Efficacy and Safety of Brexpiprazole for the Treatment of Acute Schizophrenia: A 6-Week Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Objective:
The efficacy, safety, and tolerability of brexpiprazole and placebo were compared in adults with acute schizophrenia.
Method:
This was a multicenter, randomized, double-blind, placebo-controlled study. Patients with schizophrenia experiencing an acute exacerbation were randomly assigned to daily brexpiprazole at a dosage of 0.25, 2, or 4 mg or placebo (1:2:2:2) for 6 weeks. Outcomes included change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score (primary endpoint measure), Clinical Global Impressions Scale (CGI) severity score (key secondary endpoint measure), and other efficacy and tolerability measures.
Results:
The baseline overall mean PANSS total score was 95.2, and the CGI severity score was 4.9. Study completion rates were 62.2%, 68.1%, and 67.2% for patients in the 0.25-, 2-, and 4-mg brexpiprazole groups, respectively, versus 59.2% in the placebo group. At week 6, compared with placebo, brexpiprazole dosages of 2 and 4 mg produced statistically significantly greater reductions in PANSS total score (treatment differences: –8.72 and –7.64, respectively) and CGI severity score (treatment differences: –0.33 and –0.38). The most common treatment-emergent adverse event for brexpiprazole was akathisia (2 mg: 4.4%; 4 mg: 7.2%; placebo: 2.2%). Weight gain with brexpiprazole was moderate (1.45 and 1.28 kg for 2 and 4 mg, respectively, versus 0.42 kg for placebo at week 6). There were no clinically or statistically significant changes from baseline in lipid and glucose levels and extrapyramidal symptom ratings.
Conclusions:
Brexpiprazole at dosages of 2 and 4 mg/day demonstrated statistically significant efficacy compared with placebo and good tolerability for patients with an acute schizophrenia exacerbation.
Although a broad-based symptom control is key to the effective treatment of schizophrenia, second-generation antipsychotics act mainly on positive symptoms (1). Moreover, second-generation antipsychotics can cause various adverse effects, which may worsen the patient’s ability to function, diminish subjective well-being and quality of life, and contribute to poor treatment adherence (2). Long-term antipsychotic medication adherence is crucial to preventing relapse (3). Different second-generation antipsychotics have distinct adverse effect profiles (4, 5), likely influenced by their varying pharmacological profiles (6). For example, clozapine and olanzapine are most often associated with weight gain and related metabolic abnormalities (5); clozapine, olanzapine, quetiapine, and ziprasidone may cause sedation (4); ziprasidone is associated with QTc interval prolongation (4); aripiprazole is associated with activating adverse effects, including restlessness/akathisia (7); lurasidone, paliperidone, and risperidone have been associated with extrapyramidal symptoms; and paliperidone and risperidone have been linked to an increase in prolactin and potentially resultant sexual adverse effects (4). Selecting a second-generation antipsychotic requires consideration of the balance between activating and sedating adverse effects, as well as attention to extrapyramidal symptoms and cardiovascular risk. New treatments that are better tolerated are needed to optimize physical health as well as social functioning.
Brexpiprazole is a serotonin-dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors at similar potencies and as an antagonist at 5-HT2A and noradrenaline alpha1B/2C receptors (8). Preclinical models have demonstrated brexpiprazole’s efficacious functional D2 receptor partial agonist activity and antipsychotic-like profile (9). Brexpiprazole shows partial agonism with lower intrinsic activity at the D2 receptor and stronger antagonism at the 5-HT2A receptor than the only currently available D2 partial agonist, aripiprazole (8), suggesting a relatively lower potential to induce D2 partial agonist-mediated adverse effects, e.g., akathisia, insomnia, restlessness, and nausea (10). The potential to induce D2 antagonist-like adverse effects, e.g., extrapyramidal symptoms, hyperprolactinemia, and possibly tardive dyskinesia, is also considered to be lower than with full D2 antagonists (8). Further, brexpiprazole’s balanced 5-HT2A/D2 and 5-HT1A/D2 receptor binding profile may contribute to low incidences of both activating and neuromotor adverse effects clinically (8). Finally, brexpiprazole has a moderate affinity, relative to D2/5-HT1A receptor affinity, for histamine H1 receptors (8), which may result in low sedation levels.
The objective of this study was to evaluate the efficacy, safety, and tolerability of three fixed dosages of brexpiprazole (0.25, 2, and 4 mg/day) compared with placebo in adults with acute exacerbation of schizophrenia. We hypothesized that brexpiprazole at 2 and 4 mg would be more efficacious than placebo and well tolerated; the 0.25-mg dosage was hypothesized to be ineffective on the basis of preclinical data.
Method
Patients
Patients were recruited at 65 study centers in the United States (35.8% of randomly assigned patients), Ukraine (18.1%), Romania (17.1%), Serbia (11.8%), Latvia (4.9%), Malaysia (3.3%), Japan (3.0%), Poland (2.5%), South Korea (2.4%), and Canada (1.1%). Eligible patients were 18–65 years old, had a DSM-IV-TR diagnosis of schizophrenia confirmed by the Mini International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorders Studies (11), experienced an acute exacerbation, and would benefit from hospitalization or continued hospitalization for treatment. Patients were excluded if they had a first episode of schizophrenia, a DSM-IV-TR axis I diagnosis other than schizophrenia, clinically significant tardive dyskinesia, substance abuse or dependence in the previous 180 days, or a clinically significant medical condition.
The study was conducted in compliance with the “Guideline for Good Clinical Practice” of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf). The protocol was approved by independent ethics committees. After complete study description, written informed consent was obtained from all patients.
Study Design
This randomized, double-blind, placebo-controlled phase 3 study (ClinicalTrials.gov identifier: NCT01396421; the VECTOR trial) was conducted from August 2011 to December 2013. The study comprised a 14-day screening phase, a 6-week double-blind treatment phase, and a 30-day follow-up phase, as shown in Figure S1 in the data supplement accompanying the online version of this article.
Eligible patients were randomly assigned by using an interactive voice or web-based response system to 0.25, 2, or 4 mg of oral brexpiprazole or placebo once daily (1:2:2:2). Blocks of randomization numbers based on a computer-generated permuted-block randomization schedule were assigned to each study center. In the groups receiving 2 or 4 mg of brexpiprazole, dosing began at 1 mg/day and was titrated to 2 mg on day 5 and 4 mg on day 8.
