | 24 |
| Posterior superior temporal sulcus | 16 | 0 |
| Inferior temporal gyrus | 3 | 12 |
| Fusiform gyrus | 7 | 2 |
| Parahippocampal gyrus | 11 | 0 |
| Entorhinal cortex | 31 | 0 |
| Temporal pole | 4 | 70 |
TABLE 1. Percentage of Structures of the Frontal and Temporal Lobes That Are Thinner in Children Exposed to Placental CRH Averaged Across Gestation
CRH Levels Early in Gestation: Regional Cortical Thinning and Behavioral Outcomes
Fetal exposure to pCRH early in gestation (19 weeks) was associated with significant thinning of the frontal poles. The significant association between pCRH and cortical thinning was bilateral but strongest in the right (B=−0.14, p<0.001; 78% of the structure) compared with the left frontal pole (B=−6.88, p<0.05; 69% of the structure) (see Table S3 in the data supplement). In Figure 2, pial maps depict areas of significant thinning linked with fetal pCRH exposure early in gestation, and the scatterplots illustrate the regions of the frontal cortex with the strongest associations. (Findings were essentially unchanged with log transformed pCRH values; see Table S5 in the data supplement.) Nearly identical associations were observed in a smaller subsample (N=57) of children exposed to high pCRH levels measured even earlier, at 15 weeks’ gestation (see Figure S3A–D in the data supplement).
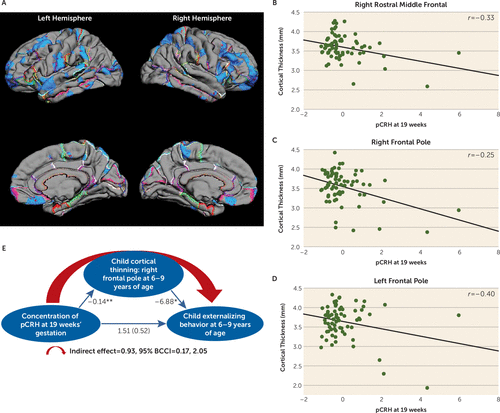
FIGURE 2. Pial Maps of Areas of Cortical Thinning Associated With Levels of pCRH at 19 Weeks’ Gestation, Representative Scatterplots of Significant Associations Between pCRH and Thinning in Cortical Regions, and a Model of the Indirect Association Among pCRH Concentrations, Cortical Thinning in the Frontal Pole, and Externalizing Behaviora
a Panel A presents pial maps illustrating statistically significant areas of cortical thinning associated with levels of placental corticotropin-releasing hormone (pCRH) at 19 weeks’ gestation. Panels B–D are representative scatterplots of the significant associations (p values, 0.04–0.001) between pCRH (plotted as z-scores, with a mean of zero and standard deviation of 1) and thinning in specific cortical regions. Panel E is a model of the indirect significant association among prenatal pCRH concentrations, child cortical thinning in the frontal pole, and child externalizing behavior. The values corresponding to each path in the model are unstandardized regression coefficients. The indirect effect was estimated with bootstrapping (1,000 samples with replacement). BCCI=bias-corrected confidence interval.
Thinning of the frontal pole has been associated with externalizing behaviors (50), defined as actions that direct energy outward and tend to harm others (51). We tested whether the effects of pCRH exposure on cortical thinning contributed to the development of these behaviors (Figure 2E). Our model supported the belief that reduced cortical volume in the frontal pole, associated with elevated fetal exposure to concentrations of pCRH at 19 weeks’ gestation, contributes to externalizing symptoms in 6- to 9-year-old children (indirect effect=0.93; 95% bias-corrected confidence interval [BCCI]=0.17, 2.05; p<0.05). Neither internalizing problems nor reaction time on the flanker task was associated with cortical thinning in this region.
