The Role of Stimulants in Late-Life Depression
In this issue, Lavretsky and colleagues report the results of a controlled study to determine whether the addition of methylphenidate to citalopram would accelerate and enhance antidepressant response in older adults (1). This was a 16-week, double-blind, three-arm, parallel-design study comparing the combination of methylphenidate and citalopram and either drug plus placebo. The study builds on previous work by the authors. The study participants had a mean age of 69 years, and all had a diagnosis of major depressive disorder. The authors found that the combination treatment accelerated response and increased the remission rate, and the differences were statistically significant and clinically meaningful. The authors also examined whether adjunctive methylphenidate improved cognition. Although cognition improved with treatment, there were no significant differences between treatments. This is a high-quality study, both in design and in the careful reporting of results.
Figure 1, from the online data supplement of the Lavretsky et al. article, provides a graphic view of the effects of the two agents. During the first 4 weeks, the rate of improvement was greater in both groups receiving methylphenidate than in the citalopram only group. During weeks 4–16, further change appeared to be driven by citalopram. The data suggest that the advantage of the combination treatment occurs in the first 4 weeks and is maintained. Although the advantage of the combination treatment involves use of citalopram, it seems unlikely that the effect would be unique to citalopram. Clinicians should bear in mind that if citalopram is employed in older patients, ECG monitoring should be considered.
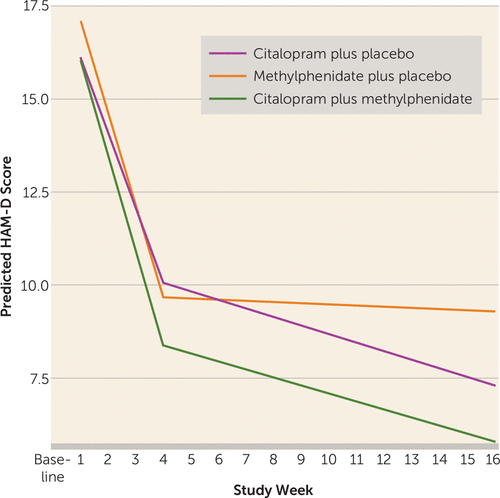
FIGURE 1. Plot of Predicted Hamilton Depression Rating Scale (HAM-D) Scores Over Time, by Treatment Group, Using a Broken-Line Mixed-Effects Model With Separate Slopes for the Three Groups From Baseline to Week 4 and Then From Week 4 to Week 16
The main limitation of the study is the low initial dosage of methylphenidate. Methylphenidate was started at 2.5 mg twice a day, and the dosage was increased by 2.5 mg twice a day every 4 days. The mean final dosage was 16 mg/day. The authors explained that safety dictated the careful titration in this older outpatient sample. Unfortunately, this limits the potential advantage of rapid effects. To understand the rationale for the use of a stimulant to accelerate response and its use in older patients, some background is in order.
Are Stimulants Especially Useful in Older Adults?
The notion that stimulants might be especially useful in older depressed patients has a long history (2). Between 1956 and 1986, at least 10 studies reported the use of stimulants in older adults, and five were placebo-controlled studies. These trials found that stimulants improved interest, apathy, and motor retardation. The findings are difficult to interpret, however, because often these patients were institutionalized or described as “senile”; it is not clear how these data apply to depressed patients.
Edwin Cassem and George Murphy, on the consultation service at Massachusetts General Hospital, were advocates of the use of stimulants in medically ill patients (3). Such patients are likely to be older. Cassem and Murphy’s group published two nonoverlapping series of cases of 66 and 180 medically ill depressed patients in whom methylphenidate and dextroamphetamine were used (4, 5). Usual dosages ranged from 12 to 15 mg/day. Moderate to marked improvement was observed in 48% and 70% of the patients in the two series, respectively. Among the responders, 85% to 93% responded within 48 hours. Most of the side effects were CNS symptoms—confusion, agitation, nervousness, hypomania, and delusions. Few cardiovascular adverse events were observed. Rapid effects are especially useful in patients with medical illness complicated by depression. Two patients of mine provide examples. One was a depressed medically ill woman on many medications who stopped taking her medications, thinking her situation was hopeless. Another was a depressed diabetic patient without appetite who stopped eating. Both responded to stimulants within days, which greatly facilitated their medical management. They then continued on conventional antidepressants. The results of two brief controlled trials (6, 7) support the efficacy of stimulants in medically ill depressed patients, but the small sample sizes (N=16 and N=23) limit the conclusions we can draw from them. The origin of the notion that stimulants are especially useful in older patients is not clear, but it may have arisen because stimulants were easier to use and better tolerated in older patients with medical comorbidity than the tricyclic antidepressants, which were the primary alternative at that time.