Assessments
Efficacy was assessed by using the Positive and Negative Syndrome Scale (PANSS) (12), the Clinical Global Impressions Scale (CGI) severity of illness and improvement scales (13, pp. 218–222), and the Personal and Social Performance scale (14, 15). The PANSS and CGI scales were completed at screening and baseline and at weekly intervals during treatment; the Personal and Social Performance scale was administered at the baseline, week 3, and week 6 visits.
The primary efficacy measure was change from baseline at week 6 in PANSS total score. Secondary efficacy measures were change from baseline at weeks 1–5 in PANSS total score; change from baseline at week 6 in the CGI severity rating (key secondary endpoint measure), Personal and Social Performance score, and PANSS positive and negative symptom subscale scores; CGI improvement rating at week 6; rate of response at week 6 (defined as change from baseline ≥30% in PANSS total score or CGI improvement score of 1 or 2); discontinuation rate due to lack of efficacy; and change from baseline at week 6 in PANSS score on the excited component (comprising excitement, hostility, tension, uncooperativeness, and poor impulse control scores) (16) and in scores on the five PANSS factors defined by Marder et al. (17). Additional post hoc exploratory analyses regarding different response definitions were conducted, based on reductions of ≥20%, ≥40%, and ≥50% in PANSS total score.
Safety and tolerability variables were adverse events, body weight, laboratory measurements, vital signs, electrocardiogram, Barnes Akathisia Rating Scale (18), Simpson Angus Scale (19) (the Drug-Induced Extrapyramidal Symptom Scale [20] was used in Japan), Abnormal Involuntary Movement Scale (AIMS) (13, pp. 534–537), and Columbia-Suicide Severity Rating Scale (21, 22).
Data Analysis
Sample size calculations were based on a predicted difference for 2 and 4 mg of brexpiprazole versus placebo of 7.5 points (SD=20) on the PANSS total score. A total sample size of 630 evaluable patients was projected to yield ≥90% power to detect treatment effects at a two-tailed alpha of 0.025.
The safety population included all randomly assigned patients taking at least one dose of study medication; the efficacy population included only patients with efficacy evaluations at baseline and on at least one occasion after baseline. The primary efficacy endpoint measure was analyzed by using a mixed model for repeated measures. The model included fixed-effect factors of treatment, site, visit, treatment–visit interaction, and fixed-effect covariates of baseline value and baseline–visit interaction. A two-step testing approach was used to control the family-wise error rate of multiple comparisons (23). If the average effect of 2 and 4 mg of brexpiprazole versus placebo was statistically significant (p≤0.05), comparisons for each individual dosage versus placebo were tested. Only if both the 2- and 4-mg dosages were statistically significant in favor of brexpiprazole versus placebo (p≤0.05) for the primary efficacy endpoint measure was the key secondary efficacy measure tested by using the same two-step strategy. Cohen’s d effect size for the primary and key secondary efficacy measures was calculated as the treatment-placebo difference divided by the pooled standard deviation. Statistical analysis of the 0.25-mg brexpiprazole dosage versus placebo was exploratory. Mixed model for repeated measures analysis was applied to changes from baseline in Personal and Social Performance scale score, PANSS subscale scores, PANSS excited component score, PANSS Marder et al. factor scores (17), body weight, and extrapyramidal symptom scale scores. CGI improvement score at week 6 was analyzed by using the Cochran-Mantel-Haenszel row mean scores test, controlled for site. Responder rates and discontinuation rates due to lack of efficacy were analyzed by the Cochran-Mantel-Haenszel general association test. The number needed to treat for responder rates was calculated as 100/absolute risk reduction. Least squares mean change in body weight at week 6 was derived from an analysis of covariance model with treatment as factor and baseline value as covariate, on observed case data. Mean changes from baseline to the last visit in laboratory measurements were analyzed using analysis of variance with treatment as an effect.
Results
All 636 randomly assigned patients received study medication, and they comprised the safety population (see Figure S2 in the online data supplement). A total of 623 patients were included in the efficacy population, of whom 87, 180, and 178 patients received 0.25, 2, and 4 mg of brexpiprazole respectively, and 178 received placebo.
Demographic and baseline clinical characteristics were similar in the four treatment groups (Table 1). The patients were markedly ill at entry to the study, with an overall mean PANSS total score of 95.2 and a CGI severity score of 4.9. All patients had previously experienced acute schizophrenia exacerbations requiring treatment.
| Brexpiprazole | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristica | Placebo (N=184) | 0.25 mg/day (N=90) | 2 mg/day (N=182) | 4 mg/day (N=180) | ||||
| Demographic characteristics | ||||||||
| N | % | N | % | N | % | N | % | |
| Female | 66 | 35.9 | 29 | 32.2 | 71 | 39.0 | 69 | 38.3 |
| Race | ||||||||
| Caucasian | 121 | 65.8 | 63 | 70.0 | 120 | 65.9 | 119 | 66.1 |
| Black/African American | 45 | 24.5 | 20 | 22.2 | 43 | 23.6 | 42 | 23.3 |
| Asian | 16 | 8.7 | 7 | 7.8 | 19 | 10.4 | 16 | 8.9 |
| Other | 2 | 1.1 | 0 | 0.0 | 0 | 0.0 | 3 | 1.7 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 39.7 | 10.8 | 40.5 | 11.4 | 39.6 | 10.2 | 40.8 | 11.0 |
| Body mass index (kg/m2) | 26.5 | 5.4 | 26.2 | 6.3 | 27.3 | 5.9 | 27.1 | 5.8 |
| Clinical characteristics | ||||||||
| Age at first diagnosis (years) | 27.4 | 9.5 | 27.5 | 8.9 | 26.6 | 8.6 | 28.0 | 9.6 |
| Duration of current exacerbation (weeks) | 2.7 | 2.6 | 2.6 | 1.8 | 2.8 | 2.3 | 2.4 | 1.6 |
| BPRS total score | 55.7 | 7.1 | 55.0 | 7.5 | 56.4 | 8.6 | 55.3 | 7.4 |
| PANSS scores | ||||||||
| Total | 95.9 | 11.5 | 93.4 | 11.7 | 95.9 | 13.7 | 94.9 | 12.2 |
| Positive subscale | 25.2 | 4.1 | 24.9 | 3.5 | 25.6 | 4.4 | 25.0 | 4.5 |
| Negative subscale | 23.5 | 4.4 | 22.7 | 4.5 | 23.2 | 4.6 | 23.3 | 4.7 |
| CGI severity total score | 4.8 | 0.7 | 4.9 | 0.6 | 4.9 | 0.6 | 4.8 | 0.6 |
| Personal and Social Performance scale score | 45.1 | 9.5 | 44.2 | 9.8 | 45.4 | 10.5 | 45.3 | 10.9 |
| PANSS excited component score | 13.0 | 3.7 | 12.7 | 3.8 | 13.4 | 4.1 | 13.1 | 3.6 |
| PANSS factor scores as defined by Marder et al. (17)b | ||||||||
| Positive symptoms | 29.6 | 4.3 | 29.0 | 3.6 | 29.7 | 4.4 | 29.0 | 4.4 |
| Negative symptoms | 22.6 | 4.8 | 21.7 | 4.7 | 22.3 | 4.8 | 22.3 | 4.8 |
| Disorganized thought | 21.9 | 4.2 | 21.5 | 4.5 | 22.1 | 4.4 | 21.7 | 4.7 |
| Uncontrolled hostility/excitement | 9.7 | 3.3 | 9.6 | 3.4 | 10.1 | 3.6 | 9.8 | 3.3 |
| Anxiety/depression | 12.0 | 3.2 | 11.8 | 3.1 | 11.7 | 3.2 | 11.9 | 2.8 |
TABLE 1. Baseline Demographic and Clinical Characteristics of Patients With a Schizophrenia Exacerbation Randomly Assigned to 6 Weeks of Placebo or 0.25, 2, or 4 mg/day of Brexpiprazole (Safety Population)
Almost all patients were taking antipsychotics before the study (579 of 636, 91.0%), while 449 of 636 (70.6%) and 73 of 636 (11.5%) were taking anxiolytics/hypnotics and antidepressants, respectively. During the study, lorazepam was the most frequently used protocol-defined rescue medication, taken by 46 of 90 (51.1%), 94 of 182 (51.6%), and 82 of 180 (45.6%) patients in the 0.25-, 2-, and 4-mg brexpiprazole groups, respectively, and 82 of 184 (44.6%) in the placebo group.
