Treatment of Maternal Depression in a Medication Clinical Trial and Its Effect on Children
Abstract
Objective:
Observational studies show that when a depressed mother’s symptoms remit, her children’s psychiatric symptoms decrease. Using randomized treatment assignment, the authors sought to determine the differential effects of a depressed mother’s treatment on her child.
Method:
The study was a randomized double-blind 12-week trial of escitalopram, bupropion, or the combination of the two in depressed mothers (N=76), with independent assessment of their children (N=135; ages 7–17 years).
Results:
There were no significant treatment differences in mothers’ depressive symptoms or remission. Children’s depressive symptoms and functioning improved significantly among those whose mothers were in the escitalopram group (compared with those whose mothers were in the bupropion and combination treatment groups). Only in the escitalopram group was significant improvement of mother’s depression associated with improvement in the child’s symptoms. Exploratory analyses suggested that this may be due to changes in parental functioning: Mothers in the escitalopram group reported significantly greater improvement, compared with the other groups, in their ability to listen and talk to their children, who as a group reported that their mothers were more caring over the 12 weeks. Maternal baseline negative affectivity appeared to moderate the effect of maternal treatment on children, although the effect was not statistically significant. Children of mothers with low negative affectivity improved in all treatment groups. Children of mothers with high negative affectivity improved significantly only for those whose mothers were in the escitalopram group.
Conclusions:
The effects of the depressed mother’s improvement on her children may depend on her type of treatment. Depressed mothers with high anxious distress and irritability may require medications that reduce these symptoms in order to show the effect of her remission on her children.
School-age offspring of mothers with major depression consistently have elevated rates of depression (1–7), and we and others have found that the remission of maternal depression is associated with a reduction in their offspring’s psychiatric symptoms (8–11). Because these studies were observational and the mothers’ treatment was not randomly assigned, we could not conclude that the improvement in the child’s symptoms was due to the mother’s treatment.
A 9-month randomized controlled study of 47 depressed mothers receiving either interpersonal psychotherapy or treatment as usual, and their 6- to 18-year-old children (12), found that symptom improvement in mothers receiving interpersonal psychotherapy was statistically significant at 12 weeks, and the treatment effects on children were significant at 9 months. Two other randomized controlled trials testing the effects of maternal treatment on children included much younger children (ages 2–4 and 4–11) and found that there were no statistically significant treatment effects on children (13, 14).
In this study, we independently assessed children of depressed mothers participating in a 12-week double-blind randomized clinical trial testing the effects of escitalopram, bupropion, or the combination of the two (15). We hypothesized that mothers receiving the combination treatment would have an earlier onset and a higher rate of remission than those receiving either of the monotherapies and that the results would be reflected in their children.
Method
Adult study participants were psychiatric outpatients 18–65 years of age with nonpsychotic major depression, without a lifetime history of bipolar disorder, schizophrenia, or schizoaffective disorder, and without a current substance use disorder. Patients with other psychiatric conditions or with medical conditions were included unless any of the study medications were contraindicated.
Parents were eligible for the child study if they participated in the adult study and had at least one child 7–17 years of age who lived at least half of the time with the treated parent and had no developmental disability that would preclude participation. All eligible parents and children were enrolled. Among the 245 adults in the study, 110 (44.9%) had age-eligible children, and 93 (84.5%) eligible parents (82 mothers and 11 fathers) entered the child study (Figure 1). These 93 parents had 175 age-eligible children, of whom 168 (96%) participated in the child study. The present analyses are limited to mothers. Seventy-six (92%) of the 82 eligible mothers and 135 (93%) of their 145 children entered the study and comprise the reported cohort. There were no significant differences in age or in baseline depressive symptom scores between the women who enrolled in the study and the six who did not.
