Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis
Abstract
Objective:
Structural brain imaging studies in obsessive-compulsive disorder (OCD) have produced inconsistent findings. This may be partially due to limited statistical power from relatively small samples and clinical heterogeneity related to variation in illness profile and developmental stage. To address these limitations, the authors conducted meta- and mega-analyses of data from OCD sites worldwide.
Method:
T1 images from 1,830 OCD patients and 1,759 control subjects were analyzed, using coordinated and standardized processing, to identify subcortical brain volumes that differ between OCD patients and healthy subjects. The authors performed a meta-analysis on the mean of the left and right hemisphere measures of each subcortical structure, and they performed a mega-analysis by pooling these volumetric measurements from each site. The authors additionally examined potential modulating effects of clinical characteristics on morphological differences in OCD patients.
Results:
The meta-analysis indicated that adult patients had significantly smaller hippocampal volumes (Cohen’s d=−0.13; % difference=−2.80) and larger pallidum volumes (d=0.16; % difference=3.16) compared with adult controls. Both effects were stronger in medicated patients compared with controls (d=−0.29, % difference=−4.18, and d=0.29, % difference=4.38, respectively). Unmedicated pediatric patients had significantly larger thalamic volumes (d=0.38, % difference=3.08) compared with pediatric controls. None of these findings were mediated by sample characteristics, such as mean age or scanning field strength. The mega-analysis yielded similar results.
Conclusions:
The results indicate different patterns of subcortical abnormalities in pediatric and adult OCD patients. The pallidum and hippocampus seem to be of importance in adult OCD, whereas the thalamus seems to be key in pediatric OCD. These findings highlight the potential importance of neurodevelopmental alterations in OCD and suggest that further research on neuroplasticity in OCD may be useful.
Obsessive-compulsive disorder (OCD) is a neurodevelopmental disorder that affects 1%–3% of the population (1, 2). In more than half of OCD cases, symptoms emerge during childhood or adolescence (1, 3), and in more than 40% of these cases, the disorder persists into adulthood (4). OCD symptoms have been associated with structural and functional brain abnormalities in the parallel cortico-striato-thalamo-cortical circuits and other related brain networks, involving fronto-parietal, fronto-limbic, and cerebellar regions (5, 6).
Several studies have shown volumetric abnormalities in different deep gray matter structures, mainly the basal ganglia (7–10). Meta-analyses have repeatedly, although not consistently, reported larger volumes in the lenticular nucleus extending to the caudate (11–14). In addition, Pujol et al. (7) showed that the relative enlargement of striatal areas in OCD patients was driven by higher age and longer illness duration, suggesting that basal ganglia alterations progress throughout the illness course, which is supported by the mega-analysis from the OCD Brain Imaging Consortium (15). These findings led to the hypothesis that preservation of basal ganglia volume resulted from neuroplastic changes due to chronic compulsivity.
Although these findings suggest ongoing neuroplasticity, a lifespan approach has seldom been used to understand the variation in structural abnormalities in OCD (5). Studying the brain characteristics of illness during childhood may minimize the potentially confounding effects of neuroplastic changes associated with chronic symptoms and long-term treatment. Pediatric studies have been sparse and small, leaving the extant findings inconclusive and variable. For example, some studies reported increased thalamus volume in adult (16, 17) and pediatric OCD patients (18), a finding supported by two meta-analyses (14, 19) showing larger thalamus volumes in OCD patients when pediatric and adult data were combined. In contrast, several recent meta-analyses showed no differences in thalamus volumes when adult and pediatric subjects were combined (11–13). The variation across studies may partially be explained by variations in the developmental and illness stages of the subjects included.
In view of the clinical heterogeneity of OCD, relatively small samples and differences in data acquisition, data processing protocols, and statistical analyses further contribute to the inconsistent findings. Different segmentation algorithms may produce variable estimates of subcortical volumes and therefore may influence their sensitivity to detect regionalized group differences (20). To overcome the heterogeneity in image processing and to increase sample sizes, especially pediatric samples, we initiated the OCD Working Group within the Enhancing NeuroImaging Genetics Through Meta-Analysis (ENIGMA) consortium (21).
The ENIGMA OCD Working Group is an international collaboration. Its current aim is to identify subcortical imaging markers that differ in OCD patients and healthy subjects, both in children and in adults. Therefore, we conducted meta- and mega-analyses on structural MRI data from 1,830 OCD patients and 1,759 healthy control subjects. The mega-analysis ensures information preservation and enables the examination of specific effects of demographic and clinical parameters. By employing meta- and mega-analysis, we sought to investigate whether the mega-analytic design has greater sensitivity to detect more subtle brain abnormalities from increased statistical power.
In this study, we investigated nine regions of interest (seven subcortical gray matter regions, the lateral ventricle, and total intracranial volume) in OCD patients compared with healthy control subjects by performing the largest meta- and mega-analyses to date. In additional exploratory analyses, we examined potential modulating effects of demographic, clinical, and methodological characteristics on subcortical brain volume in OCD. Based on previous meta- and mega-analyses, we expected subcortical brain volumes to vary across developmental stage, showing differences between pediatric and adult OCD, and across illness profile and stage, including comorbidity.
