An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline
Abstract
Objective:
Major depressive disorder has been linked with inflammatory processes, but it is unclear whether individual differences in levels of inflammatory biomarkers could help match patients to treatments that are most likely to be beneficial. The authors tested the hypothesis that C-reactive protein (CRP), a commonly available marker of systemic inflammation, predicts differential response to escitalopram (a serotonin reuptake inhibitor) and nortriptyline (a norepinephrine reuptake inhibitor).
Method:
The hypothesis was tested in the Genome-Based Therapeutic Drugs for Depression (GENDEP) study, a multicenter open-label randomized clinical trial. CRP was measured with a high-sensitivity method in serum samples from 241 adult men and women with major depressive disorder randomly allocated to 12-week treatment with escitalopram (N=115) or nortriptyline (N=126). The primary outcome measure was the score on the Montgomery-Åsberg Depression Rating Scale (MADRS), administered weekly.
Results:
CRP level at baseline differentially predicted treatment outcome with the two antidepressants (CRP-drug interaction: β=3.27, 95% CI=1.65, 4.89). For patients with low levels of CRP (<1 mg/L), improvement on the MADRS score was 3 points higher with escitalopram than with nortriptyline. For patients with higher CRP levels, improvement on the MADRS score was 3 points higher with nortriptyline than with escitalopram. CRP and its interaction with medication explained more than 10% of individual-level variance in treatment outcome.
Conclusions:
An easily accessible peripheral blood biomarker may contribute to improvement in outcomes of major depressive disorder by personalizing treatment choice.
Inadequate response to treatment in a substantial proportion of affected individuals contributes to the large burden of disability associated with major depressive disorder (1). It has been proposed that outcomes of depression could be improved by personalizing treatment with the use of biomarkers that are easily measurable by noninvasive methods and are differentially predictive of outcomes with alternative treatments, with clinically significant effect sizes (2). While replicable general predictors of poor treatment outcomes have been identified (3, 4), clinically significant differential predictors are lacking.
Several lines of evidence implicate immunity and inflammation in the pathogenesis of depression and mechanisms of antidepressant response (5). Increased levels of systemic inflammation have been associated with depression (6). Inflammatory biomarkers have also been associated with an adverse course of depression at the population level (7). Different effects of norepinephrine and serotonin on inflammation suggest that inflammatory biomarkers may differentially predict outcomes of treatment with antidepressants that affect the levels of these neuromediators (8). Norepinephrine inhibits production of Th1 proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), by white blood cells and microglia through their action on the β2 receptors (8–10). Serotonin inhibits the production of Th2 cytokines such as interleukin 6 (IL-6) (8). Antidepressant drugs that affect norepinephrine and serotonin also distinctly affect immunity: while norepinephrine reuptake inhibitor antidepressants suppress Th1-type cytokines and shift the balance toward humoral immunity, serotonin reuptake inhibitors reduce the production of Th2-type cytokines and shift the balance toward cellular immune response (8, 11). Nortriptyline, a tricyclic antidepressant whose predominant mode of action is inhibition of norepinephrine reuptake, has also been found to inhibit migration of polymorphonuclear white blood cells toward an inflammation site, an effect not replicated with serotonin reuptake inhibitors (12). Inflammatory cytokines also affect the metabolism of serotonin and norepinephrine in different ways: while TNF-α increases the expression of serotonin transporter by astrocytes, potentially reducing the levels of intracellular brain serotonin (13), interleukin 1 (IL-1), another Th1 proinflammatory cytokine, increases the production of norepinephrine in the hypothalamus (14). These data led us to formulate the hypothesis that pretreatment levels of systemic inflammation will differentially predict therapeutic response to the serotonin reuptake inhibitor escitalopram and the norepinephrine reuptake inhibitor nortriptyline in major depressive disorder. While previous studies have shown that baseline levels of inflammatory biomarkers predicted response to a single treatment (15–17) and were related to overall resistance to antidepressants (18, 19) or persistence of depression among antidepressant-treated individuals (7), to our knowledge no previous study has examined inflammatory biomarkers as differential predictors of response to alternative treatments.