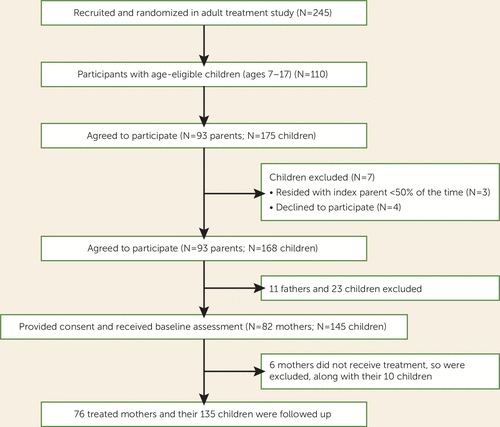
FIGURE 1. Flow of Participants in a Study of Treatment of Maternal Depression and Its Effect on Children
Children were referred for treatment if they or a parent requested it, and children were not excluded if they were in treatment. The study protocol was approved by the institutional review boards of the involved institutions, and the study took place in New York City and Ottawa. Each site had two clinics under the direction of the principal investigator, with the same protocol and institutional review board.
Adult Study
Mothers received a battery of assessments at baseline and at 1, 2, 3, 4, 6, 8, 10, and 12 weeks. The mother’s diagnosis was established by the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (16). The severity of depressive symptoms was estimated with the 17-item Hamilton Depression Rating Scale (HAM-D) (17). Remission was defined as a HAM-D score ≤7, and relapse as a score ≥14 after remission (18).
The Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR), a self-report measure designed to assess the severity of depressive symptoms, and which can be transformed to HAM-D scores (19), was used if a mother missed a HAM-D rating. This occurred with five mothers in week 4, six in week 8, and seven in week 12, and did not vary by maternal treatment. The Montgomery-Åsberg Depression Rating Scale (MADRS) (20) was also used to assess depressive symptoms.
The Social Adjustment Scale–Self-Report was used to assess performance in work functioning, social and leisure activities, and in parental, marital, and extended family roles (21, 22). Parenting items include questions about interest in child activities, ability to talk and listen to one’s child, getting along with one’s child, and feeling affection toward one’s child.
Nutt et al. (23) and Stahl et al. (24) have reported that high negative affect might result from serotonin deficiency. Gerra et al. (25), based on the adult study, developed a negative affectivity score and hypothesized that patients with high negative affectivity would preferentially respond to selective serotonin reuptake inhibitors (SSRIs). A negative affectivity measure was composed of items from the HAM-D, MADRS, QIDS-SR, and Social Adjustment Scale–Self-Report and included three dimensions: guilt, hostility/irritability, and fear/anxiety (see the Supplemental Methods section in the data supplement that accompanies the online edition of this article). Because of the different ranges of item scores, the variables were recoded into a 7-point scale. The mean affect scores were used, and negative affectivity was derived by summing the three dimension scores. The sample median (2.10) was used to dichotomize scores into low and high negative affectivity.
Medication side effects were recorded on a checklist at each appointment. The daily dose for escitalopram ranged from 10 mg to 40 mg, and for bupropion from 150 mg to 450 mg. Treating physicians were encouraged to reach doses of 40 mg for escitalopram and 450 mg for bupropion in order to maximize the effect of treatment (see reference 15 for details). (The study was designed before the U.S. Food and Drug Administration issued a recommendation not to exceed daily doses of 20 mg for escitalopram.) To maintain the blind, medication and placebo looked identical, and the same number were used for each treatment.
Child Study
The child study assessments were conducted by independent assessors who knew that participating mothers were depressed but were blind to their assessments. Children were assessed at baseline and at 4, 8, and 12 weeks.
Children’s psychiatric disorders were established by independent interviews of mothers and children using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (26). Depressive symptoms were assessed with the 27-item self-report Children’s Depression Inventory (CDI) (27, 28). Children’s functioning was assessed with the child version of the Columbia Impairment Scale (CIS) (29).
Children’s perception of their mothers’ parenting was assessed with the self-report Parental Bonding Inventory (30). The 12-item dimension of care and affection (see Table S5 in the online data supplement) was used to replicate the findings of Garber et al. (11).
Mental health treatment received by the child historically at baseline and during the 12 weeks of the study was recorded. Six interviewers with child clinical experience received assessment training under the supervision of child psychiatrists (D.J.P. and M.F.F.) and completed the child assessments (see the Supplemental Methods section in the online data supplement).