Method
Samples
The ENIGMA OCD Working Group includes 35 data sets from 25 international research institutes, with neuroimaging and clinical data from OCD patients and healthy control subjects, including both children and adults. We considered subjects age 18 and older as adults and those under age 18 as children. Because the literature has suggested differential effects between pediatric and adult samples, we performed separate meta- and mega-analyses for adult and pediatric data. The demographic and clinical characteristics of the participants at each center are summarized in Tables 1 and 2. In total, we analyzed data from 3,589 subjects, including 1,830 OCD patients (335 children and 1,495 adults) and 1,759 control subjects (287 children and 1,472 adults). All local institutional review boards permitted the use of extracted measures of the completely anonymized data.
| Study Principal Investigator | Site | Field Strength (teslas) | Age (years) | Control Subjects (N) | OCD Patients (N) | Total (N) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Subjects | OCD Patients | Male (%) | |||||||||
| Mean | SD | Mean | SD | Control Subjects | OCD | ||||||
| Adult samples | |||||||||||
| Benedetti | Milan | 3.0 | 34.0 | 12.3 | 35.0 | 10.4 | 73 | 71 | 62 | 66 | 128 |
| Beucke | Berlin | 1.5 | 31.9 | 9.5 | 32.4 | 9.7 | 49 | 50 | 104 | 92 | 196 |
| Cheng | Kunming I | 1.5 | 31.4 | 8.0 | 30.6 | 10.2 | 33 | 38 | 40 | 24 | 64 |
| Kunming II | 3.0 | 26.2 | 4.2 | 32.9 | 10.6 | 28 | 55 | 95 | 56 | 151 | |
| Denys | Amsterdam | 3.0 | 39.6 | 10.3 | 33.8 | 9.6 | 44 | 21 | 25 | 24 | 49 |
| van den Heuvel | Amsterdam I | 1.5 | 31.6 | 7.7 | 33.5 | 9.2 | 39 | 30 | 49 | 54 | 103 |
| Amsterdam II | 3.0 | 39.6 | 11.4 | 38.3 | 10.1 | 47 | 48 | 38 | 42 | 80 | |
| Hoexter | São Paulo I | 1.5 | 27.6 | 7.8 | 31.5 | 10.1 | 35 | 44 | 37 | 50 | 87 |
| Koch | Munich | 3.0 | 30.2 | 9.0 | 31.1 | 9.7 | 40 | 33 | 75 | 72 | 147 |
| Kwon | Seoul I | 1.5 | 24.0 | 3.6 | 24.8 | 5.4 | 56 | 76 | 104 | 45 | 149 |
| Seoul II | 1.5 | 24.9 | 5.3 | 28.8 | 6.8 | 64 | 56 | 45 | 34 | 79 | |
| Seoul III | 3.0 | 26.3 | 6.9 | 26.3 | 6.8 | 61 | 61 | 89 | 90 | 179 | |
| Mataix-Cols | Stockholm | 1.5 | 36.1 | 11.3 | 38.7 | 10.9 | 36 | 43 | 33 | 44 | 77 |
| Menchon | Barcelona | 1.5 | 33.1 | 10.2 | 34.8 | 9.2 | 45 | 50 | 66 | 117 | 183 |
| Nakamae | Kyoto I | 1.5 | 30.3 | 7.8 | 31.7 | 9.3 | 52 | 49 | 48 | 82 | 130 |
| Kyoto II | 3.0 | 30.0 | 7.4 | 33.3 | 9.7 | 48 | 35 | 42 | 34 | 76 | |
| Nakao | Fukuoka | 3.0 | 39.3 | 13.0 | 36.6 | 10.0 | 39 | 42 | 41 | 81 | 122 |
| Reddy | Bangalore I | 1.5 | 27.2 | 6.4 | 27.5 | 6.3 | 74 | 59 | 46 | 44 | 90 |
| Bangalore II | 3.0 | 26.3 | 5.0 | 29.6 | 8.0 | 62 | 52 | 156 | 208 | 364 | |
| Simpson | New York | 3.0 | 28.3 | 8.0 | 29.6 | 8.0 | 52 | 52 | 33 | 33 | 66 |
| Spalletta | Rome | 3.0 | 36.5 | 10.5 | 36.7 | 11.6 | 59 | 67 | 128 | 84 | 212 |
| Stein | Cape Town | 3.0 | 30.6 | 10.8 | 30.7 | 10.8 | 38 | 50 | 29 | 22 | 51 |
| Tolin | Connecticut | 3.0 | 48.0 | 11.9 | 32.1 | 12.0 | 22 | 67 | 32 | 27 | 59 |
| Walitza | Zurich I | 3.0 | 32.9 | 9.2 | 31.2 | 7.7 | 28 | 47 | 18 | 17 | 35 |
| Wang | Shanghai | 3.0 | 26.2 | 7.5 | 29.6 | 9.3 | 54 | 57 | 37 | 53 | 90 |
| Total, adult samples | 1,472 | 1,495 | 2,967 | ||||||||
| Pediatric samples | |||||||||||
| Arnold | Ontario | 3.0 | 12.3 | 2.2 | 12.9 | 2.4 | 54 | 58 | 13 | 40 | 53 |
| Fitzgerald | Michigan | 3.0 | 12.9 | 2.9 | 13.9 | 2.6 | 52 | 49 | 67 | 74 | 141 |
| Gruner | Connecticut | 3.0 | 14.2 | 2.2 | 14.3 | 2.1 | 52 | 57 | 23 | 23 | 46 |
| Hoexter | São Paulo II | 3.0 | 12.0 | 2.4 | 12.6 | 2.5 | 57 | 61 | 28 | 28 | 56 |
| Huyser | Amsterdam | 3.0 | 13.3 | 2.5 | 13.6 | 2.5 | 36 | 37 | 25 | 27 | 52 |
| Lazaro | Barcelona I | 1.5 | 14.6 | 2.3 | 14.6 | 2.0 | 47 | 58 | 32 | 31 | 63 |
| Barcelona II | 3.0 | 14.6 | 2.1 | 14.6 | 2.0 | 55 | 60 | 44 | 58 | 102 | |
| Reddy | Bangalore III | 3.0 | 13.1 | 2.1 | 14.6 | 2.0 | 50 | 56 | 14 | 18 | 32 |
| Soreni | Ontario | 3.0 | 11.2 | 3.1 | 13.4 | 2.5 | 52 | 40 | 21 | 20 | 41 |