A number of proteins in peripheral blood can be used as markers of systemic inflammation. We chose C-reactive protein (CRP), an acute-phase protein produced by the liver in response to interleukin 1β (IL-1β) (20). The choice was based on pragmatic considerations. High-sensitivity measurement of CRP is a readily available test in most medical biochemistry laboratories, the level of CRP does not change with time of day or time since last meal, and CRP is stable in stored biological samples (21, 22). Of all evaluated inflammatory biomarkers, CRP has the largest body of evidence for consistent associations with depression, its risk factors, and its treatment outcomes (6, 16, 23). Therefore, we specified and tested the practical hypothesis that CRP level will differentially predict response to escitalopram and nortriptyline in depression.
Method
Study Design
We analyzed data from the Genome-Based Therapeutic Drugs for Depression (GENDEP) study, a multicenter open-label randomized clinical trial carried out at nine European academic psychiatry centers to compare treatment with escitalopram and nortriptyline in depression (24). The study was approved by the research ethics boards of participating institutions, and participants provided written informed consent.
Participants
GENDEP included 811 adult men and women with a diagnosis of major depressive disorder, established with the semistructured Schedules for Clinical Assessment in Neuropsychiatry interview (25). Recruitment was by clinical referrals from primary and secondary care. GENDEP had broad inclusion criteria and included patients with comorbid disorders, severe illness, and suicidal ideation (24). Participants were excluded if they had a personal or family (first-degree relative) history of mania, hypomania, or mood-incongruent psychotic symptoms. Because of the genetic component, GENDEP included only participants of European ancestry. A total of 468 participants had no contraindications to either drug and were randomly allocated to receive escitalopram or nortriptyline. The present study includes 241 (51.5%) of these participants, for whom baseline serum samples of CRP were available (for a participant flow chart, see Figure S1 in the data supplement that accompanies the online edition of this article). Availability of serum samples depended on recruitment center and medication-free status at baseline. Seven of the nine GENDEP centers (Aarhus, Bonn, Brussels, Ljubljana, London, Poznan, and Zagreb) collected serum samples. Because the sampling protocol prioritized genetic testing and individuals who were free of psychotropic medication at baseline, serum samples were available for the majority (72%) of medication-free participants but only for a minority (40%) of participants who were taking antidepressants at the time of recruitment. Patients with available serum samples were younger on average (mean age, 40.7 years [SD=11.4], compared with 43.5 years [SD=11.6]; this was a result of the overrepresentation of medication-free participants) but did not differ significantly from those with no serum samples in drug allocation, depression severity, or treatment outcome. Patients who were allocated to escitalopram and nortriptyline were comparable in depression severity and in clinical and demographic variables (Table 1).
| Variable | Escitalopram Group (N=115) | Nortriptyline Group (N=126) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 40.1 | 11.6 | 41.2 | 11.4 |
| Education (years) | 12.8 | 3.2 | 12.1 | 3.4 |
| Body mass index | 25.4 | 4.3 | 25.7 | 5.6 |
| Montgomery-Åsberg Depression Rating Scale | 29.3 | 5.8 | 29.9 | 6.3 |
| Hamilton Depression Rating Scale | 21.9 | 4.5 | 22.2 | 5.2 |
| Beck Depression Inventory | 28.8 | 9.0 | 28.8 | 9.8 |
| CRP level (mg/L) | 1.30 | 1.58 | 1.66 | 2.17 |
| lnCRP (ln[mg/L]) | –0.46 | 1.29 | –0.27 | 1.33 |
| N | % | N | % | |
| Female | 71 | 61.7 | 80 | 63.5 |
| Marital status | ||||
| Married or cohabiting | 70 | 60.9 | 73 | 57.9 |
| Separated | 13 | 11.3 | 16 | 12.7 |
| Widowed | 4 | 3.5 | 6 | 4.8 |
| Single | 28 | 24.4 | 31 | 24.6 |
| Occupational status | ||||
| Employed full-time | 46 | 40.0 | 54 | 42.9 |
| Employed part-time | 12 | 10.4 | 12 | 9.5 |
| Unemployed | 27 | 23.5 | 29 | 23.0 |
| Retired | 15 | 13.0 | 16 | 12.7 |
| Student | 11 | 9.6 | 7 | 5.6 |
| Homemaker | 4 | 3.5 | 8 | 6.4 |
| Episodes | ||||
| 1 | 38 | 33.0 | 39 | 31.0 |
| 2 | 60 | 52.2 | 59 | 46.8 |
| 3 or more | 17 | 14.8 | 28 | 22.2 |
| Smoker | 41 | 35.7 | 33 | 26.2 |