Data Analysis
Differences in means of continuous variables by treatment for mother’s baseline characteristics were determined using analysis of variance; differences in categorical variables by treatment were analyzed using contingency table analyses and associated chi-square tests or Fisher’s exact test when expected counts were low. Differences in children’s baseline characteristics by treatment were analyzed using linear mixed models for continuous variables and logistic regression analyses in the context of generalized estimating equations (31) for categorical variables, to allow adjustment for correlation between siblings. Baseline characteristics found to be significantly different were adjusted for in subsequent analyses.
Differences in rates of maternal remission from depression during the 12 weeks by treatment assignment were determined using logistic regression analysis as follows. Maternal remission status was the binary outcome variable; treatment assignment, represented by two dummy variables, was considered the set of independent variables; and age of mother as well as other baseline demographic and clinical characteristics that had been previously shown to have significant differences in distribution by treatment assignment were included as potential confounding variables. Differential effects of treatment on change in mother’s depressive symptoms and functioning were analyzed using linear mixed-effects regression analyses, which account for the nesting of time within person, to test linear and curvilinear (quadratic) trends over time and their interaction with treatment.
The differential effect of maternal treatments on child outcomes was investigated as follows.
Model 1.
When the child outcome was a continuous variable, linear mixed-effects models were fitted to the data with child outcome as the dependent variable, maternal treatment and time (study week) as independent variables, and an interaction term representing treatment by time. In addition, age and gender of child and study site were included as covariates. Other child and maternal baseline characteristics that were significantly different between treatments were also tested but were dropped from the model if they did not contribute to an appreciable difference in the results. A statistically significant interaction term indicated a differential treatment effect. Correlations between related measures over time, as well as nonindependence of observations among siblings, were handled by including nested random effects in the model (i.e., within-subject observations nested within family) (32). When child outcomes were binary variables (child diagnoses) or count variables (child symptoms), logistic random-effects regression models (for binary measures) and Poisson random-effects regression (for count measures) were used to determine differential effects of maternal treatment on these outcomes (33). Repeated measures over time and nonindependence of siblings as well as potential confounding variables were handled as described for continuous outcomes.
Model 2.
For child outcomes that showed statistically significant differences in trend parameters over time, we investigated whether differential effects of changes in mother’s depressive symptoms over time could explain (i.e., mediated) these differences. In model 2, we included mother’s depressive symptoms as time-varying covariates in models with relevant child outcomes as dependent variables, and maternal treatment effects as the independent variables; we tested for main effects and/or interactions of mother’s depressive symptoms over time by treatment, including a main effect for treatment in the models that we used to estimate the effect of mother’s treatment on child outcomes implicitly controlled for baseline differences by treatment (if any) (34). Exploratory analyses of the potential moderating effect of mother’s baseline negative affect score on the patterns of differential effects of mother’s treatment on child outcomes (if any) were conducted by including main effects and two- and three-way interaction terms for negative affectivity (coded as a binary variable) and time, treatment, and time by treatment in model 1.
Results
Characteristics of Depressed Mothers and Their Children
There were no significant differences in mothers’ or children’s demographic or clinical characteristics at baseline by maternal treatment (see Table S1 in the online data supplement), with the following exceptions. Mothers who received combination treatment were older on average than those who received one of the monotherapies (mean age, 43.7 years [SD=6.4] for the combination treatment group, compared with 39.9 years [SD=5.6] for the bupropion group and 38.2 [SD=5.7] for the escitalopram group; p=0.003), and 20% of mothers treated with bupropion were married or cohabitating, as compared with 52% of mothers who received either escitalopram or combination treatment. A significantly lower proportion of children of mothers who received bupropion were girls (20% in the bupropion group, compared with 50% in the combination treatment group and 61% in the escitalopram group; p<0.001). There were no significant differences in children’s depressive symptom scores (on the CDI) or impairment scores (on the CIS) or in proportion with current or lifetime psychiatric diagnoses at baseline. Analyses of both mothers’ and children’s outcomes were adjusted for these demographic differences.