| Walitza | Zurich II | 3.0 | 14.6 | 1.3 | 15.7 | 1.4 | 50 | 81 | 20 | 16 | 36 |
| Total, pediatric samples | 287 | 335 | 622 | ||||||||
| Total, adult and pediatric samples | 1,759 | 1,830 | 3,589 | ||||||||
TABLE 1. Breakdown, by Site, of Numbers, Age, and Sex of Patients With Obsessive-Compulsive Disorder (OCD) and Healthy Control Subjects in the ENIGMA OCD Working Group Samples
| Study Principal Investigator | Site | Medicated (%) | YBOCSaScore | Age at Onset (years) | Lifetime Comorbid Disorders | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Anxiety (%) | Depression (%) | |||
| Adult samples | ||||||||
| Benedetti | Milan | 64 | 30.9 | 5.6 | 16.0 | 6.1 | 1.5 | 10.6 |
| Beucke | Berlin | 40 | 20.1 | 7.1 | 17.2 | 7.8 | 12.0 | 18.5 |
| Cheng | Kunming I | 71 | 31.0 | 6.1 | 26.8 | 10.4 | 50.0 | 16.7 |
| Kunming II | 68 | 28.2 | 6.3 | 27.2 | 10.7 | 89.3 | 28.6 | |
| Denys | Amsterdam | 63 | 26.6 | 6.2 | 18.1 | 6.9 | 4.2 | 41.7 |
| van den Heuvel | Amsterdam I | 0 | 22.7 | 6.1 | 14.4 | 7.7 | 22.2 | 33.3 |
| Amsterdam II | 0 | 21.5 | 6.1 | 15.5 | 6.9 | 40.5 | 52.4 | |
| Hoexter | São Paulo I | 20 | 27.2 | 6.1 | 13.1 | 7.0 | 62.0 | 54.0 |
| Koch | Munich | 60 | 20.9 | 6.2 | 17.0 | 6.7 | — | — |
| Kwon | Seoul I | 24 | 20.2 | 6.0 | 17.4 | 5.2 | 0.0 | 0.0 |
| Seoul II | 0 | 23.9 | 6.5 | 18.9 | 6.6 | 0.0 | 2.9 | |
| Seoul III | 2 | 26.5 | 6.5 | 19.0 | 6.4 | 1.1 | 2.2 | |
| Mataix-Cols | Stockholm | 41 | 25.9 | 7.7 | 18.4 | 9.2 | 27.3 | 34.1 |
| Menchon | Barcelona | 97 | 25.5 | 5.8 | 21.4 | 8.5 | 20.5 | 18.8 |
| Nakamae | Kyoto I | 49 | 25.2 | 6.4 | 25.1 | 9.4 | 9.8 | 22.0 |
| Kyoto II | 0 | 22.4 | 6.9 | 25.2 | 9.1 | 8.8 | 20.6 | |
| Nakao | Fukuoka | 88 | 22.5 | 5.6 | 24.6 | 9.5 | — | 35.8 |
| Reddy | Bangalore I | 0 | 25.8 | 7.3 | 21.7 | 7.5 | 15.9 | 18.2 |
| Bangalore II | 40 | 25.8 | 6.3 | 22.0 | 7.6 | 7.7 | 15.4 | |
| Simpson | New York | 0 | 25.5 | 3.7 | 15.0 | 7.0 | 21.2 | 30.3 |
| Spalletta | Rome | 88 | 23.4 | 8.9 | 18.9 | 10.9 | 9.5 | 9.5 |
| Stein | Cape Town | 41 | 22.9 | 4.2 | 13.6 | 6.6 | 0.0 | 0.0 |
| Tolin | Connecticut | 78 | 22.7 | 4.8 | — | — | 44.4 | 40.7 |
| Walitza | Zurich I | 59 | 17.1 | 9.9 | 16.7 | 7.8 | 47.1 | 47.1 |
| Wang | Shanghai | 0 | 25.5 | 5.1 | 23.3 | 10.3 | 0.0 | 0.0 |
| Pediatric samples | ||||||||
| Arnold | Ontario | 53 | 20.9 | 7.8 | 8.7 | 2.6 | 25.0 | 17.5 |
| Fitzgerald | Michigan | 50 | 18.7 | 7.8 | 9.9 | 3.0 | 50.0 | 6.8 |
| Gruner | Connecticut | 52 | 26.9 | 4.5 | — | — | 43.5 | 39.1 |
| Hoexter | São Paulo II | 46 | 26.9 | 5.4 | 7.2 | 3.0 | 21.4 | 0.0 |
| Huyser | Amsterdam | 0 | 25.1 | 5.0 | 10.9 | 2.8 | 48.2 | 25.9 |
| Lazaro | Barcelona I | 55 | 22.2 | 6.0 | 12.4 | 2.2 | 16.1 | 3.2 |
| Barcelona II | 79 | 18.6 | 7.4 | 12.0 | 2.4 | 25.9 | 5.2 | |
| Reddy | Bangalore III | 83 | 22.6 | 7.3 | 13.1 | 2.1 | 22.2 | 5.6 |
| Soreni | Ontario | 0 | 22.8 | 4.3 | — | — | — | — |
| Walitza | Zurich II | 56 | 14.7 | 1.0 | 11.1 | 2.2 | 50.0 | 6.3 |
TABLE 2. Breakdown, by Site, of Clinical Characteristics of Patients With Obsessive-Compulsive Disorder (OCD) in the ENIGMA OCD Working Group Samples
Image Acquisition and Processing
Structural T1-weighted MRI brain scans were acquired and analyzed locally. Images were acquired at different field strengths (1.5-T and 3-T). The acquisition parameters of each sample are listed in Table S1 in the data supplement that accompanies the online edition of this article. The images were analyzed using the fully automated and validated segmentation program FreeSurfer, version 5.3 (22), following standardized protocols to harmonize analysis and quality control processes across multiple sites (see http://enigma.ini.usc.edu/protocols/imaging-protocols/). Segmentation of nine regions of interest, including seven subcortical gray matter structures (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus), the lateral ventricle volumes (mean bilateral volume and right and left volumes separately), and total intracranial volume, were visually inspected for accuracy (see the Supplementary Methods section in the online data supplement).