| Inflammatory illness | 3 | 2.6 | 5 | 4.0 |
| Autoimmune illness | 2 | 1.7 | 3 | 2.4 |
| Use of anti-inflammatory drugs | 25 | 21.7 | 19 | 15.1 |
| CRP level, by reference category | ||||
| <1 mg/L | 64 | 55.7 | 67 | 53.2 |
| 1–2.9 mg/L | 33 | 28.7 | 30 | 23.8 |
| 3–9.9 mg/L | 14 | 12.2 | 23 | 18.3 |
| >10 mg/L | 4 | 3.5 | 6 | 4.8 |
TABLE 1. Baseline Demographic and Clinical Characteristics of Participants in a Study of C-Reactive Protein (CRP) Level as a Predictor of Response to Antidepressant Treatment With Escitalopram or Nortriptyline
Interventions
Eligible participants without contraindications were allocated to receive a 12-week treatment with protocol-guided escitalopram and nortriptyline; an independently operated random number generator stratified by center was used for allocation. Escitalopram is a selective serotonin reuptake inhibitor that has no effect on norepinephrine transport (26). It was initiated at a dosage of 10 mg/day and titrated to a mean dosage of 17 mg/day (range, 5–30 mg/day). Nortriptyline is a tricyclic antidepressant with an affinity more than 100 times stronger for the norepinephrine transporter than for the serotonin transporter, and it is therefore considered primarily a norepinephrine reuptake inhibitor (27). We chose nortriptyline in preference to a more selective norepinephrine reuptake inhibitor because of its superior efficacy record (28, 29). Nortriptyline was initiated at a dosage of 50 mg/day and titrated to a mean dosage of 106 mg/day (range, 50–200 mg/day). Of the participants for whom CRP data were available, 115 were allocated to escitalopram and 126 to nortriptyline. Of these, 179 (74.3%) completed at least 8 weeks of treatment with the allocated drug. Completion rates were higher for the escitalopram group (N=93, 80.9%) than for the nortriptyline group (N=86, 68.3%), but the two treatments showed comparable efficacy in reducing depressive symptoms (24).
Outcome Measures
The primary outcome measure was the total score on the Montgomery-Åsberg Depression Rating Scale (MADRS) (30), administered weekly by trained psychiatrists and psychologists with high interrater reliability (31, 32). Secondary outcome measures included the 17-item Hamilton Rating Scale for Depression (HAM-D) (33), the Beck Depression Inventory (BDI) (34), and symptom dimensions derived from factor analysis: observed mood (mood, psychomotor retardation), cognitive symptoms (pessimism, loss of interest, anhedonia, and reduced activity), and neurovegetative symptoms (insomnia and loss of appetite) (24, 31, 32).
Measurement of C-Reactive Protein
We measured CRP in the serum separated from peripheral blood samples obtained by antecubital venipuncture at baseline (before initiation of antidepressant treatment), using a high-sensitivity immunoturbidimetry assay (Cormay, Lublin, Poland) on the Siemens Advia 2400 analyzer. This assay has the sensitivity to detect CRP levels as low as 0.1 mg/L, with high reproducibility, with an intra-assay coefficient of variation of 2.96% and an interassay coefficient of variation of 3.85%. All measurements were carried out at the same time at the same laboratory (the Department of Biochemistry, King’s College Hospital, London), blind to treatment allocation and outcomes.
Statistical Analysis
As in previous GENDEP analyses, we used mixed-effects models for repeated measures, applied in the STATA environment (35). Mixed-effects models for repeated measures allow inclusion of all available measurements across the follow-up weeks and provide unbiased valid estimates under a broader range of assumptions about missing data than more traditional approaches (36–39). Since the distribution of CRP was skewed, we used a natural logarithm transformation, consistent with previous investigations (16), which led to an approximately normal distribution (see Figure S2 in the online data supplement). The primary hypothesis was tested as a statistical interaction between logarithmically transformed baseline CRP levels (lnCRP) and the antidepressant drug (escitalopram versus nortriptyline). Continuous MADRS score in each follow-up week was the primary outcome measure. Clustering of repeated measurements from the same participant was modeled with a random effect of the individual. All models controlled for age, sex, baseline depression severity, and recruitment center. Time of each measurement (as number of weeks in the trial at time of each of the repeated measurements) was modeled by linear and quadratic effects of time (model A).