Maternal Outcomes
The overall rate of maternal remission during the 12 weeks following treatment initiation was high (67%), and remission rates did not vary by treatment. Relapse rates were low and did not vary by treatment; only seven mothers of 15 children relapsed over the 12 weeks (see Table S2 in the data supplement). Dropout rates were low, with only four mothers and their five children dropping out of the study (see Table S3 in the data supplement).
The results of fitting a mixed-effects regression model with linear and quadratic terms to estimate changes in maternal depression symptoms over time by maternal treatment are presented in Figure S1 in the data supplement. Both linear and quadratic terms were found to be significant but did not vary significantly with treatment. Taken together, the negative linear component (beta1=−2.62, SE=0.17; t=−15.22, p<0.001) and the positive quadratic component (beta2=0.14, SE=0.01; t=9.85, p<0.001) suggest that maternal HAM-D scores decreased significantly and then leveled off for all treatment groups over the 12 weeks.
Child Outcomes
We compared child outcomes over the 12 weeks by maternal treatment, adjusting for child’s age and gender, within-family correlation, and study site. Statistically significant differential treatment effects were observed on CDI and CIS scores. Additional models were tested that included maternal baseline characteristics that were significantly different between treatment groups (age and marital status); these additional variables did not confound results or qualify as moderators and thus were subsequently omitted from the analyses.
We first determined whether there were significant changes among child outcome measures in each of the maternal treatments separately during the 12 weeks. As shown in Table 1, mean CDI scores decreased significantly over time among children of mothers who received monotherapy (reflected in the negative beta coefficients and associated p values), indicating that these children became less depressed over time, and those whose mothers received the combination treatment did not. The group-by-time interaction was significant (F=7.28, df=2, 227, p<0.001), suggesting a difference in treatment effect. Post hoc tests revealed that the time trend for children of mothers who received escitalopram monotherapy was statistically different from that of children whose mothers received the combination treatment (t=3.81, p<0.001) or bupropion monotherapy (t=2.04, p=0.04). There were no significant differences between the combination treatment and bupropion monotherapy (Figure 2).
| Measure and Treatment Group | Baseline Score | Beta | SE | p | Change Over Time |
|---|---|---|---|---|---|
| Children’s Depression Inventory Scoreb | |||||
| Bupropion | 8.6 | –0.20 | 0.07 | 0.006 | –2.38 |
| Escitalopram | 10.5 | –0.39 | 0.06 | <0.001 | –4.68 |
| Combination treatment | 7.2 | –0.06 | 0.06 | 0.35 | –0.68 |
| Pairwise comparisonsc | |||||
| Bupropion versus escitalopram | 0.19 | 0.09 | 0.04 | ||
| Combination versus bupropion | 0.14 | 0.09 | 0.13 | ||
| Combination versus escitalopram | 0.33 | 0.09 | <0.001 | ||
| Columbia Impairment Scale Scored | |||||
| Bupropion | 10.6 | –0.04 | 0.09 | 0.62 | –0.53 |
| Escitalopram | 12.6 | –0.44 | 0.08 | <0.001 | –5.22 |