Overall, 410 patients completed the study: 56 of 90 (62.2%), 124 of 182 (68.1%), and 121 of 180 (67.2%) in the 0.25-, 2-, and 4-mg brexpiprazole groups, respectively, compared with 109 of 184 (59.2%) in the placebo group (see Figure S2 in the online data supplement).
Efficacy
The average effect of the 2- and 4-mg brexpiprazole dosages on the primary efficacy endpoint measure was statistically significant compared with placebo (p<0.0001), allowing subsequent comparison of individual dosage groups.
Patients in the 2- and 4-mg brexpiprazole groups had statistically significantly greater mean improvements in PANSS total score than the placebo group at week 6 (Table 2). At week 6, compared with placebo, the treatment differences were –8.72 (Cohen’s d: 0.41, p<0.0001) and –7.64 (Cohen’s d: 0.36, p=0.0006) for the 2-mg and 4-mg brexpiprazole dosages, respectively. The difference between brexpiprazole and placebo in mean change from baseline reached statistical significance at week 1 in the 2-mg group and at week 2 in the 4-mg group, and the effect was maintained throughout the remainder of the study (Figure 1A).
| Measure | Value | Difference From Placebo | Statistical Analysis | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SE | Score Difference | 95% CI | pb | Effect Size (Cohen’s d) | |
| Changes from baselineb | |||||||
| Primary endpoint measure: PANSS total score | |||||||
| Placebo | 178 | –12.01 | 1.60 | ||||
| Brexpiprazole 0.25 mgc | 87 | –14.90 | 2.23 | –2.89 | –8.27, 2.49 | 0.30 | 0.14 |
| Brexpiprazole 2 mg | 180 | –20.73 | 1.55 | –8.72 | –13.1, –4.37 | <0.0001 | 0.41 |
| Brexpiprazole 4 mg | 178 | –19.65 | 1.54 | –7.64 | –12.0, –3.30 | 0.0006 | 0.36 |
| Key secondary endpoint measure: CGI severity total score | |||||||
| Placebo | 181 | –0.82 | 0.09 | ||||
| Brexpiprazole 0.25 mgc | 89 | –0.85 | 0.12 | –0.03 | –0.31, 0.26 | 0.85 | 0.03 |
| Brexpiprazole 2 mg | 181 | –1.15 | 0.08 | –0.33 | –0.56, –0.10 | 0.006 | 0.29 |
| Brexpiprazole 4 mg | 178 | –1.20 | 0.08 | –0.38 | –0.61, –0.15 | 0.002 | 0.33 |
| Other secondary endpoint measures | |||||||
| Personal and Social Performance scale score | |||||||
| Placebo | 170 | 10.26 | 0.98 | ||||
| Brexpiprazole 0.25 mgc | 86 | 11.84 | 1.33 | 1.58 | –1.58, 4.74 | 0.33 | 0.12 |
| Brexpiprazole 2 mg | 173 | 13.15 | 0.93 | 2.89 | 0.37, 5.42 | 0.03 | 0.23 |
| Brexpiprazole 4 mg | 168 | 12.72 | 0.93 | 2.46 | –0.06, 4.98 | 0.06 | 0.19 |
| PANSS positive subscale score | |||||||
| Placebo | 178 | –4.35 | 0.54 | ||||
| Brexpiprazole 0.25 mgc | 87 | –5.46 | 0.74 | –1.11 | –2.90, 0.68 | 0.23 | 0.16 |
| Brexpiprazole 2 mg | 180 | –6.57 | 0.52 | –2.22 | –3.67, –0.77 | 0.003 | 0.31 |
| Brexpiprazole 4 mg | 178 | –6.78 | 0.51 | –2.44 | –3.88, –0.99 | 0.001 | 0.35 |
| PANSS negative subscale score | |||||||
| Placebo | 178 | –2.24 | 0.38 | ||||
| Brexpiprazole 0.25 mgc | 87 | –3.31 | 0.53 | –1.07 | –2.33, 0.20 | 0.10 | 0.21 |
| Brexpiprazole 2 mg | 180 | –4.02 | 0.36 | –1.78 | –2.81, –0.76 | 0.0007 | 0.36 |
| Brexpiprazole 4 mg | 178 | –3.65 | 0.36 | –1.41 | –2.44, –0.39 | 0.007 | 0.29 |
| PANSS excited component score | |||||||
| Placebo | 178 | –1.64 | 0.36 | ||||
| Brexpiprazole 0.25 mgc | 87 | –1.99 | 0.49 | –0.34 | –1.53, 0.85 | 0.58 | 0.07 |
| Brexpiprazole 2 mg | 180 | –2.87 | 0.34 | –1.22 | –2.19, –0.26 | 0.02 | 0.26 |
| Brexpiprazole 4 mg | 178 | –2.75 | 0.34 | –1.10 | –2.06, –0.14 | 0.03 | 0.24 |
| PANSS factor scores as defined by Marder et al. (17) | |||||||
| Positive symptoms | |||||||
| Placebo | 178 | –4.89 | 0.53 | ||||
| Brexpiprazole 0.25 mgc | 87 | –5.78 | 0.73 | –0.89 | –2.66, 0.89 | 0.33 | 0.13 |
| Brexpiprazole 2 mg | 180 | –7.37 | 0.51 | –2.47 | –3.91, –1.04 | 0.0008 | 0.36 |