CRH Levels in Late Gestation: Regional Cortical Thinning and Cognitive Outcomes
Exposure to elevated levels of pCRH later in gestation (31 weeks) was associated with cortical thinning in the lateral temporal and paracentral regions (Figure 3A). The effect was localized to the left paracentral region and was bilateral in the temporal cortex. Remarkably, 98% of the lateral surface of the right temporal pole and 66% of the left temporal pole was significantly thinner in children exposed to high levels of pCRH at 31 weeks’ gestation (see Table S4 in the data supplement). The scatterplots in Figure 3 illustrate these regional associations. (Similar associations were observed at 25 weeks’ gestation in a smaller subsample [N=50].)
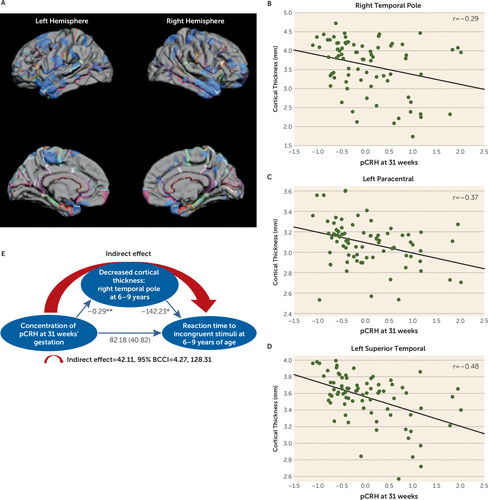
FIGURE 3. Pial Maps of Areas of Cortical Thinning Associated With Levels of pCRH at 31 Weeks’ Gestation, Representative Scatterplots of Significant Associations Between pCRH and Thinning in Cortical Subregions, and a Model of the Indirect Association Among pCRH Concentrations, Cortical Thinning in the Right Temporal Pole, and Child Reaction Time to a Behavioral Challenge Taska
a Panel A presents pial maps illustrating statistically significant areas of cortical thinning associated with levels of placental corticotropin-releasing hormone (pCRH) at 31 weeks’ gestation. Panels B–D are representative scatterplots of the significant associations (p values, 0.01–0.001) between pCRH (plotted as z-scores, with a mean of zero and standard deviation of 1) and thinning in cortical subregions. Panel E is a model of the indirect association among prenatal pCRH concentrations, child cortical thinning in the right temporal pole, and child reaction time in a behavioral challenge task (a visual processing and sustained attention test). The values corresponding to each path in the model are unstandardized regression coefficients. The indirect effect was estimated with bootstrapping (1,000 samples with replacement). BCCI=bias-corrected confidence interval.
Structures comprising the temporal cortex subserve numerous cognitive and emotional functions. Temporal cortical thinning has been reported in individuals with attentional deficits (52), especially in the right temporal pole (53), which is active in tasks requiring attention to relevant stimulation (54). We employed a statistical model (55) to test whether the effects of exposure to pCRH late in prenatal life on cortical thinning contributed to impairment of attention in 6- to 9-year-olds. The model (Figure 3E) indicated that the reduced right temporal pole volume associated with exposure to pCRH at 31 weeks’ gestation may partially account for poorer performance on a visual processing and sustained attention test (indirect effect=42.11; 95% BCCI=4.27, 128.31; p<0.01) (56, 57).
Sex Differences
Exploratory analyses suggest significant sex differences in the nature and degree of association between pCRH levels and global as well as regional cortical thinning. At both 19 and 31 weeks’ gestation, the associations were stronger in girls (see Figure S2 in the data supplement). The association between fetal exposure to pCRH at 19 weeks’ gestation and cortical thinning involved most cortical areas in girls (see Figure S2A) but minimal areas in boys (see Figure S2B). Fetal exposure to pCRH at 31 weeks affected cortical thinning globally in boys (see Figure S2C) but locally in the temporal pole in girls (see Figure S2D; similar to the findings for the combined sexes).
Discussion
The principal and novel findings in this series of studies are that human fetal exposure to pCRH, even at concentrations that are insufficient to initiate labor or early delivery, is associated with regional cortical thinning and commensurate cognitive and emotional problems in a sex-specific manner in school-age children. Notably, such problems often are prodromal events that are associated with eventual neuropsychiatric outcomes.