Mixed-effects models for repeated measures allow the decomposition of variance in outcome between individually consistent effects and variance due to time in the study, baseline severity, and other factors (40). We took advantage of mixed-effects models for repeated measures variance decomposition to estimate what proportion of the variance in outcome that was explainable by individual-level factors was due to the predictor of interest (CRP and its interaction with drug) (40–42). To estimate whether a prediction might be clinically significant, we compared the proportion of the variance explained to a simulation-based benchmark of 6.3% of the variance, which corresponds to a previously established consensus rule that an average difference of 3 points or more on the HAM-D is clinically significant (43, 44).
Based on mixed-effects models for repeated measures, we further estimated the relative advantage of each antidepressant over the other at various levels of CRP (45). For graphing, we grouped participants according to the cutoffs for CRP recommended by the U.S. Centers for Disease Control and Prevention and the American Heart Association: <1 mg/L=low cardiovascular risk/low level of systemic inflammation; 1–2.9 mg/L=average cardiovascular risk/average level of systemic inflammation; 3–10 mg/L=high cardiovascular risk/high level of systemic inflammation; >10 mg/L=acute inflammation. In sensitivity analyses, we tested the effects of potential confounders, including known inflammatory or autoimmune medical conditions, use of anti-inflammatory drugs (46), smoking, medication-free status at recruitment, and body mass index (BMI) (47) as covariates. These covariates were included in two steps (models B and C). To completely exclude the influence of known inflammatory disease or anti-inflammatory medication, we carried out a final sensitivity analysis restricted to individuals who did not have inflammatory or autoimmune disease, were not using anti-inflammatory drugs, and had a baseline CRP level below the threshold suggestive of acute inflammation (<10 mg/L) (model D). To explore how the predictive effects generalize across different depression rating scales and symptom dimensions, the analyses were repeated with secondary outcome measures.
Power Calculation
We aimed to detect a differential prediction that explains 6.3% of the variance in outcome, a previously established threshold for a prediction to be clinically significant (44). A power calculation indicated that a sample of at least 180 is required to detect such an effect at an alpha level of 0.01 with a power of 90%. The available sample of 241 individuals with CRP values provided a power of 97% to detect a prediction explaining 6.3% of the variance and a power of 68% to detect a prediction explaining 3.17% of the variance (half of what is clinically significant) at an alpha of 0.01. We concluded that the available sample was of an adequate size to test our hypothesis.
Results
Baseline Correlates of Systemic Inflammation
Of the 241 participants with baseline CRP data, 131 (54%) had values in the range indicating low levels of systemic inflammation, 63 (26%) had values in the moderate range, 37 (15%) had values in the high range, and only 10 (4%) had values suggesting acute inflammation. Baseline level of systemic inflammation (indexed by lnCRP) showed the expected positive correlations with BMI (r=0.35, 95% CI=0.23, 0.45, p<0.001) and with age (r=0.16, 95% CI=0.04, 0.28, p=0.011) but was unrelated to baseline depression severity or symptom dimensions (see Table S1 in the online data supplement) or to sex or recruitment center. Baseline levels of inflammation did not differ significantly between the escitalopram and nortriptyline groups (Table 1).