Are Stimulants Rapidly Acting?
Amid recent reports of the rapid antidepressant effects of intravenous ketamine, early reports of rapid effects of stimulants in depression are all but forgotten. Between 1971 and 1983, six controlled trials that examined rapid effects of stimulants (reviewed elsewhere in 1989 [8]) were reported. Changes were observed with both intravenous and oral administration within 2 to 48 hours in responding patients. Starting oral dosages were high—for example, 15 mg b.i.d. or 30 mg q.d. of dextroamphetamine. In the single study of methylphenidate, the starting dosage was 5–10 mg b.i.d., increased to 30 mg b.i.d. over 5 days (9). In that study, 26 of 43 patients showed improved mood. The focus of these trials, however, was whether improvement with stimulants predicted antidepressant response. To capture rapid effects, various mood scales were employed. None of these trials characterized clinical response to the stimulant using standard definitions of response or remission with a conventional depression scale.
In our 1989 review, we suggested that the acute effects of stimulants might be used to jump-start treatment with a traditional antidepressant (8). In 1994, a systematic trial reported on the use of methylphenidate to accelerate response with tricyclic antidepressants, but the study was not controlled (10). The Lavretsky et al. group is the first to demonstrate acceleration of response in a prospective controlled stimulant trial.
Are Stimulants Effective Antidepressants With Chronic Dosing?
The logical next question is whether stimulants would have sustained antidepressant effects. As early as 1937, Wilbur et al. (11) noted beneficial effects of stimulants in depressed patients, but the effects dissipated over a few weeks. In our 1989 review of the 10 published placebo-controlled treatment trials of stimulants in depression (8), we found that only one trial demonstrated clear efficacy. While the methods of these trials were not rigorous by current standards, during the same period, 14 of 23 controlled trials of imipramine showed clear evidence of efficacy. We concluded that stimulants are not effective as monotherapy for depression.
Are Stimulants Contraindicated in Patients With Anxiety?
Several monotherapy trials excluded patients with anxiety because it was thought that stimulants exacerbated anxiety; however, in two of the controlled stimulant trials, emergent anxiety was reported as frequently in the patients on placebo as in those on stimulants (8). In the Lavretsky et al. study, it is noteworthy that anxiety scores declined in all groups, and there appeared to be no difference between those on methylphenidate and those on citalopram only. Thus, controlled data challenge the view that stimulants are more likely to trigger anxiety than other antidepressants or placebo.
Are Adjunctive Stimulants Useful in Treatment-Resistant Depression?
Early open trials of adjunctive stimulants were promising, but subsequent controlled trials of adjunctive stimulants with second-generation antidepressants in treatment-resistant depression have failed to show efficacy. Patkar et al. (12) found a suggestive but nonsignificant effect of methylphenidate on outcome in 60 patients. In a trial of adjunctive osmotic-release methylphenidate in 284 patients (13), greater improvement occurred with drug compared with placebo at 1–2 weeks but not at the end of the 8-week trial. Three trials of lisdexamfetamine in patients who had an incomplete response to antidepressant treatment failed to show a significant effect (14, 15). Thus, to date there is no evidence from controlled studies supporting stimulant efficacy in treatment-resistant depression. I have not included modafinil or armodafinil studies, since they are categorized as wakefulness-promoting agents and have a different mechanism of action.
Are Stimulants Dangerous?