| Brexpiprazole 4 mg | 178 | –7.23 | 0.51 | –2.34 | –3.77, –0.91 | 0.002 | 0.34 |
| Negative symptoms | |||||||
| Placebo | 178 | –2.80 | 0.39 | ||||
| Brexpiprazole 0.25 mgc | 87 | –3.66 | 0.54 | –0.86 | –2.17, 0.44 | 0.20 | 0.17 |
| Brexpiprazole 2 mg | 180 | –4.48 | 0.37 | –1.68 | –2.73, –0.62 | 0.002 | 0.33 |
| Brexpiprazole 4 mg | 178 | –4.10 | 0.37 | –1.30 | –2.35, –0.25 | 0.02 | 0.26 |
| Disorganized thought | |||||||
| Placebo | 178 | –1.97 | 0.37 | ||||
| Brexpiprazole 0.25 mgc | 87 | –2.69 | 0.52 | –0.72 | –1.96, 0.52 | 0.26 | 0.15 |
| Brexpiprazole 2 mg | 180 | –3.94 | 0.36 | –1.98 | –2.98, –0.97 | 0.0001 | 0.40 |
| Brexpiprazole 4 mg | 178 | –3.72 | 0.36 | –1.75 | –2.76, –0.75 | 0.0007 | 0.36 |
| Uncontrolled hostility/excitement | |||||||
| Placebo | 178 | –0.82 | 0.30 | ||||
| Brexpiprazole 0.25 mgc | 87 | –1.15 | 0.41 | –0.33 | –1.31, 0.66 | 0.52 | 0.08 |
| Brexpiprazole 2 mg | 180 | –1.91 | 0.28 | –1.08 | –1.88, –0.28 | 0.009 | 0.28 |
| Brexpiprazole 4 mg | 178 | –1.90 | 0.28 | –1.07 | –1.87, –0.28 | 0.009 | 0.28 |
| Anxiety/depression | |||||||
| Placebo | 178 | –3.05 | 0.26 | ||||
| Brexpiprazole 0.25 mgc | 87 | –3.27 | 0.35 | –0.21 | –1.07, 0.64 | 0.63 | 0.06 |
| Brexpiprazole 2 mg | 180 | –3.70 | 0.25 | –0.65 | –1.34, 0.04 | 0.07 | 0.19 |
| Brexpiprazole 4 mg | 178 | –3.40 | 0.25 | –0.34 | –1.03, 0.35 | 0.33 | 0.10 |
| Measures at week 6 | |||||||
| CGI improvement score | |||||||
| Placebo | 181 | 3.48 | 1.47 | ||||
| Brexpiprazole 0.25 mgc | 89 | 3.37 | 1.46 | –0.14 | –0.50, 0.22 | 0.46d | 0.01 |
| Brexpiprazole 2 mg | 181 | 2.94 | 1.34 | –0.54 | –0.82, –0.26 | 0.0002d | 0.29 |
| Brexpiprazole 4 mg | 178 | 2.94 | 1.29 | –0.50 | –0.77, –0.22 | 0.0004d | 0.31 |
| N | Rate (%) | Relative Risk | 95% CI | pe | Number Needed to Treat | ||
| Rates of response, by response definition | |||||||
| ≥20% improvementf | |||||||
| Placebo | 178 | 34.8 | |||||
| Brexpiprazole 0.25 mgc | 87 | 44.8 | 1.28 | 0.96, 1.71 | 0.11 | 10 | |
| Brexpiprazole 2 mg | 180 | 52.2 | 1.51 | 1.19, 1.90 | 0.0005 | 6 | |
| Brexpiprazole 4 mg | 178 | 53.9 | 1.51 | 1.21, 1.90 | 0.0003 | 6 | |
| ≥30% improvementf | |||||||
| Placebo | 178 | 30.3 | |||||
| Brexpiprazole 0.25 mgc | 87 | 39.1 | 1.27 | 0.92, 1.76 | 0.16 | 12 | |
| Brexpiprazole 2 mg | 180 | 47.8 | 1.59 | 1.23, 2.05 | 0.0004 | 6 | |
| Brexpiprazole 4 mg | 178 | 46.1 | 1.48 | 1.14, 1.91 | 0.004 | 7 | |
| ≥40% improvementf | |||||||
| Placebo | 178 | 30.3 | |||||
| Brexpiprazole 0.25 mgc | 87 | 35.6 | 1.16 | 0.82, 1.64 | 0.41 | 19 | |
| Brexpiprazole 2 mg | 180 | 46.7 | 1.55 | 1.19, 2.01 | 0.0009 | 7 | |
| Brexpiprazole 4 mg | 178 | 44.4 | 1.42 | 1.10, 1.85 | 0.009 | 8 | |
| ≥50% improvementf | |||||||
| Placebo | 178 | 30.3 | |||||
| Brexpiprazole 0.25 mgc | 87 | 33.3 | 1.09 | 0.76, 1.55 | 0.66 | 34 | |
| Brexpiprazole 2 mg | 180 | 46.7 | 1.55 | 1.19, 2.01 | 0.0009 | 7 | |
| Brexpiprazole 4 mg | 178 | 44.4 | 1.42 | 1.10, 1.85 | 0.009 | 8 | |
| Discontinuation due to lack of efficacyg | |||||||
| Placebo | 178 | 10.1 | |||||
| Brexpiprazole 0.25 mgc | 87 | 8.1 | 0.77 | 0.35, 1.68 | 0.52 | ||
| Brexpiprazole 2 mg | 180 | 9.4 | 0.87 | 0.46, 1.65 | 0.67 | ||
| Brexpiprazole 4 mg | 178 | 3.9 | 0.39 | 0.18, 0.85 | 0.02 | ||
TABLE 2. Efficacy Endpoint Measures for Patients With a Schizophrenia Exacerbation Randomly Assigned to 6 Weeks of Placebo or 0.25, 2, or 4 mg/day of Brexpiprazole (Efficacy Population)a
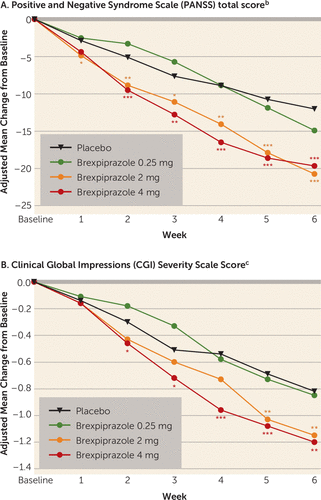
FIGURE 1. Least Squares Mean Change in Symptom and Severity Scores for Patients With a Schizophrenia Exacerbation Randomly Assigned to 6 Weeks of Placebo or 0.25, 2, or 4 mg/day of Brexpiprazolea
a The significance of the difference from placebo was determined by mixed-model repeated measures analysis.