Baseline Inflammation and Outcome of Treatment
As in the entire GENDEP sample, nortriptyline and escitalopram were equally effective, resulting in no significant effect of drug on the primary outcome measure (β=0.21, 95% CI=−1.39, 1.81). A mixed-effects model for repeated measures without interaction terms detected a weak overall effect of increasing lnCRP leading to worse outcome across the entire sample, with a worsening of approximately 1 point on the MADRS per standard deviation of lnCRP (β=0.96, 95% CI=0.09, 1.83, p=0.031). A mixed-effects model with an interaction term detected a significant interaction between baseline lnCRP and antidepressant drug on the primary outcome measure (β=3.27, 95% CI=1.65, 4.89, p<0.001) (Table 2). The interaction was primarily due to the fact that escitalopram was less effective for individuals with high levels of systemic inflammation (see Figure S3 in the online data supplement). Among participants treated with escitalopram, a one-standard-deviation increase in baseline lnCRP was associated with a worsening of outcome by 2.7 points on the MADRS (95% CI=1.02, 2.85, p<0.001). Among participants treated with nortriptyline, higher levels of systemic inflammation at baseline were associated with a nonsignificantly better outcome by 0.6 points on the MADRS per standard deviation of lnCRP (95% CI=−1.73, 0.60). Marginal effect estimates suggested that at low levels of systemic inflammation, escitalopram led to an improvement of 3 more points on the MADRS than nortriptyline, and at moderate to high levels of systemic inflammation, nortriptyline led to an improvement of 3 more points on the MADRS than escitalopram (Figure 1).The differential prediction generalized across the three outcome measures: the effects were similar with HAM-D scores and were even stronger with scores on the self-reported BDI (Table 2). The interaction between antidepressant drug and baseline systemic inflammation affected all three previously identified symptom dimensions, including observed mood, cognitive symptoms, and neurovegetative symptoms (see Table S2 in the online data supplement).
| Outcome Measure and Model | β | 95% CI | p | Variance Explained (%) |
|---|---|---|---|---|
| Montgomery-Åsberg Depression Rating Scale | ||||
| A | 3.27 | 1.65, 4.89 | <0.001 | 10.6 |
| B | 3.19 | 1.57, 4.80 | <0.001 | |
| C | 3.24 | 1.62, 4.86 | <0.001 | |
| D | 2.95 | 1.21, 4.68 | <0.001 | |
| Hamilton Rating Scale for Depression | ||||
| A | 2.34 | 1.08, 3.59 | <0.001 | 9.2 |
| B | 2.27 | 1.02, 3.52 | <0.001 | |
| C | 2.26 | 1.01, 3.51 | <0.001 | |
| D | 2.09 | 0.71, 3.48 | 0.003 | |
| Beck Depression Inventory | ||||
| A | 4.86 | 2.90, 6.82 | <0.001 | 13.8 |
| B | 4.75 | 2.83, 6.67 | <0.001 | |
| C | 4.90 | 2.95, 6.84 | <0.001 | |
| D | 4.42 | 2.29, 6.56 | <0.001 |
TABLE 2. Interaction Between C-Reactive Protein Level and Antidepressant on Treatment Outcome Tested With Mixed-Effects Models for Repeated Measuresa
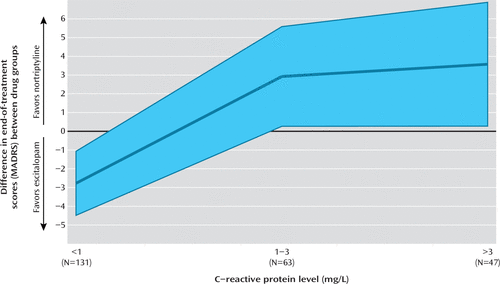
FIGURE 1. Effects of Antidepressant Choice on Depression Severity According to C-Reactive Protein (CRP) Levela
a MADRS=Montgomery-Åsberg Depression Rating Scale. The relative advantage of each antidepressant (marginal effect) at different CRP levels was estimated based on mixed-effects model A. CRP level is categorized according to the cutoffs recommended by the U.S. Centers for Disease Control and Prevention and the American Heart Association. Estimates for the three categories of CRP levels are drawn from the same model (rather than subgroup analyses), as indicated by the lines connecting the estimation points. Estimate of marginal effect on the MADRS is traced with a thick blue line; the pale blue area is the 95% confidence interval.
Role of Potential Confounders
A number of factors are known to influence serum CRP level: inflammatory medical illness, autoimmune conditions, anti-inflammatory medication, smoking, and body weight. If these factors were associated with both CRP and outcome, confounding could occur. To examine the possibility that the observed differential prediction of response to escitalopram and nortriptyline was a result of confounding, we carried out a series of sensitivity analyses that either controlled for these factors (models B and C) or excluded cases that were most likely to be affected (model D). The results of these sensitivity analyses were similar to those of the original analyses (Table 2), suggesting that confounding by measured variables did not significantly affect the results.