The human placenta expresses pCRH by the eighth week of gestation, and concentrations of pCRH increase geometrically as pregnancy advances to regulate the timing and onset of labor and delivery (30, 58). Indeed, extremely high levels of pCRH, often a result of adverse events during pregnancy, stimulate a cascade of events that results in preterm labor and delivery. Premature birth is a significant contributing factor to impaired neurological and psychiatric outcomes. However, pCRH levels also rise in response to a variety of maternal stresses, and the novel finding here is the profound consequence of fetal exposure to pCRH at levels that are not associated with early delivery in full-term school-age children. Specifically, we identify pronounced thinning in discrete cortical areas with implications for emotional and cognitive functions. Pronounced cortical thinning was associated with fetal exposure to pCRH across gestation, with some evidence of localization in the prefrontal and temporal poles. Examination of discrete gestational intervals clarified the fact that prefrontal thinning was associated with exposure at early midgestation, and thinning of the temporal pole was associated with exposure later in gestation.
Our findings raise the novel possibility that CRH is causally linked to cortical thinning rather than merely signifying the presence of a harsh, stressful milieu for the developing fetal brain. A causal role for CRH in cortical thinning is supported by recent findings in an animal model (22). Cortical volume is largely made up of dendritic trees of cortical neurons (22, 59), and changes in dendritic arborization can be measured as volume loss in MR imaging (60). Our recent experiments (22) demonstrate that exposure of developing cortical neurons to physiological levels of CRH results in a dose-dependent reduction and impoverishment of dendritic arborization. In addition, exposure to early postnatal CRH in vivo (61) and to maternal stress signals (62, 63) both provoke similarly impoverished dendritic trees in the hippocampus. Indeed, exposure to nanomolar levels of CRH has been shown to reduce dendritic length and complexity via CRH receptor type 1 (64), which is expressed on the dendrites (65, 66). Eliminating the actions of endogenous CRH, both in transgenic mice lacking the CRH receptor and via chronic exposure of organotypic slice cultures to CRH receptor blockers (64, 65), has been shown to lead to exuberant dendritic trees. Thus, it appears that the role of physiological levels of CRH is to modulate—perhaps in concert with glucocorticoids (67, 68)—neuronal dendritic development in the perinatal hippocampus and neocortex.
The sex differences observed here are intriguing and consistent with increased prevalence of stress-related disorders in women compared with men—tendencies also observed in prepubertal children (69). Our novel findings here suggest that sex-specific structural consequences of early-life adversity and potentially of CRH may be most apparent in females and are consistent with conclusions that females exposed to prenatal stress are more likely than males to exhibit increased levels of anxiety, impaired executive function, and neurological markers associated with these behaviors (69).
In summary, our findings uncover a novel and unexpected result of prenatal elevation of pCRH: reduction in cortical volume in typically developing children, perhaps related to stunting of normal neuronal growth, and consequent commensurate subtle but significant emotional and cognitive impairments. The behavioral assessments were not designed a priori for assessing the unexpected cortical thinning observed in the temporal lobes, and this limitation should be addressed in future studies. Even when CRH levels are not sufficient to trigger premature birth and children are born at term, fetuses exposed to increased levels of pCRH carry less well developed cortical neurons, apparent from extensive yet selective areas of cortical thinning. The cortical thinning is biologically significant because it is associated with both cognitive and emotional deficits.