b For the PANSS, the baseline mean total scores were 95.9 for placebo (N=183), 93.4 for 0.25 mg of brexpiprazole (N=90), 95.9 for 2 mg of brexpiprazole (N=181), and 94.9 for 4 mg brexpiprazole (N=180). The difference in endpoint change between the average for the 2- and 4-mg brexpiprazole groups and the placebo group was –8.18 (p<0.0001).
c For the CGI severity rating, the baseline mean scores were 4.84 for placebo (N=184), 4.86 for 0.25 mg of brexpiprazole (N=90), 4.90 for 2 mg of brexpiprazole (N=182), and 4.82 for 4 mg of brexpiprazole (N=180). The difference in endpoint change between the average for the 2- and 4-mg brexpiprazole groups and the placebo group was –0.36 (p=0.0006).
*p<0.05. **p<0.01. ***p<0.001.
The average effect of the 2- and 4-mg brexpiprazole dosages, compared with placebo, on the key secondary efficacy endpoint measure was statistically significant (p=0.0006), allowing subsequent comparison of individual dosage groups.
The mean change from baseline at week 6 in the CGI severity total score was statistically significantly greater in both the 2- and 4-mg brexpiprazole groups than in the placebo group (Table 2, Figure 1B). Improvements (p<0.05) from baseline to week 6 that were significantly greater in the 2-mg and 4-mg brexpiprazole groups than in the placebo group were seen in the following secondary efficacy measures: PANSS positive and negative subscale scores, PANSS excited component score, and PANSS Marder et al. factor scores (17) relating to positive and negative symptoms, disorganized thought, and uncontrolled hostility/excitement (Table 2). Mean change from baseline at week 6 in the Personal and Social Performance scale score was greater than that for placebo (p<0.05) in the 2-mg brexpiprazole group (Table 2) but not in the 4-mg group (p=0.06). Improvement at week 6 evaluated by the CGI improvement score was greater in the 2- and 4-mg brexpiprazole groups than in the placebo group (p<0.05, Table 2). Responder rates were also higher in the 2-mg and 4-mg brexpiprazole groups than in the placebo group (p<0.05), whether response was defined as improvement in PANSS total score of ≥30% (prespecified analysis; number needed to treat: 6 and 7) or as an improvement in score of ≥20%, ≥40%, or≥50% (exploratory analyses; number needed to treat: 6 to 8) (Table 2). Fewer patients discontinued because of lack of efficacy in the 4-mg brexpiprazole group than in the placebo group (p=0.02) (Table 2). In the 0.25-mg brexpiprazole group, there were only minimal changes from baseline at week 6 in the primary and secondary efficacy measures (Table 2).
Safety and Tolerability
The overall incidence of treatment-emergent adverse events was lower in the three brexpiprazole groups (48.9%−56.7%) than in the placebo group (62.0%) (Table 3). The same was true for discontinuation due to adverse events (8.2%−13.3% versus 17.4%). Most treatment-emergent adverse events with placebo were related to the underlying condition. Akathisia was more frequently reported in the 2- and 4-mg brexpiprazole groups than in the placebo group (4.4% and 7.2% versus 2.2%, respectively) (Table 3). Akathisia occurred most often during the first 3 weeks of treatment; all incidences were mild or moderate in severity, and none resulted in treatment discontinuation. The incidences of other activating (restlessness, insomnia, anxiety) and sedating (somnolence, fatigue, sedation) treatment-emergent adverse events in patients receiving brexpiprazole were similar to or lower than the rates in patients receiving placebo (Table 3). The most frequently reported serious adverse events in all treatment groups were psychiatric disorders (schizophrenia, psychotic disorder). There were no deaths during the study.
| Brexpiprazole | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurea | Placebo (N=184) | 0.25 mg (N=90) | 2 mg (N=182) | 4 mg (N=180) | ||||||||
| N | % | N | % | N | % | N | % | |||||
| Incidence of treatment-emergent adverse events | ||||||||||||
| At least one treatment-emergent adverse event | 114 | 62.0 | 44 | 48.9 | 103 | 56.6 | 102 | 56.7 | ||||
| Serious adverse eventb | 7 | 3.8 | 4 | 4.4 | 4 | 2.2 | 2 | 1.1 | ||||
| Discontinuation due to treatment-emergent adverse eventc | 32 | 17.4 | 12 | 13.3 | 15 | 8.2 | 17 | 9.4 | ||||
| Treatment-emergent adverse events occurring in ≥5% of patients in any group | ||||||||||||
| Headache | 15 | 8.2 | 9 | 10.0 | 17 | 9.3 | 22 | 12.2 | ||||
| Insomnia | 18 | 9.8 | 8 | 8.9 | 16 | 8.8 | 15 | 8.3 | ||||
| Agitation | 19 | 10.3 | 4 | 4.4 | 11 | 6.0 | 13 | 7.2 | ||||
| Akathisia | 4 | 2.2 | 0 | 0.0 | 8 | 4.4 | 13 | 7.2 | ||||
| Schizophrenia | 20 | 10.9 | 8 | 8.9 | 9 | 4.9 | 11 | 6.1 | ||||
| Diarrhea | 3 | 1.6 | 5 | 5.6 | 3 | 1.6 | 7 | 3.9 | ||||
| Nausea | 8 | 4.3 | 1 | 1.1 | 10 | 5.5 | 6 | 3.3 | ||||
| Other relevant treatment-emergent adverse events | ||||||||||||
| Restlessness | 2 | 1.1 | 1 | 1.1 | 1 | 0.5 | 5 | 2.8 | ||||