Meta-Analysis of Subcortical Brain Volumes
We examined differences between OCD patients and controls across samples by performing a meta-analysis on the mean of the left and right hemisphere measures of each subcortical structure. The meta-analysis was based on multiple linear regression models, with the mean subcortical brain volume as the outcome measure and a binary indicator of diagnosis (0=controls, 1=patients) as the predictor of interest. All models were controlled for age, sex, and intracranial volume. Effect size estimates, adjusted for age, sex, and intracranial volume, were calculated using Cohen’s d, computed from the t statistic of the diagnosis indicator variable from the regression models.
To explore the influence of sex and age on between-group subcortical volume differences, we assessed the significance of diagnosis-by-sex and diagnosis-by-age interaction effects within each sample. Multiple linear regression models were used to investigate the within-group effects of age at onset, illness duration, illness severity (using the total severity score from the Yale-Brown Obsessive Compulsive Scale [YBOCS] and the Children’s YBOCS [23, 24]) as continuous variables. To further study the neurodevelopmental aspects of illness within the adult samples, we performed separate stratified meta-analyses comparing early-onset OCD patients (onset before age 18) to controls, and late-onset OCD patients (onset at age 18 or older) to controls. Stratified meta-analyses were also performed for medicated and unmedicated patients. Likewise, separate stratified analyses were performed to investigate comorbid major depressive disorder, comorbid anxiety disorders, and OCD symptom dimensions (using the YBOCS symptom checklist; see Supplementary Methods in the data supplement).
All regression models and effect size estimates were fitted at each site separately. Subsequently, a final Cohen’s d estimate was obtained using an inverse variance-weighted random-effect meta-analysis model with the R package metafor, version 1.9-1. The meta-analysis of illness severity, age at onset, and illness duration were exceptions. The scores on these variables were considered as continuous variables, so effect sizes are reported using Pearson’s r, a partial correlation after removing nuisance variables (age, sex, and intracranial volume). The final meta-analyzed Pearson’s r was estimated following the same inverse variance-weighted random-effect meta-analysis models used for the other meta-analyses (see Supplementary Methods in the data supplement).
Moderator Analyses
Meta-regressions were performed to examine the effects of moderator variables on meta-analysis effect sizes. We tested whether hypothesized moderating factors, such as the mean age of each sample, scanner field strength, percentage of patients taking antidepressants, and percentage of patients taking antipsychotics, influenced the effect size estimates of the comparison of OCD patients with controls on all subcortical volumes across samples included in the meta-analysis. Each moderator variable was separately included as a fixed-effect predictor in a meta-regression model. We report uncorrected p values with a significance threshold determined by Bonferroni correction for testing nine regions of interest (p=0.05/9=5.6×10−3).
Power Analysis
Sample sizes that achieve 80% power to detect group differences given the presented effect sizes were calculated based on two-sided t tests assuming unequal variance, with G*Power, version 3.2.1 (25). See Supplementary Methods in the data supplement for full details of the power analysis.
Mega-Analysis of Subcortical Brain Volumes
We also performed a mega-analysis by pooling all volumetric measurements. The mega-analysis of each mean ([left+right]/2) subcortical volume was performed using the following model: Brain volume=βageXage+βsexXsex+βintracranial volumeXintracranial volume+βdiagnosisXdiagnosis+βcohort1Xcohort1+….+βcohort35Xcohort35+ε. Similar to the meta-analysis, several covariates of interest were investigated using this regression model. Results were considered significant if they exceeded the Bonferroni-corrected p value threshold 5.6×10−3.
Results
We included data from 25 adult cohorts and 10 pediatric cohorts. The adult meta- and mega-analyses contained 1,495 OCD patients and 1,472 controls, and the pediatric meta- and mega-analyses contained 335 OCD patients and 287 controls. An overview of the cohorts is provided in Tables 1 and 2. The Supplementary Methods section in the data supplement describes which sites were included in the analyses of clinical characteristics, and what was considered a sufficient amount of data.