| Combination treatment | 10.2 | –0.19 | 0.07 | 0.01 | –2.27 |
| Pairwise comparisonse | |||||
| Bupropion versus escitalopram | 0.39 | 0.12 | 0.001 | ||
| Combination versus bupropion | –0.15 | 0.12 | 0.22 | ||
| Combination versus escitalopram | 0.25 | 0.11 | 0.03 |
TABLE 1. Time Trends for Child-Reported Symptoms and Impairment Over 12 Weeks, by Maternal Treatment Assignmenta
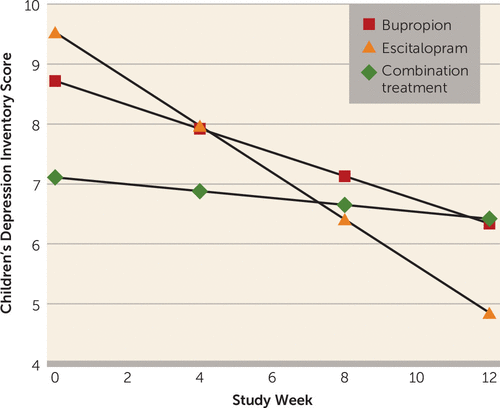
FIGURE 2. Estimated Trends in Scores Over 12 Weeks on the Children’s Depression Inventory, by Maternal Treatment Assignmenta
a The figure shows the mean scores for 35 children of 20 mothers treated with bupropion, 46 children of 29 mothers treated with escitalopram, and 54 children of 27 mothers receiving combination treatment with bupropion and escitalopram. Lower scores indicate improvement. All analyses were adjusted for child age (centered), gender, site, and within-family correlation. The time trends by treatment imply that children of mothers treated with escitalopram improved the most (beta=−0.39, SE=0.06; t=−6.27, p<0.001), followed by children of mothers treated with bupropion (beta=−0.20, SE=0.07; t=−2.82, p=0.006) and children of mothers who received the combination treatment (beta=−0.06, SE=0.06; t=−0.93, p=0.35). The overall week-by-treatment interaction was statistically significant (F=7.28, df=2, 227, p<0.001), implying that the change in child depression score over time differed significantly between treatment groups. Pairwise comparisons showed significant differences between children of mothers in the combination treatment group compared with the escitalopram group (beta=0.33, SE=0.09; t=3.81, p<0.001) and the bupropion group compared with the escitalopram group (beta=0.19, SE=0.09; t=2.04, p=0.04), and no significant differences between children of mothers who received the combination treatment compared with those of mothers who received bupropion (beta=0.14, SE=0.09; t=1.51, p=0.13).
There was a statistically significant decrease over time in mean CIS scores among children of mothers who received the combination treatment or escitalopram monotherapy (Table 1), indicating that these children became less impaired over time, as reflected in the statistically significant group-by-time interaction (F=5.57, df=2, 238, p=0.004). Post hoc tests revealed that the time trend for children of mothers who received escitalopram monotherapy was statistically different from that of children whose mothers received the combination treatment (t=2.25, p=0.03) or bupropion monotherapy (t=3.25, p=0.001). There were no significant differences between combination treatment and bupropion monotherapy (see Figure S2 in the online data supplement).
Changes in Maternal Depressive Symptoms and Treatment Effects on Child Outcomes
Tables 2 and 3 present results of the analysis to determine the relationship between the changes in maternal depressive symptoms over time and the observed effect of treatment on CDI scores. Analyses reported in these tables are based on participants for whom HAM-D data were available. Table 2 presents models in which the main predictors are maternal treatment assignment, time, and their interaction, and Table 3 presents models in which mothers’ HAM-D scores were added as a main predictor, along with interactions among HAM-D scores, treatment assignment, and time.