| Anxiety | 3 | 1.6 | 0 | 0.0 | 2 | 1.1 | 0 | 0.0 | ||||
| Somnolence | 5 | 2.7 | 0 | 0.0 | 3 | 1.6 | 7 | 3.9 | ||||
| Fatigue | 3 | 1.6 | 4 | 4.4 | 3 | 1.6 | 3 | 1.7 | ||||
| Sedation | 1 | 0.5 | 1 | 1.1 | 0 | 0.0 | 4 | 2.2 | ||||
| Hypersomnia | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Change from baseline to last visit | ||||||||||||
| Fasting metabolic variables (mg/dL) | ||||||||||||
| Cholesterol | 163 | –3.07 | 29.05 | 75 | –5.25 | 25.36 | 163 | 0.63 | 31.74 | 160 | 4.24 | 30.33 |
| HDL cholesterol | 163 | –1.21 | 8.91 | 75 | –1.87 | 9.84 | 163 | 1.33 | 9.91 | 160 | 0.48 | 7.26 |
| LDL cholesterol | 163 | –2.12 | 24.70 | 74 | –2.47 | 21.14 | 162 | –0.70 | 28.00 | 157 | 2.57 | 26.79 |
| Triglycerides | 163 | –0.79 | 72.54 | 75 | –0.85 | 70.28 | 163 | –1.45 | 66.35 | 160 | 6.76 | 69.54 |
| Glucose | 163 | 0.13 | 15.40 | 75 | 3.80 | 19.98 | 162 | 0.70 | 17.84 | 160 | 1.13 | 14.72 |
| Prolactin (ng/mL) | ||||||||||||
| Women | 60 | –5.82 | 31.73 | 27 | 3.42 | 18.67 | 67 | –4.89 | 34.18 | 66 | 1.47 | 18.46 |
| Men | 108 | –1.36 | 9.31 | 57 | –1.71 | 10.32 | 106 | –1.34 | 12.35 | 101 | 1.37 | 9.71 |
| Electrocardiogram variables (ms) | ||||||||||||
| QTcB | 170 | 3.1 | 21.1 | 85 | 5.7 | 22.2 | 176 | 1.1 | 21.2 | 167 | 2.3 | 19.1 |
| QTcF | 170 | 0.9 | 18.5 | 85 | 2.5 | 17.3 | 176 | –0.8 | 16.5 | 167 | 1.3 | 14.9 |
| QTcN | 170 | 1.4 | 18.4 | 85 | 3.2 | 17.9 | 176 | –0.4 | 16.8 | 167 | 1.6 | 15.2 |
TABLE 3. Treatment-Emergent Adverse Events and Changes in Metabolic, Prolactin, and ECG Measures for Patients With a Schizophrenia Exacerbation Randomly Assigned to 6 Weeks of Placebo or 0.25, 2, or 4 mg/day of Brexpiprazole (Safety Population)
Increased body weight was reported as a treatment-emergent adverse event by 2.7% and 3.9% of the patients who received 2 and 4 mg of brexpiprazole, respectively, versus 1.6% of the placebo patients. Mean body weight change at week 6 was 1.45 kg and 1.28 kg for the 2- and 4-mg brexpiprazole groups and 0.42 kg for the placebo group; the least squares mean differences from placebo were 1.03 kg for the 2-mg brexpiprazole group (p=0.03) and 0.86 kg for the 4-mg group (p=0.07). An increase in body weight of ≥7% from baseline at any visit was seen in 8.8% and 9.0% of the 2- and 4-mg brexpiprazole groups and 4.4% of the placebo patients.
Changes in fasting-state metabolic measurements are shown in Table 3. Slight increases occurred in total cholesterol and high-density lipoprotein cholesterol from baseline to the last visit for 2 mg and 4 mg of brexpiprazole, compared with slight decreases in the placebo group, but these changes were neither clinically relevant nor statistically significant. There were slight increases in low-density lipoprotein cholesterol and triglycerides in the 4-mg brexpiprazole group, but these differences were also not clinically relevant. All four groups had minimal increases in glucose. Shifts to abnormal fasting lipid or glucose values were similar in the brexpiprazole and placebo groups, with no significant difference between the groups, as shown in Table S1 in the online data supplement. Metabolic-related treatment-emergent adverse events were reported by three brexpiprazole patients: diabetes mellitus (0.25 mg), hypertriglyceridemia (4 mg), and increased glycosylated hemoglobin level (4 mg). Treatment-emergent metabolic syndrome (three or more criteria) occurred in none of 144 placebo patients and seven of the brexpiprazole patients: 3.0% (2/67) of those taking 0.25 mg, 1.5% (2/136) of those taking 2 mg, and 2.3% (3/132) of those taking 4 mg.
Mean prolactin concentrations increased from baseline to the last visit in both female and male patients in the 4-mg brexpiprazole group, while reductions were seen in the 2-mg brexpiprazole group and the placebo group (Table 3). Shifts to abnormal prolactin values were similar in the placebo and brexpiprazole groups, without significant differences between the groups (online Table S1). Hyperprolactinemia was reported as a treatment-emergent adverse event for one patient in the 2-mg brexpiprazole group.
The mean changes in QT interval from baseline to the last visit, as corrected by Bazett’s formula, were 1.1 and 2.3 msec for 2 and 4 mg of brexpiprazole, respectively, compared with 3.1 msec for placebo (Table 3).
There were no consistent differences between treatment groups in clinical laboratory results (with no significant differences between the groups, online Table S2), vital signs, and ECG measures. Six patients (two each in the 2-mg brexpiprazole group, the 4-mg group, and the placebo group) discontinued the study because of liver-related adverse events (“drug-induced liver injury,” “hepatic enzyme increased,” or “liver function test abnormal”).
Changes from baseline to the last visit in scores on the extrapyramidal symptom scales were minimal in all treatment groups, without statistically significant treatment differences (Table 4).