Meta-Analysis
OCD patients versus healthy controls.
Adult comparison:
The results from the analysis comparing all adult OCD patients (N=1,495) with all adult controls (N=1,472) across volumes of nine regions of interest are summarized in Figure 1A and Table 3. Compared with controls, adult OCD patients had significantly smaller hippocampal volumes (Cohen’s d=−0.13, 95% CI=−0.23, −0.04; p=5.08×10−3, % difference=−2.80) and larger pallidum volumes (d=0.16, 95% CI=0.06, 0.26; p=1.60×10−3, % difference=3.16). No significant diagnosis-by-sex or diagnosis-by-age interaction effect was observed for any of the subcortical volumes.
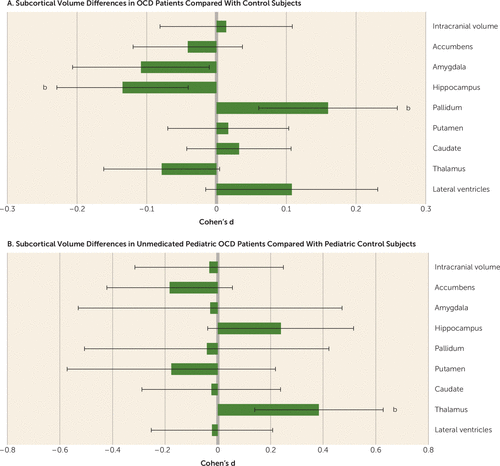
FIGURE 1. Effect Sizes for Differences in Subcortical Brain Volumes Between Adult Patients With Obsessive-Compulsive Disorder (OCD) and Healthy Control Subjects and Between Unmedicated Pediatric OCD Patients and Pediatric Healthy Control Subjectsa
a Effect sizes were corrected for age, sex, and intracranial volume. Error bars indicate 95% confidence interval.
b Significant effect, p<5.6×10−3.
| Structure | Adjusted Cohen’s da | SE | 95% CI | % Difference | p | I2 | Control Subjects (N) | OCD Patients (N) |
|---|---|---|---|---|---|---|---|---|
| Lateral ventricles | 0.108 | 0.063 | –0.016, 0.231 | 1.712 | 0.087 | 61.327 | 1,466 | 1,491 |
| Thalamus | –0.079 | 0.042 | –0.162, 0.005 | –1.851 | 0.064 | 12.542 | 1,387 | 1,375 |
| Caudate | 0.032 | 0.038 | –0.043, 0.107 | 0.844 | 0.399 | 0.003 | 1,424 | 1,441 |
| Putamen | 0.017 | 0.044 | –0.070, 0.103 | 0.380 | 0.704 | 16.141 | 1,335 | 1,365 |
| Pallidum | 0.160 | 0.051 | 0.061, 0.259 | 3.156 | 1.60×10–3 | 32.877 | 1,312 | 1,336 |
| Hippocampus | –0.135 | 0.048 | –0.229, –0.040 | –2.802 | 5.08×10–3 | 32.692 | 1,440 | 1,444 |
| Amygdala | –0.108 | 0.050 | –0.206, –0.010 | –2.163 | 0.031 | 37.194 | 1,418 | 1,452 |
| Accumbens | –0.041 | 0.040 | –0.120, 0.037 | –1.025 | 0.305 | 8.384 | 1,446 | 1,465 |
| Intracranial volume | 0.014b | 0.048 | –0.081, 0.109 | 0.286 | 0.775 | 35.547 | 1,470 | 1,493 |
TABLE 3. Meta-Analytic Results for Mean Volume of Each Structure in Patients With Obsessive-Compulsive Disorder (OCD) Compared With Healthy Control Subjects
Pediatric comparison:
None of the subcortical volumes were significantly different between pediatric OCD cases (N=335) and controls (N=287) after Bonferroni correction (see Table S2 in the data supplement).
Influence of medication on subcortical volume.
Adult comparisons:
Compared with controls, medicated OCD patients (N=654) had larger lateral ventricles (d=0.24, 95% CI=0.08, 0.41; p=2.95×10−3, % difference=2.97) and larger pallidum volumes (d=0.29, 95% CI=0.16, 0.42; p=1.20×10−5, % difference=4.38) as well as smaller hippocampal volumes (d=−0.29, 95% CI=−0.43, −0.16; p=2.39×10−5, % difference=−4.18). We did not detect any significant differences between unmedicated OCD patients (N=821) and healthy controls or between medicated OCD patients and unmedicated OCD patients. See Table S3A–C in the data supplement for full meta-analytic details regarding medication influence on the adult comparisons.
Pediatric comparisons:
As shown in Figure 1B and Table 4, the unmedicated pediatric OCD patients (N=159) had larger thalamic volumes compared with controls (d=0.38, 95% CI=0.14, 0.63; p=2.09×10−3, % difference=3.08). We also observed smaller nucleus accumbens volumes in medicated pediatric OCD patients (N=170) compared with controls (d=−0.32, 95% CI=−0.54, −0.09; p=5.25×10−3, % difference=−2.79). No significant differences were detected between medicated and unmedicated pediatric OCD patients (see Table S4A,B in the data supplement).