1 : A common cause for a common phenotype: the gatekeeper hypothesis in fetal programming. Med Hypotheses 2012; 78:88–94Crossref, Medline, Google Scholar
2 : Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 2010; 167:1305–1320Link, Google Scholar
3 : Early life programming and neurodevelopmental disorders. Biol Psychiatry 2010; 68:314–319Crossref, Medline, Google Scholar
4 : Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem 2011; 96:79–88Crossref, Medline, Google Scholar
5 : NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol Psychiatry (Epub ahead of print, Jan 10, 2017)Google Scholar
6 : Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017; 356:1185–1188Crossref, Medline, Google Scholar
7 : Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 2012; 379:445–452Crossref, Medline, Google Scholar
8 : MR-based in vivo hippocampal volumetrics, 2: findings in neuropsychiatric disorders. Mol Psychiatry 2005; 10:160–184Crossref, Medline, Google Scholar
9 : Brain anatomy of major depression, II: focus on amygdala. Epidemiol Psychiatr Sci 2011; 20:33–36Crossref, Medline, Google Scholar
10 : Sex differences in the relationship between white matter connectivity and creativity. Neuroimage 2014; 101:380–389Crossref, Medline, Google Scholar
11 : The role of childhood parent figure loss in the etiology of adult depression: findings from a prospective longitudinal study. Attach Hum Dev 2009; 11:445–470Crossref, Medline, Google Scholar
12 : The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001; 49:1023–1039Crossref, Medline, Google Scholar
13 : Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry 2000; 48:778–790Crossref, Medline, Google Scholar
14 : Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev 2010; 35:23–32Crossref, Medline, Google Scholar
15 : Broader focus required to understand the effects of the perinatal environment on child neurodevelopment: response to Bell and Chimata (letter). Am J Psychiatry 2017; 174:999–1000Link, Google Scholar
16 : Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc Natl Acad Sci USA 2017; 114:10390–10395Crossref, Medline, Google Scholar
17 : Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213:1394–1397Crossref, Medline, Google Scholar
18 : Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis: the corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am 1992; 21:833–858Crossref, Medline, Google Scholar
19 : Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci 2001; 21:7171–7181Crossref, Medline, Google Scholar
20 : Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Exp Brain Res 1998; 123:334–340Crossref, Medline, Google Scholar
21 : Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature 1995; 378:284–287Crossref, Medline, Google Scholar
22 : Abnormal dendritic maturation of developing cortical neurons exposed to corticotropin releasing hormone (CRH): insights into effects of prenatal adversity? PLoS One 2017; 12:e0180311Crossref, Medline, Google Scholar
23 : The regulation of human corticotrophin-releasing hormone gene expression in the placenta. Peptides 2001; 22:1941–1947Crossref, Medline, Google Scholar
24 : Human corticotropin releasing hormone gene is located on the long arm of chromosome 8. Cytogenet Cell Genet 1988; 47:113–116Crossref, Medline, Google Scholar
25 : The corticotropin releasing hormone gene is expressed in human placenta. Biochem Biophys Res Commun 1987; 148:1208–1214Crossref, Medline, Google Scholar
26 : Expression of the corticotropin-releasing hormone (CRH) gene in human placenta and amniotic membrane. Horm Metab Res 1990; 22:394–397Crossref, Medline, Google Scholar
27 : Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci 2008; 30:419–426Crossref, Medline, Google Scholar
28 : Functions of corticotropin-releasing hormone in anthropoid primates: from brain to placenta. Am J Hum Biol 2006; 18:431–447Crossref, Medline, Google Scholar
29 : Fetal programming of children’s obesity risk. Psychoneuroendocrinology 2015; 53:29–39Crossref, Medline, Google Scholar
30 : Corticotrophin releasing hormone and the timing of birth. Front Biosci 2007; 12:912–918Crossref, Medline, Google Scholar
31 : The endocrinology of human pregnancy and fetal-placental neuroendocrine development, in Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Edited by Strauss J, Barbieri R. Philadelphia, WB Saunders, 2014, pp 243–271Crossref, Google Scholar
32 : Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol 1989; 160:247–251Crossref, Medline, Google Scholar
33 : Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J 2001; 5:119–125Crossref, Medline, Google Scholar
34 : Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci 2009; 31:285–292Crossref, Medline, Google Scholar
35 : Prenatal programming of human neurological function. Int J Pept 2011; 2011:1–9Crossref, Google Scholar