| Predictor | Betab | SE | p | Overall p |
|---|---|---|---|---|
| Week | –0.40 | 0.06 | <0.001 | <0.001 |
| Treatment | 0.097 | |||
| Bupropion | –0.71 | 1.37 | 0.60 | |
| Combination | –2.55 | 1.21 | 0.04 | |
| Escitalopram (reference) | ||||
| Week-by-treatment interaction | <0.001 | |||
| Week by bupropion | 0.21 | 0.10 | 0.03 | |
| Week by combination | 0.34 | 0.09 | <0.001 | |
| Week by escitalopram (reference) |
TABLE 2. Effect of Maternal Treatment Assignment on Trends in Children’s Depression Inventory Scores Over 12 Weeksa
| Maternal Treatment Effect (HAM-D Score) | ||||
|---|---|---|---|---|
| Predictor | Betab | SE | p | Overall p |
| Week | 0.08 | 0.14 | 0.57 | 0.63 |
| Treatment | 0.003 | |||
| Bupropion | 5.73 | 2.15 | 0.008 | |
| Combination | –1.50 | 2.02 | 0.46 | |
| Escitalopram (reference) | ||||
| Week by treatment | 0.13 | |||
| Week by bupropion | –0.37 | 0.21 | 0.08 | |
| Week by combination | 0.02 | 0.19 | 0.93 | |
| Week by escitalopram (reference) | ||||
| HAM-D score | 0.24 | 0.09 | <0.001 | 0.008 |
| HAM-D score by treatment | <0.001 | |||
| HAM-D score by bupropion | –0.35 | 0.09 | <0.001 | |
| HAM-D score by combination | –0.09 | 0.09 | 0.32 | |
| HAM-D score by escitalopram (reference) | ||||
| Week by HAM-D score | –0.03 | 0.01 | 0.001 | 0.07 |
| Week by HAM-D score by treatment | 0.02 | |||
| Week by HAM-D score by bupropion | 0.03 | 0.01 | 0.03 | |
| Week by HAM-D score by combination | 0.03 | 0.01 | 0.006 | |
| Week by HAM-D score by escitalopram (reference) | ||||
TABLE 3. Explaining Maternal Treatment Effect on Children’s Depression Inventory Outcome Over 12 Weeks by Mother’s Hamilton Depression Rating Scale (HAM-D) Scorea
There was a statistically significant interaction between change in maternal depression symptoms over time and treatment (i.e., the week-by-HAM-D score-by-treatment interaction in Table 3), implying that the association between maternal depressive symptoms and child depressive symptoms over time varied significantly by maternal treatment assignment (p=0.02).
To investigate these results further, we examined each treatment arm separately (Table 4). This investigation revealed that a reduction in mothers’ HAM-D scores was associated with a reduction in their children’s CDI scores over time, as originally hypothesized, but only for mothers who received escitalopram monotherapy. That is, there was a positive association between HAM-D scores and child CDI scores (beta=0.11, SE=0.05, p=0.03), and the coefficient corresponding to week (number of weeks from baseline) decreased in magnitude and statistical significance with the addition of HAM-D scores as the time-dependent covariate (i.e., the beta coefficient decreased in absolute magnitude from 0.40 [SE=0.06, p<0.001] to 0.27 [SE=0.08, p=0.002]). Furthermore, when the effects of HAM-D scores on child CDI scores were allowed to vary over time (by inclusion of an additional HAM-D score-by-week interaction term) this effect was found to be significant (p=0.001). These results suggest that as maternal HAM-D scores decrease over time, there is a corresponding decrease in child depressive symptoms (CDI scores), but the strength of this association decreased over the first 8 weeks of the study until it leveled off during the last 4 weeks. The effect of HAM-D scores did not vary with time for CDI scores of children whose mothers received bupropion monotherapy (i.e., the week-by-HAM-D score-by-treatment interactions were nonsignificant). Furthermore, there was a negative association between mother’s HAM-D scores and child’s CDI scores on average, and the magnitude of the slope coefficient increased rather than decreased with the addition of mothers’ HAM-D scores as time-dependent covariates, suggesting that a decrease in mother’s HAM-D scores does not explain the decrease in child’s CDI scores for mothers receiving bupropion monotherapy. There was no significant change over time in CDI scores for children whose mothers received the combination treatment, so the question of mediation of change in child CDI scores over time by change in mothers’ depressive symptoms over time became irrelevant for this group.
| HAM-D Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model | Add Main Effect | Add Interaction | |||||||
| Predictor | Beta | SE | p | Beta | SE | p | Beta | SE | p |
| Combination treatmentb | |||||||||
| Week | –0.05 | 0.06 | 0.43 | ||||||
| HAM-D score | |||||||||
| HAM-D score by week | |||||||||
| Bupropion | |||||||||
| Week | –0.19 | 0.08 | 0.03 | –0.31 | 0.10 | 0.004 | –0.28 | 0.18 | 0.12 |
| HAM-D score | –0.12 | 0.05 | 0.03 | –0.11 | 0.07 | 0.10 | |||
| HAM-D score by week | 0.00 | 0.01 | 0.88 | ||||||
| Escitalopram | |||||||||
| Week | –0.40 | 0.06 | <0.001 | –0.27 | 0.08 | 0.002 | 0.06 | 0.14 | 0.65 |
| HAM-D score | 0.11 | 0.05 | 0.03 | 0.24 | 0.07 | <0.001 | |||
| HAM-D score by week | –0.03 | 0.01 | 0.003 | ||||||
TABLE 4. Mother’s Score on the Hamilton Depression Rating Scale (HAM-D) and Children’s Score on the Children’s Depression Inventory, by Maternal Treatmenta
Similar analyses to determine whether reduction in maternal depressive scores explained the differential treatment effects on child impairment (CIS scores) revealed no significant relationship between improvement in child CIS scores and reduction in mother’s HAM-D scores for any of the three treatments.