| Change in Score Between Baseline and Last Visita | ||||||
|---|---|---|---|---|---|---|
| Scale and Group | N | Mean | SE | Difference From Placebo | 95% CI | p |
| Barnes Akathisia Rating Scale | ||||||
| Placebo | 181 | 0.01 | 0.03 | |||
| Brexpiprazole 0.25 mg | 89 | 0.03 | 0.04 | 0.02 | –0.09, 0.13 | 0.74 |
| Brexpiprazole 2 mg | 181 | 0.01 | 0.03 | 0.00 | –0.09, 0.09 | 1.00 |
| Brexpiprazole 4 mg | 178 | 0.05 | 0.03 | 0.05 | –0.04, 0.13 | 0.32 |
| Simpson-Angus Scale | ||||||
| Placebo | 174 | –0.02 | 0.08 | |||
| Brexpiprazole 0.25 mg | 85 | –0.04 | 0.12 | –0.02 | –0.30, 0.27 | 0.91 |
| Brexpiprazole 2 mg | 175 | –0.07 | 0.08 | –0.05 | –0.28, 0.18 | 0.68 |
| Brexpiprazole 4 mg | 172 | 0.12 | 0.08 | 0.14 | –0.09, 0.38 | 0.23 |
| Drug-Induced Extrapyramidal Symptom Scaleb | ||||||
| Placebo | 6 | –0.77 | 0.78 | |||
| Brexpiprazole 0.25 mg | 3 | –3.56 | 1.11 | –2.79 | –5.69, 0.11 | 0.06 |
| Brexpiprazole 2 mg | 5 | –1.85 | 0.85 | –1.08 | –3.55, 1.39 | 0.37 |
| Brexpiprazole 4 mg | 5 | 0.30 | 0.86 | 1.07 | –1.42, 3.56 | 0.38 |
| Abnormal Involuntary Movement Scale | ||||||
| Placebo | 181 | –0.05 | 0.05 | |||
| Brexpiprazole 0.25 mg | 89 | –0.06 | 0.07 | –0.01 | –0.19, 0.16 | 0.89 |
| Brexpiprazole 2 mg | 181 | –0.11 | 0.05 | –0.06 | –0.21, 0.08 | 0.38 |
| Brexpiprazole 4 mg | 178 | –0.05 | 0.05 | –0.00 | –0.14, 0.14 | 1.00 |
TABLE 4. Change in Extrapyramidal Symptom Rating Scale Scores for Patients With a Schizophrenia Exacerbation Randomly Assigned to 6 Weeks of Placebo or 0.25, 2, or 4 mg/day of Brexpiprazole (Safety Population)
The occurrence of suicidal ideation or behavior, as recorded on the Columbia-Suicide Severity Rating Scale, was low. One patient in the 2-mg brexpiprazole group reported suicidal behavior and serious active suicidal ideation at week 1. A different patient in the 2-mg brexpiprazole group had active suicidal ideation at week 1. One placebo patient reported suicidal behavior.
Discussion
In this 6-week randomized, double-blind, placebo-controlled study of markedly ill adults with acutely exacerbated schizophrenia, brexpiprazole at a dosage of either 2 or 4 mg/day resulted in statistically significantly greater improvement than placebo as indicated by the primary outcome measure, change in PANSS total score. Statistically significant improvement was seen in these two brexpiprazole groups within 1–2 weeks of initiating treatment and was maintained throughout the treatment period. The 2- and 4-mg doses were reached by days 5 and 8, respectively. While titration to 4 mg on day 8 could be perceived as relatively late, the difference between the 2-mg arm and placebo reached statistical significance for the primary outcome measure at week 1, with the difference from placebo in the 4-mg arm being close to statistical significance at day 7 (p=0.06), i.e., prior to reaching the 4-mg dose. The key secondary efficacy endpoint measure, mean change from baseline at week 6 in CGI severity total score, was also statistically significantly greater than the change with placebo in the 2- and 4-mg brexpiprazole groups. Other secondary efficacy measures supported the results of the primary and key secondary analyses. Brexpiprazole at dosages of 2 and 4 mg demonstrated efficacy in treating both positive and negative symptoms of schizophrenia, and they reduced agitation as measured by the PANSS excited component. Rates of response (≥30% improvement in PANSS total score or CGI improvement rating of 1 or 2) were higher with the 2- and 4-mg brexpiprazole dosages than with placebo (p=0.0004 and p=0.004, respectively). The 0.25-mg dosage of brexpiprazole did not have any clinically relevant effects on any of the efficacy measures, supporting its purpose in the study, i.e., to explore the lower end of the effective dosage range.
The 2- and 4-mg dosages demonstrated efficacy in four of five PANSS dimensions as defined by Marder et al. (17): positive symptoms, negative symptoms, disorganized thought, and uncontrolled hostility/excitement. Although brexpiprazole had a limited effect on the anxiety/depression dimension, the enrollment criteria were not designed to select patients with marked levels of anxiety or depression.
The superiority of 2 and 4 mg of brexpiprazole in change in PANSS total score compared with placebo was not only statistically significant but also clinically relevant, indicated by a significantly greater change in CGI severity score as well as Cohen’s d effect sizes of 0.36 to 0.41 for PANSS total score. Moreover, the numbers needed to treat for response (≥30% improvement in PANSS total score or CGI improvement rating of 1 or 2) were 6 for 2 mg and 7 for 4 mg. Both the effect sizes and numbers needed to treat are within the midrange across examined antipsychotics (4, 24). However, one needs to be careful with indirect comparisons of effect size in placebo-controlled trials across antipsychotics, as an increase in placebo response has been observed (25). In fact, the 12-point improvement in PANSS total score with placebo in the current study is on the higher side when compared with changes in other regulatory trials of second-generation antipsychotics (26). Thus, the effect size may be a conservative estimate when compared with those for antipsychotics studied previously, when placebo response was less marked; yet efficacy relative to that of other antipsychotics will need to be tested in future head-to-head trials.
In addition to its demonstrated efficacy, brexpiprazole was well tolerated in this study and had a high completion rate. Notably, more patients randomly assigned to placebo than brexpiprazole discontinued the study because of lack of efficacy or adverse events. Many of the adverse events reported by patients in the placebo group were related to their underlying condition, reported as worsening of schizophrenia. Incidences of activating and sedating adverse events were low and comparable in the brexpiprazole and placebo groups. Consistent with this finding, changes in extrapyramidal symptom scale scores were minimal. A meta-analysis of second-generation antipsychotics has suggested that there are differences between agents in their ability to induce extrapyramidal symptoms (27), although the only currently available dopamine D2 partial agonist, aripiprazole, was reported to have a relatively low risk (4). In this study, the incidence of akathisia was low (4.4% and 7.2% at brexpiprazole doses of 2 and 4 mg, respectively). A moderate increase in body weight in the brexpiprazole groups over 6 weeks was noted, with a difference from placebo of 1 kg or less, but there was no evidence of statistically significant or clinically relevant adverse effects on metabolic measures and prolactin when compared with those for placebo. This finding is important, given that metabolic side effects are a serious concern with some second-generation antipsychotics, namely clozapine, olanzapine, and quetiapine (5, 28, 29). Preliminary data on newer second-generation antipsychotics suggest that asenapine and iloperidone may also have weight gain potential and metabolic risk, at least in the short term (30). Some antipsychotics, particularly sertindole and ziprasidone, have been associated with QTc prolongation (4). However, like lurasidone (4), brexpiprazole actually resulted in numerically lower QTc changes than placebo when the QTc interval is corrected by using Bazett’s formula. Overall, the tolerability profile of brexpiprazole appears to be consistent with its pharmacological profile, whereby it shows a balanced 5-HT2A and 5-HT1A receptor binding affinity relative to D2, with less intrinsic activity at the D2 receptor than aripiprazole and moderately low affinity for receptors that have been associated with sedation and weight gain (8).