36 : Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Dev Neurosci 2003; 25:41–49Crossref, Medline, Google Scholar
37 : Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol 2008; 50:232–241Crossref, Medline, Google Scholar
38 : Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci 2005; 27:299–305Crossref, Medline, Google Scholar
39 : Fetal exposure to placental corticotropin-releasing hormone is associated with child self-reported internalizing symptoms. Psychoneuroendocrinology 2016; 67:10–17Crossref, Medline, Google Scholar
40 : Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med 2014; 76:355–362Crossref, Medline, Google Scholar
41 Rodbard D, Munson PJ, DeLean A: Improved curve-fitting, parallelism testing, characterisation of sensitivity and specificity, validation, and optimization for radioligand assays, in Radioimmunoassay and Related Procedures in Medicine 1977: Proceedings of an International Symposium on Radioimmunoassay and Related Procedures in Medicine held by the International Atomic Energy Agency in Co-operation with the World Health Organization in Berlin (West), 31 October–4 November 1977. Vienna, International Atomic Energy Agency, 1978, pp 469–503Google Scholar
42 : The confluence of mental, physical, social, and academic difficulties in middle childhood, I: exploring the “head waters” of early life morbidities. J Am Acad Child Adolesc Psychiatry 2002; 41:580–587Crossref, Medline, Google Scholar
43 : The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
44 : A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998; 17:87–97Crossref, Medline, Google Scholar
45 : Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14:11–22Crossref, Medline, Google Scholar
46 : High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999; 8:272–284Crossref, Medline, Google Scholar
47 : Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97:11050–11055Crossref, Medline, Google Scholar
48 : Manual for the ASEBA School-Age Forms and Profiles: An Integrated System of Multi-Informant Assessment. Burlington, University of Vermont, Research Center for Children, Youth, and Families, 2001Google Scholar
49 : Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 1974; 16:143–149Crossref, Google Scholar
50 : Right frontal pole cortical thickness and social competence in children with chronic traumatic brain injury: cognitive proficiency as a mediator. J Head Trauma Rehabil 2015; 30:E24–E31Crossref, Medline, Google Scholar
51 : Process, mechanism, and explanation related to externalizing behavior in developmental psychopathology. J Abnorm Child Psychol 2002; 30:431–446Crossref, Medline, Google Scholar
52 : Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry Clin Neurosci 2010; 64:394–402Crossref, Medline, Google Scholar
53 : Cortical thinning of temporal pole and orbitofrontal cortex in medication-naïve children and adolescents with ADHD. Psychiatry Res 2014; 224:8–13Crossref, Medline, Google Scholar
54 : Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex 2008; 18:262–271Crossref, Medline, Google Scholar
55 : Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behav Res 2010; 45:627–660Crossref, Medline, Google Scholar
56 : The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol 2002; 17:235–272Crossref, Medline, Google Scholar
57 : Isolating attentional systems: a cognitive-anatomical analysis. Psychobiology 1987; 15:107–121Google Scholar
58 : A placental clock controlling the length of human pregnancy. Nat Med 1995; 1:460–463Crossref, Medline, Google Scholar
59 : Brain development, in Handbook of Adolescent Psychology. Edited by Lerner RM, Steinberg L. Hoboken, NJ, John Wiley & Sons, 2009, pp 95–115Crossref, Google Scholar
60 : MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus 2016; 26:1618–1632Crossref, Medline, Google Scholar
61 : Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA 2001; 98:8856–8861Crossref, Medline, Google Scholar
62 : Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 2005; 25:9328–9338Crossref, Medline, Google Scholar
63 : Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci 2010; 30:13005–13015Crossref, Medline, Google Scholar
64 : Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA 2004; 101:15782–15787Crossref, Medline, Google Scholar
65 : Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci 2008; 28:2903–2911Crossref, Medline, Google Scholar
66 : Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci 2012; 6:13Crossref, Medline, Google Scholar
67 : Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA 2011; 108:16074–16079Crossref, Medline, Google Scholar
68 : Converging, synergistic actions of multiple stress hormones mediate enduring memory impairments after acute simultaneous stresses. J Neurosci 2016; 36:11295–11307Crossref, Medline, Google Scholar
69 : Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res 2013; 75:327–335Crossref, Medline, Google Scholar