| Structure | Adjusted Cohen’s da | SE | 95% CI | % Difference | p | I2 | Control Subjects (N) | OCD Patients (N) |
|---|---|---|---|---|---|---|---|---|
| Lateral ventricles | –0.022 | 0.118 | –0.253, 0.209 | –0.189 | 0.850 | 0.000 | 216 | 115 |
| Thalamus | 0.384 | 0.125 | 0.139, 0.628 | 3.078 | 2.09×10–3 | 0.000 | 201 | 103 |
| Caudate | –0.024 | 0.134 | –0.288, 0.239 | –0.182 | 0.855 | 14.641 | 198 | 109 |
| Putamen | –0.177 | 0.202 | –0.572, 0.219 | –0.875 | 0.382 | 59.152 | 204 | 104 |
| Pallidum | –0.042 | 0.237 | –0.506, 0.423 | –0.176 | 0.860 | 66.561 | 174 | 87 |
| Hippocampus | 0.239 | 0.141 | –0.038, 0.516 | 1.688 | 0.091 | 22.715 | 210 | 107 |
| Amygdala | –0.029 | 0.256 | –0.530, 0.473 | –0.112 | 0.911 | 72.254 | 188 | 89 |
| Accumbens | –0.183 | 0.122 | –0.422, 0.056 | –1.500 | 0.134 | 0.004 | 203 | 111 |
| Intracranial volume | –0.033b | 0.144 | –0.314, 0.249 | –0.226 | 0.821 | 29.531 | 219 | 116 |
TABLE 4. Meta-Analytic Results for Mean Volume of Each Structure in Pediatric Unmedicated Patients With Obsessive-Compulsive Disorder (OCD) Compared With Pediatric Healthy Control Subjects
Influence of comorbid major depression on subcortical volume in adult OCD.
Adult comparisons:
As shown in Table S5A–C in the data supplement, compared with controls, OCD patients with a comorbid lifetime diagnosis of depression (N=325) had smaller hippocampal volumes (d=−0.27, 95% CI=−0.43, −0.12; p=6.43×10−4, % difference=−3.41) and larger lateral ventricles (d=0.29, 95% CI=0.14, 0.44; p=1.16×10−4, % difference=3.85). OCD patients without a comorbid lifetime diagnosis of major depressive disorder (N=1,041) had larger pallidum volumes (d=0.19, 95% CI=0.09, 0.29; p=1.56×10−4, % difference=3.78) and smaller hippocampal volumes (d=−0.16, 95% CI=−0.25, −0.06; p=1.04×10−3, % difference=−3.28). No significant subcortical volume differences were observed between OCD patients with and without a comorbid lifetime diagnosis of depression.
Pediatric comparisons:
Too few pediatric samples had sufficient numbers of subjects with major depressive disorder to permit analyses (see the Supplementary Methods section of the data supplement).
Influence of a comorbid anxiety disorder on subcortical volume.
Adult comparisons:
Compared with controls, patients without a comorbid anxiety diagnosis (N=1,002) had larger pallidum volumes (d=0.17, 95% CI=0.05, 0.28; p=4.70×10−3, % difference=2.83) and smaller hippocampal volumes (d=−0.20, 95% CI=−0.30, −0.10; p=1.51×10−4, % difference=−3.79). We did not detect any significant differences between OCD patients with a comorbid anxiety diagnosis (N=291) and controls. The comparison between OCD patients with and without a comorbid anxiety diagnosis showed that OCD patients with a comorbid lifetime anxiety diagnosis had larger intracranial volumes (d=0.41, 95% CI=0.12, 0.70; p=5.08×10−3, % difference=2.80) (see Table S6A–C in the data supplement).
Pediatric comparisons:
Too few pediatric samples had sufficient numbers of subjects with comorbid anxiety disorders to permit analyses (see the Supplementary Methods section of the data supplement).
Influence of symptom dimensions on subcortical volume.
Adult comparisons:
Regression analyses within OCD patients on symptom dimensions (N=1,151) showed no association of the presence of a particular symptom dimension and volume of any of the subcortical structures.
Pediatric comparisons:
Insufficient data on the symptom dimensions were available to perform meta-analyses (see Supplementary Methods in the data supplement).
Influence of age at onset and illness duration on subcortical volume.
Stratified analyses (see Table S7A–C in the data supplement) showed that adult OCD patients with an early illness onset (N=626) exhibited larger pallidum volumes (d=0.25, 95% CI=0.12, 0.38; p=2.30×10−4, % difference=3.68) and that patients with a late illness onset (N=794) exhibited smaller hippocampal volumes (d=−0.18, 95% CI=−0.29, −0.08; p=7.87×10−4, % difference=−3.36) than controls. No significant differences in subcortical brain volume were found when comparing early-onset with late-onset adult OCD patients. In addition, we did not observe any significant association between age at onset or illness duration (as continuous variables) and subcortical volumes in the adult (N=1,420) or pediatric (N=285) OCD groups (see Tables S8A,B and S9A,B in the data supplement).
Association of illness severity with subcortical volumes.
We did not detect any significant associations, in either the adult (N=1,455) or the pediatric (N=328) OCD patients, between illness severity and subcortical volumes (see Tables S10 and S11 in the data supplement).
Moderator analyses.
The mean age of each sample and scanner field strength did not moderate case-control differences in subcortical volumes in the adult or pediatric meta-analyses. The percentage of patients using a selective serotonin reuptake inhibitor or an antipsychotic medication in each adult sample did not moderate the subcortical volume differences (see Tables S12 and S13 in the data supplement).
Mega-Analysis
Adult OCD.