Brexpiprazole is also in clinical development for adjunctive treatment of patients with major depressive disorder and inadequate response to antidepressants. Phase 2 and 3 studies have indicated that adjunctive brexpiprazole is effective and well tolerated in this population (31, 32). Additional studies are under way for agitation associated with dementia of the Alzheimer's type (33, 34) and as an adjunct to paroxetine or sertraline in subjects with posttraumatic stress disorder (35).
The results of this study need to be interpreted within its limitations. These include the lack of an active comparator, short study duration, and inclusion of patients with schizophrenia without other psychiatric comorbidities. However, this study was designed as an acute, short-term regulatory approval study in which comorbidities were excluded to isolate the efficacy signal for schizophrenia symptoms. Clearly, longer-term studies that include a comparator, as well as patients with common comorbid conditions, will be needed to further evaluate the efficacy, safety, and effectiveness of brexpiprazole in the treatment of patients with schizophrenia commonly encountered in clinical care and in comparison with other first-line antipsychotics.
In conclusion, in this 6-week randomized, double-blind, placebo-controlled study, brexpiprazole at doses of 2 mg and 4 mg once daily demonstrated statistically significant efficacy compared with placebo and good tolerability for patients with an acute exacerbation of schizophrenia.
1 : Schizophrenia. Lancet 2009; 374:635–645Crossref, Medline, Google Scholar
2 : Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011; 25:567–620Crossref, Medline, Google Scholar
3 : Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010; 176:109–113Crossref, Medline, Google Scholar
4 : Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382:951–962Crossref, Medline, Google Scholar
5 : Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012; 8:114–126Crossref, Google Scholar
6 : From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry 2010; 25(suppl 2):S12–S21Crossref, Medline, Google Scholar
7 : Evaluation of akathisia in patients with schizophrenia, schizoaffective disorder, or bipolar I disorder: a post hoc analysis of pooled data from short- and long-term aripiprazole trials. J Psychopharmacol 2010; 24:1019–1029Crossref, Medline, Google Scholar
8 : Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 2014; 350:589–604Crossref, Medline, Google Scholar
9 : Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 2014; 350:605–614Crossref, Medline, Google Scholar
10 : Aripiprazole. Expert Opin Pharmacother 2005; 6:2091–2101Crossref, Medline, Google Scholar
11 Sheehan DV, Janavs J, Harnett-Sheehan K, et al: Mini International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorders Studies, Version 6.0.0. https://medical-outcomes.com/Google Scholar
12 : Positive and Negative Syndrome Scale (PANSS) Rating Criteria. North Tonawanda, NY, Multi-Health Systems, 1999Google Scholar
13 (ed): ECDEU Assessment Manual for Psychopharmacology: US Department of Health, Education and Welfare Publication (ADM) 76-338. Rockville, Md, National Institute of Mental Health, 1976Google Scholar
14 : The impact of insight on functioning in patients with schizophrenia or schizoaffective disorder receiving risperidone long-acting injectable. J Nerv Ment Dis 2007; 195:976–982Crossref, Medline, Google Scholar
15 : Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000; 101:323–329Crossref, Medline, Google Scholar
16 : Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes 2011; 9:18Crossref, Medline, Google Scholar
17 : The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997; 58:538–546Crossref, Medline, Google Scholar
18 : A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Crossref, Medline, Google Scholar
19 : A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
20 : Extrapyramidal symptom profiles assessed with the Drug-Induced Extrapyramidal Symptom Scale: comparison with Western scales in the clinical double-blind studies of schizophrenic patients treated with either olanzapine or haloperidol. Int Clin Psychopharmacol 2003; 18:39–48Crossref, Medline, Google Scholar
21 : The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011; 168:1266–1277Link, Google Scholar
22 : Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale; reply of (letter). Am J Psychiatry 2012; 169:662–663Link, Google Scholar
23 : Performance of some multiple testing procedures to compare three doses of a test drug and placebo. Pharm Stat 2005; 4:25–35Crossref, Google Scholar
24 : Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry 2014; 71:706–715Crossref, Medline, Google Scholar
25 : Increasing placebo response in antipsychotic drug trials: let’s stop the vicious circle. Am J Psychiatry 2013; 170:1232–1234Link, Google Scholar
26 : Placebo-related effects in clinical trials in schizophrenia: what is driving this phenomenon and what can be done to minimize it? Int J Neuropsychopharmacol 2012; 15:1003–1014Crossref, Medline, Google Scholar
27 : Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull 2012; 38:167–177Crossref, Medline, Google Scholar
28 : Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123:225–233Crossref, Medline, Google Scholar
29 : Cardiometabolic risk in first episode schizophrenia-spectrum disorder patients: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014; 71:1350–1363Crossref, Medline, Google Scholar
30 : Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs 2012; 26:733–759Crossref, Medline, Google Scholar
31 : Efficacy of adjunctive OPC-34712 across multiple outcome measures in major depressive disorder: a phase II, randomized, placebo-controlled study. Neuropsychopharmacology 2011; 36:S302–S304Google Scholar
32 : Efficacy and safety of adjunctive brexpiprazole (OPC-34712) in major depressive disorder (MDD): a phase 3, randomized, placebo-controlled study (abstract). Eur Psychiatry 2014; 29(suppl 1):EPA-0808Crossref, Google Scholar
33 Otsuka Pharmaceutical Development & Commercialization: Safety and Tolerability Study of Flexible Dosing of Brexpiprazole in the Treatment of Subjects With Agitation Associated With Dementia of the Alzheimer's Type. http://clinicaltrials.gov/ct2/show/NCT01922258?term=brexpiprazole&rank=5Google Scholar
34 Otsuka Pharmaceutical Development & Commercialization: A Study of Two Fixed-Doses of Brexpiprazole in the Treatment of Subjects With Agitation Associated With Dementia of the Alzheimer's Type. http://clinicaltrials.gov/ct2/show/NCT01862640?term=brexpiprazole&rank=2Google Scholar
35 H Lundbeck: Brexpiprazole as an Additional Treatment to Paroxetine or Sertraline in Adult Patients Suffering From Post-Traumatic Stress Disorder (PTSD). http://clinicaltrials.gov/ct2/show/NCT01987960?term=brexpiprazole&rank=10Google Scholar