Clinical Significance of Inflammation in Antidepressant Choice
Levels of CRP and their interaction with drug explained 10.6% of individual-level variance in the primary outcome measure (MADRS score), 9.2% of individual-level variance in change on HAM-D score, and 13.8% of the variance in score on the self-report BDI. All these values are above the clinical significance benchmark of 6.3% variance explained (44). Baseline levels of systemic inflammation were not strongly related to other known predictors of antidepressant response. For example, baseline lnCRP was only weakly correlated with the symptom dimension of interest and activity, falling short of significance (r=0.11, p=0.09; see Table S1 in the online data supplement for correlations with all symptom dimensions), although this dimension was previously found to strongly predict outcome of treatment with antidepressants (4). Consequently, these two factors contribute independently to the prediction of outcomes. Baseline lnCRP and interest-activity symptoms jointly explained 15.8% of the variance in the primary outcome measure.
Discussion
In a study of 241 patients with depression, we found that CRP, an easily accessible biomarker of systemic inflammation, differentially predicted response to escitalopram and nortriptyline. The effect size of the prediction suggested that the use of this biomarker in the selection of an antidepressant may meaningfully improve outcomes in the treatment of depression.
A major advantage of CRP lies in its easy availability. CRP can be obtained from a nonfasting peripheral blood sample, and high-sensitivity assays are routine in most medical laboratories (22). In GENDEP, CRP differentially predicted therapeutic response to the two antidepressants, suggesting that it could be used to inform the selection of antidepressant drug (2). The effect size of the differential prediction met criteria for clinical significance (43, 44), suggesting that the prediction can be meaningful in individual cases. The even stronger effects with the secondary self-report outcome measure (BDI) suggests that the differential prediction will be as meaningful to patients as to clinicians. Additivity with other predictors, such as interest-activity symptoms (4), suggests that an even more accurate prediction of outcome can be achieved by the combination of biomarkers and additional clinical variables (3). While the prediction of outcome by interest-activity symptoms replicated across studies (4), the contribution of CRP level and the effect size of the joint prediction will require replication to establish generalizability. This promising finding contrasts with the limited effects of pharmacogenetics (48, 49) and suggests that state-dependent biomarkers may be needed to personalize treatment choice (50). The added value of combining state-dependent biomarkers with genetic ones is a field for future investigation.
While this is the first study that shows a differential prediction by an inflammatory biomarker of response to serotonin reuptake inhibitor and norepinephrine reuptake inhibitor antidepressants, the results are consistent with the literature. Two previous studies (17, 19) found that higher levels of CRP or proinflammatory cytokines at baseline were associated with poorer response to the predominantly serotonin reuptake inhibiting antidepressants, including escitalopram, fluoxetine, and low-dosage venlafaxine. Although one study (51) found no significant effect of baseline inflammation on the therapeutic response to sertraline in individuals with cardiovascular disease, the mean CRP levels in that selected sample were four times those in the present study, putting most subjects in the high-risk range of CRP levels and making the data sets incomparable. In previous studies using nortriptyline or related tricyclic antidepressants, elevated CRP level either was not associated with outcome or predicted better antidepressant response, consistent with the present findings (15, 52). Baseline CRP levels were also found to moderate the antidepressant effects of a treatment that directly targets immune pathogenic mechanisms, which was beneficial only for individuals with high CRP levels (16). Additional hope for individuals with high levels of systemic inflammation comes from a study that shows that they may benefit more from physical exercise (53). Taken together, the evidence to date is consistent with systematic inflammation acting as a moderator of antidepressant response.
The hypothesis that led to this investigation stemmed from known contrasting effects of norepinephrine and serotonin on the immune system and hence focused on the differential action of norepinephrine reuptake inhibitor and serotonin reuptake inhibitor antidepressants (8–10, 12). However, the differential prediction of response to these types of antidepressants is currently based on a single study of two drugs and must be interpreted with several limitations kept in mind.