The results of the adult mega-analysis are summarized in Table S14 in the data supplement. Overall, the mega-analysis yielded results similar to those of the meta-analysis. The case-control mega-analysis indicated larger pallidum volumes (β=0.06; p=1.02×10−4) and smaller hippocampal volumes (β=−0.05; p=4.66×10−4). The pallidum (β=0.09; p=5.50×10−7) and hippocampus (β=−0.09; p=1.99×10−7) effects were more pronounced in the comparison between medicated OCD patients and controls. Early-onset patients had larger pallidum volumes (β=0.08; p=8.42×10−6) than controls. Patients with a late illness onset (β=−0.06; p=8.23×10−5) and patients with comorbid depression (β=−0.07; p=2.75×10−4) had smaller hippocampal volumes compared with controls.
Pediatric OCD.
The results of the pediatric mega-analysis are summarized in Table S15 in the data supplement. Pediatric OCD patients, compared with controls, had larger thalamus volumes (β=0.08; p=5.47×10−3). The thalamic effect was more pronounced in patients without a comorbid anxiety disorder (β=0.11; p=9.60×10−4) and in patients without comorbid depression (β=0.09; p=2.16×10−3).
Discussion
This worldwide collaborative analysis identified distinct subcortical volume alterations in pediatric and adult OCD. The adult meta- and mega-analyses were consistent, and the results showed that, compared with controls, adult OCD patients had significantly smaller hippocampal and larger pallidum volumes. Both findings were more pronounced in the subsample of medicated OCD patients compared with controls. Furthermore, the smaller hippocampal volume seemed to be driven, at least partly, by the OCD patients with comorbid depression and a late illness onset. Indeed, jackknife resampling showed a robust pallidum effect and a hippocampal effect dependent on site characteristics (data not shown). The larger pallidum finding was more pronounced in the adult OCD patients with an early illness onset. The pediatric mega-analysis showed larger thalamus volume in OCD based on the main group comparison, whereas the meta-analysis showed this only in unmedicated pediatric OCD patients compared with controls. The pediatric mega-analysis also suggests that larger thalamic volume in pediatric OCD patients is specific to those without comorbid anxiety or depression. The finding of larger thalamic volume in pediatric OCD is in line with some previous research in pediatric OCD patients (18, 26). Notably, Gilbert et al. (18) suggested a normalizing effect of pharmacological treatment on thalamic volume in pediatric OCD. Our adult meta- and mega-analyses did not reveal group differences in thalamic volume, consistent with the most recent meta-analyses of OCD (11–13). The only meta-analytic findings of thalamic enlargement in OCD included pediatric patients (14, 19). Our results provide evidence of a clear distinction in thalamic volume across pediatric and adult OCD, and they suggest that an increased thalamic volume may be an early marker of the disease, unrelated to illness severity, and may be related to altered neurodevelopment. Indeed, patients with other neurodevelopmental disorders, such as Tourette’s syndrome (27) and ADHD (28), have also been shown to have a morphologically enlarged thalamus.
Most previous research (11, 13–15, 19) did not report volumetric differences in the hippocampal complex of OCD patients. The (para)hippocampal regions are specifically vulnerable to stress-related toxic changes (29). Greater volume loss in these regions may thus be related to chronic stress and the exaggerated emotional responsiveness seen in OCD (30). The hippocampal effect in OCD patients was more pronounced in medicated patients and seemed to be driven, at least partly, by the OCD patients with comorbid major depression (31). These two findings are probably not independent, since patients with comorbidities are often the patients who receive medication. Furthermore, Selles et al. (32) showed that comorbid depression is associated with a late onset of OCD. This is in line with our finding that the hippocampal effect seemed to be driven by late-onset OCD patients. Other ENIGMA disease working groups, such as those focusing on major depression (33), schizophrenia (34), and bipolar disorder (35), have also observed smaller hippocampal volumes in patients, which suggests that the hippocampal abnormalities in OCD are disease nonspecific and possibly related to chronic stress and comorbid depression.
Our results suggest a key role for the pallidum in adult OCD patients. Previous meta-analyses have reported greater lenticular (i.e., putamen and pallidum) volume in OCD patients (11–14) but decreased lenticular nucleus volume in patients with other anxiety disorders (13). Since repetitive behaviors differentiate OCD from other anxiety disorders, the increased lenticular volume in OCD may reflect these unique symptoms (13). Our analyses also suggest that the early-onset adult OCD patients drive the pallidum effect. We therefore hypothesize that a larger pallidum in OCD patients could be the consequence of illness chronicity. Notably, the ENIGMA Schizophrenia Working Group (34) also observed a larger pallidum in schizophrenia patients compared with controls. Future ENIGMA research will enable cross-diagnosis analyses to further investigate common and distinct neural substrates across psychiatric disease groups.
Our analyses could not replicate the findings of increased putamen and caudate nucleus volumes that have been reported in smaller meta-analyses (11–14). Note that these studies used different segmentation techniques. It is possible that the technique influences findings in cases of adjacent structures such as the pallidum and putamen (36). Our observations in this study suggest that subcortical alterations in adult OCD may be limited to the pallidum and hippocampus rather than being widespread.
This study constitutes the largest meta- and mega-analyses of subcortical brain volumes in OCD to date. Strengths of this study include the sample size (N=3,589) and inclusion of both adults and children. Another strength is our strategy of ensuring methodological homogeneity by standardizing brain segmentation techniques and statistical models across all participating samples, which increased the power to detect small effects. A similar strategy has been used in parallel by other ENIGMA working groups (33–35). This method generates highly significant findings and allows us to systematically investigate the effects of clinical characteristics on brain alterations in OCD patients.