In 2006, based on reports of sudden death and other cardiovascular problems, the FDA issued a class-specific black box warning for stimulants. Since then, several large population-based studies of cardiovascular risk with stimulant treatment have been published. A review of these studies reported that six of seven studies in children found no increase in cardiovascular risk (16). The three studies in adults reported mixed results (17–19). None of the studies found an increased risk of stroke or myocardial infarction. One study found an increased risk of sudden death (17), but another did not (18). The third study found that the risk of transient ischemic attacks (TIAs) increased with stimulant use but also noted that ADHD was associated with cardiovascular risk factors (19). These factors complicate attribution of sudden death or TIAs to stimulant use. Harbeck-Seu et al. recently reviewed 10 controlled studies of stimulants for treatment of poststroke patients (20). Although neurotrophic effects of stimulants have been suggested, the authors concluded that the evidence for efficacy does not support their use in poststroke patients. Stimulants can have modest effects on blood pressure and pulse, so monitoring is advised.
In summary, the study by Lavretsky et al. is the first controlled trial to successfully demonstrate both acceleration of antidepressant effects and enhanced endpoint outcome with adjunctive stimulants. Previous studies suggested that higher starting dosages might have rapid effects, but older patients may not be the best candidates for such dosing. Adjunctive stimulants have not been effective in treatment-resistant depression in previous controlled trials. In the Lavretsky et al. study, 40% of the patients had treatment-resistant depression. It would be interesting to know how those patients fared, but the trial was not powered to address efficacy in that group.
1 : Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 2015; 172:561–569Link, Google Scholar
2 : Stimulants, in Late Life Depression. Edited by Roose SP, Sackheim HA. New York, Oxford University Press, 2004, pp 222–231Google Scholar
3 : Use of stimulants in depressed patients with medical illness, in Geriatric Psychopharmacology. Edited by Nelson JC. New York, Marcel Dekker, 1998, pp 245–257Google Scholar
4 : Psychostimulant treatment of depressive disorders secondary to medical illness. J Clin Psychiatry 1986; 47:12–15Medline, Google Scholar
5 : Psychostimulants for secondary depression in medical illness. Psychosomatics 1991; 32:203–208Crossref, Medline, Google Scholar
6 : Effects of dextroamphetamine on depression and fatigue in men with HIV: a double-blind, placebo-controlled trial. J Clin Psychiatry 2000; 61:436–440Crossref, Medline, Google Scholar
7 : Double-blind, placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am J Psychiatry 1995; 152:929–931Link, Google Scholar
8 : Stimulants in the treatment of depression: a critical overview. J Clin Psychiatry 1989; 50:241–249Medline, Google Scholar
9 : The methylphenidate test for differentiating desipramine-responsive from nortriptyline-responsive depression. Am J Psychiatry 1983; 140:212–214Link, Google Scholar
10 : The antidepressant response to tricyclics in major depressives is accelerated with adjunctive use of methylphenidate. Psychopharmacol Bull 1994; 30:157–164Medline, Google Scholar
11 : Clinical observations on the effect of benzedrine sulfate. JAMA 1937; 109:549–554Crossref, Google Scholar
12 : A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J Clin Psychopharmacol 2006; 26:653–656Crossref, Medline, Google Scholar
13 : Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 2008; 69:87–94Crossref, Medline, Google Scholar
14 : A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J Clin Psychiatry 2013; 74:802–809Crossref, Medline, Google Scholar
15 Press release: Shire reports top-line results from two phase 3 studies for Vyvanse (lisdexamfetamine dimesylate) capsules (CII) as an adjunctive treatment for adults with major depressive disorder. Jersey, UK, Shire, Feb 6, 2014 (http://www.shire.com/shireplc/uploads/press/MDDPhase3release06Feb2014.pdf)Google Scholar
16 : Do prescription stimulants increase the risk of adverse cardiovascular events? A systematic review. BMC Cardiovasc Disord 2012; 12:41Crossref, Medline, Google Scholar
17 : Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry 2012; 169:178–185Link, Google Scholar
18 : ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 2011; 306:2673–2683Crossref, Medline, Google Scholar
19 : Atomoxetine and cerebrovascular outcomes in adults. J Clin Psychopharmacol 2009; 29:453–460Crossref, Medline, Google Scholar
20 : A speedy recovery: amphetamines and other therapeutics that might impact the recovery from brain injury. Curr Opin Anaesthesiol 2011; 24:144–153Crossref, Medline, Google Scholar