First, the two antidepressants investigated here differ in a number of respects. Escitalopram is a highly selective serotonin reuptake inhibitor with no important effects on other receptors or transporters and no effect on the production of proinflammatory cytokines (26, 54). Nortriptyline is a tricyclic antidepressant; although it preferentially blocks the reuptake of norepinephrine and has only a weak affinity to the serotonin transporter (27), it also binds several receptors, including the histamine H1 receptor, the muscarinic acetylcholine receptor M1, and serotonin receptors 5-HT2A and 5-HT2C (55). Given the pleiotropy of nortriptyline effects, the present study cannot separate the effects of norepinephrine reuptake blockage from other compound-specific effects or class effects of tricyclic antidepressants. It remains to be investigated whether other antidepressants with similar effects on the immune system (8) can substitute for nortriptyline in patients with high levels of systemic inflammation.
Second, this study is limited to testing a pragmatic hypothesis regarding prediction of treatment outcomes. In the absence of serial measurements of multiple cytokines, it does not directly address the molecular mechanisms underlying the observed effects. We are unable to differentiate between effects of antidepressants on cytokine production (8) and effects of cytokines on the monoaminergic neurotransmitter systems affecting the therapeutic action of antidepressants (13). Studies with serial collection of samples over time are needed to elucidate the molecular mechanisms underlying the observed effect.
Third, GENDEP was primarily a pharmacogenetic study (56, 57), and collection of serum samples was not a priority. As a result, serum samples were available for just over half of the participants. Participants with and without serum samples were comparable on depression severity, drug allocation, and treatment outcome, suggesting no selection bias. However, the smaller sample size may reduce the generalizability of findings in spite of high levels of statistical significance. Replication is a necessary step before clinical implementation. Future studies should test the replicability of the differential prediction of escitalopram and nortriptyline efficacy by CRP level and extend the findings to other antidepressants and other markers of inflammation.
Conclusions
A hypothesis based on differential effects of norepinephrine and serotonin on the immune system has led to the identification of the strongest differential predictor of response to a serotonin reuptake inhibitor versus a norepinephrine reuptake inhibitor to date. The easy accessibility of the biomarker and the substantial effect size suggest that, if the results are replicated, CRP level may help in selection of an antidepressant more likely to benefit a given individual. Individuals with high levels of inflammation may benefit from the noradrenergic nortriptyline more than from a selective serotonin reuptake inhibitor. Further studies are needed to test how the differential prediction replicates and extends to other antidepressants.
1 : Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med 2013; 10:e1001547Crossref, Medline, Google Scholar
2 : Personalized medicine for depression: can we match patients with treatments? Am J Psychiatry 2010; 167:1445–1455Link, Google Scholar
3 : A clinical risk stratification tool for predicting treatment resistance in major depressive disorder. Biol Psychiatry 2013; 74:7–14Crossref, Medline, Google Scholar
4 : Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 2012; 42:967–980Crossref, Medline, Google Scholar
5 : Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65:732–741Crossref, Medline, Google Scholar
6 : Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71:171–186Crossref, Medline, Google Scholar
7 : Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology 2014; 39:1624–1634Crossref, Medline, Google Scholar
8 : Immunomodulation mechanism of antidepressants: interactions between serotonin/norepinephrine balance and Th1/Th2 balance. Curr Neuropharmacol 2012; 10:97–123Crossref, Medline, Google Scholar
9 : Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci USA 2010; 107:6058–6063Crossref, Medline, Google Scholar
10 : A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun 2012; 26:469–479Crossref, Medline, Google Scholar
11 : The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011; 36:2452–2459Crossref, Medline, Google Scholar
12 : Chlorimipramine and nortriptyline but not fluoxetine and fluvoxamine inhibit human polymorphonuclear cell chemotaxis in vitro. Gen Pharmacol 1994; 25:409–412Crossref, Medline, Google Scholar
13 : The pro-inflammatory cytokine TNF-α regulates the activity and expression of the serotonin transporter (SERT) in astrocytes. Neurochem Res 2013; 38:694–704Crossref, Medline, Google Scholar
14 : Cytokine-induced change in hypothalamic norepinephrine turnover: involvement of corticotropin-releasing hormone and prostaglandins. Brain Res 1993; 622:257–261Crossref, Medline, Google Scholar