This study also had limitations. First, the individual sites in our study varied in workstation vendor and operating system version, which have been shown to have effects on estimates of brain volume and cortical thickness (37). Additionally, Schoemaker et al. (38) showed that FreeSurfer tends to overestimate subcortical volumes in children. However, this nonsystematic error probably affects patients and controls equally. Second, although we have pooled an enormous amount of data, subjects with comorbidities and subjects categorized to each specific symptom dimension, especially in the pediatric data sets, were still limited. However, the key variable—the Children’s YBOCS score, the gold-standard clinical instrument in pediatric OCD research—was available for all subjects. Third, the structure labeled as “thalamus” by FreeSurfer’s segmentation algorithm may contain both white matter and gray matter. We therefore cannot conclude that thalamic enlargement involves solely gray matter enlargement. Fourth, our findings indicate medication effects. It should be noted, however, that only current medication status was taken into consideration. It is difficult to attribute the results to direct effects of the medication itself. Furthermore, the range of medications that are generally prescribed for OCD patients is broad. Although we tested whether different types of medication influenced our findings, we were not able to calculate relative dosages of different medication types and analyze medication effects in a more fine-grained manner because of the retrospective nature of our study. Thus, we need to interpret these findings with caution.
Despite these limitations, the results of this first initiative of the ENIGMA OCD Working Group clearly indicate a key role of the thalamus and the pallidum in the pathophysiology of pediatric and adult OCD, respectively. Our findings suggest a different pattern of subcortical abnormalities in pediatric and adult OCD patients, which is in line with the developmental nature of OCD and neuroplastic changes during the course of the illness. The present study is a first step toward identifying robust brain volume alterations in OCD patients. An important next step is to apply similar methods in order to identify robust cortical imaging markers on cortical thickness and surface area measures associated with OCD.
1 : The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010; 15:53–63Crossref, Medline, Google Scholar
2 : Size and burden of mental disorders in Europe: a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol 2005; 15:357–376Crossref, Medline, Google Scholar
3 : A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57:358–363Crossref, Medline, Google Scholar
4 : Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand 2004; 110:4–13Crossref, Medline, Google Scholar
5 : Brain circuitry of compulsivity. Eur Neuropsychopharmacol 2016; 26:810–827Crossref, Medline, Google Scholar
6 : Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012; 16:43–51Crossref, Medline, Google Scholar
7 : Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61:720–730Crossref, Medline, Google Scholar
8 : Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: a preliminary voxel-based morphometry study. Neurosci Lett 2008; 435:45–50Crossref, Medline, Google Scholar
9 : Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: an optimized voxel-based morphometry study. Am J Psychiatry 2008; 165:1299–1307Link, Google Scholar
10 : Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry 2011; 70:1083–1090Crossref, Medline, Google Scholar
11 : Brain structural abnormalities in obsessive-compulsive disorder: converging evidence from white matter and grey matter. Asian J Psychiatr 2012; 5:290–296Crossref, Medline, Google Scholar
12 : Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009; 195:393–402Crossref, Medline, Google Scholar
13 : Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry 2010; 67:701–711Crossref, Medline, Google Scholar
14 : Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology 2010; 35:686–691Crossref, Medline, Google Scholar
15 : Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry 2014; 171:340–349Link, Google Scholar
16 : Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30:1051–1057Crossref, Medline, Google Scholar
17 : Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:46–52Crossref, Medline, Google Scholar
18 : Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Arch Gen Psychiatry 2000; 57:449–456Crossref, Medline, Google Scholar
19 : Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry 2009; 65:75–83Crossref, Medline, Google Scholar
20 : A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 2009; 45:855–866Crossref, Medline, Google Scholar
21 : ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage (Epub ahead of print, Dec 4, 2015)Google Scholar
22 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
23 : Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852Crossref, Medline, Google Scholar
24 : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
25 : GPOWER: A general power analysis program. Behav Res Methods Instrum Comput 1996; 28:1–11Crossref, Google Scholar
26 : Thalamic volume in pediatric obsessive-compulsive disorder patients before and after cognitive behavioral therapy. Biol Psychiatry 2000; 48:294–300Crossref, Medline, Google Scholar
27 : Enlargement of thalamic nuclei in Tourette syndrome. Arch Gen Psychiatry 2010; 67:955–964Crossref, Medline, Google Scholar
28 : Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 2010; 167:397–408Link, Google Scholar
29 : Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol 2013; 47:645–661Crossref, Medline, Google Scholar
30 : Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 2008; 32:525–549Crossref, Medline, Google Scholar
31 : Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage 2007; 38:413–421Crossref, Medline, Google Scholar
32 : Variations in symptom prevalence and clinical correlates in younger versus older youth with obsessive-compulsive disorder. Child Psychiatry Hum Dev 2014; 45:666–674Crossref, Medline, Google Scholar
33 : Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2016; 21:806–812Crossref, Medline, Google Scholar
34 : Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2016; 21:547–553Crossref, Medline, Google Scholar
35 : ENIGMA Bipolar Disorder Working Group findings from 1,747 cases and 2,615 controls. Mol Psychiatry 2014; 3:66–68Google Scholar
36 : Amygdalar and hippocampal volume: a comparison between manual segmentation, Freesurfer, and VBM. J Neurosci Methods 2015; 253:254–261Crossref, Medline, Google Scholar
37 : The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One 2012; 7:e38234Crossref, Medline, Google Scholar
38 : Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage 2016; 129:1–14Crossref, Medline, Google Scholar