15 : Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22:370–379Crossref, Medline, Google Scholar
16 : A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41Crossref, Medline, Google Scholar
17 : Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:445–450Crossref, Medline, Google Scholar
18 : Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res 2007; 41:326–331Crossref, Medline, Google Scholar
19 : Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav Immun 2012; 26:90–95Crossref, Medline, Google Scholar
20 : Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NFkB- and C/EBPβ-dependent autocrine interleukin-6 loop. Mol Immunol 2008; 45:2678–2689Crossref, Medline, Google Scholar
21 : Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 2013; 19:259–264 Crossref, Medline, Google Scholar
22 : Cardiology patient page: C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation 2003; 108:e81–e85Crossref, Medline, Google Scholar
23 : Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand 2013; 129:180–192 Crossref, Medline, Google Scholar
24 : Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry 2009; 194:252–259Crossref, Medline, Google Scholar
25 : SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
26 : Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology (Berl) 2003; 167:353–362Crossref, Medline, Google Scholar
27 : Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol 1999; 19:467–489Crossref, Medline, Google Scholar
28 : Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010; 341:c4737Crossref, Medline, Google Scholar
29 : The noradrenergic action in antidepressant treatments: pharmacological and clinical aspects. CNS Neurosci Ther 2011; 17:723–732Crossref, Medline, Google Scholar
30 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
31 : Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med 2008; 38:289–300Crossref, Medline, Google Scholar
32 : Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety 2012; 29:1043–1049Crossref, Medline, Google Scholar
33 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
34 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
35 : Multilevel and Longitudinal Modeling Using Stata. College Station, Tex, Stata Press, 2008Google Scholar
36 : Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat 2008; 7:93–106Crossref, Medline, Google Scholar
37 : The efficacy of duloxetine: a comprehensive summary of results from MMRM and LOCF_ANCOVA in eight clinical trials. BMC Psychiatry 2004; 4:26Crossref, Medline, Google Scholar
38 : MMRM vs LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat 2009; 19:227–246Crossref, Medline, Google Scholar
39 : MMRM versus MI in dealing with missing data: a comparison based on 25 NDA data sets. J Biopharm Stat 2011; 21:423–436Crossref, Medline, Google Scholar
40 : Estimating variance components in Stata. Stata J 2006; 6:1–21Crossref, Google Scholar
41 : An R2 statistic for fixed effects in the linear mixed model. Stat Med 2008; 27:6137–6157Crossref, Medline, Google Scholar
42 : Measuring explained variation in linear mixed effects models. Stat Med 2003; 22:3527–3541Crossref, Medline, Google Scholar
43
44 : Biomarkers predicting treatment outcome in depression: what is clinically significant? Pharmacogenomics 2012; 13:233–240Crossref, Medline, Google Scholar
45 : Estimating of marginal effects using margeff. Stata J 2005; 5:309–329Crossref, Google Scholar
46 : Non-steroidal anti-inflammatory drugs and efficacy of antidepressants in major depressive disorder. Psychol Med 2012; 42:2027–2035Crossref, Medline, Google Scholar
47 : Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord 2009; 118:147–154Crossref, Medline, Google Scholar
48
49 : Contribution of common genetic variants to antidepressant response. Biol Psychiatry 2013; 73:679–682Crossref, Medline, Google Scholar
50 : Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline “predictors” and longitudinal “targets.” Neuropsychopharmacology 2013; 38:377–385Crossref, Medline, Google Scholar
51 : Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. J Psychosom Res 2011; 71:13–17Crossref, Medline, Google Scholar
52 : Elevated C-reactive protein in depression: a predictor of good long-term outcome with antidepressants and poor outcome with psychotherapy. J Psychopharmacol 2010; 24:625–626Crossref, Medline, Google Scholar
53 : Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry 2013; 18:1119–1124Crossref, Medline, Google Scholar
54 : No evidence for an anti-inflammatory effect of escitalopram intervention in healthy individuals with a family history of depression. J Neuroimmunol 2012; 243:69–72Crossref, Medline, Google Scholar
55 : Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 2007; 151:737–748Crossref, Medline, Google Scholar
56 : Genetic predictors of response to antidepressants in the GENDEP project. Pharmacogenomics J 2009; 9:225–233Crossref, Medline, Google Scholar
57 : Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010; 167:555–564Link, Google Scholar