Possible Reasons for Maternal Treatment Effect on Children
The next exploratory analyses were carried out to examine the effect of maternal treatment on the decrease in children’s depressive symptoms. Results of fitting a regression model to overall maternal social functioning (Social Adjustment Scale–Self-Report) scores showed a significant linear decrease in overall scores over time (beta1=−0.05, SE=0.01; t=−5.84, p<0.001), but the rate of improvement did not vary significantly with treatment. However, when we fitted the same model to the parenting role scores over time, we found a differential treatment effect over time that fell short of statistical significance (p=0.09) (see Figure S3 in the online data supplement), with mothers receiving escitalopram monotherapy exhibiting greater improvement in parental functioning over the study period as compared with those who received bupropion monotherapy or the combination treatment. This effect was primarily due to a differential effect of escitalopram monotherapy as compared with the other treatments (p=0.02) on mothers’ ratings on the item “being able to talk to and listen to my child” (Figure 3). The results with parental role scores and the individual parenting items are presented in Table S4 in the data supplement.
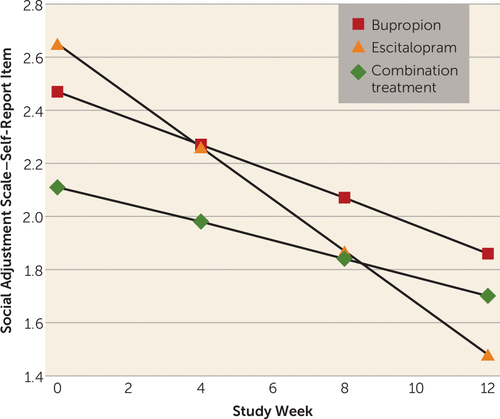
FIGURE 3. Estimated Trends in Scores Over 12 Weeks on the Social Adjustment Scale–Self-Report Parenting Item “Been Able to Talk to and Listen to Your Child”a
a The figure shows the mean scores for 20 mothers treated with bupropion, 29 mothers treated with escitalopram, and 27 mothers who received combination treatment with bupropion and escitalopram. Lower scores indicate improvement. All analyses were adjusted for site. Time trends by treatment imply that mothers treated with escitalopram improved the most (beta=−0.10, SE=0.02; t=−5.83, p<0.001), followed by mothers treated with bupropion (beta=−0.05, SE=0.02; t=−2.94, p=0.004) and those who received the combination treatment (beta=−0.03, SE=0.01; t=−2.35, p=0.02). The overall week-by-treatment interaction was statistically significant (F=4.12, df=2, 150, p=0.02), implying that change in score on this item over time differed significantly between treatment groups. Pairwise comparisons showed significant differences between mothers in the combination treatment group compared with those in the escitalopram group (beta=0.06, SE=0.02; t=2.82, p=0.005), near-significance between mothers in the bupropion group compared with those in the escitalopram group (beta=0.05, SE=0.02; t=1.92, p=0.057), and no significant difference between mothers in the combination treatment group compared with those in the bupropion group (beta=0.02, SE=0.02; t=0.73, p=0.47).
We next examined the child’s report of mother’s care and affection on the Parental Bonding Inventory. The results showed that over time, children of mothers receiving escitalopram monotherapy reported a significant increase in maternal care and affection (beta=0.17, SE=0.08; t=2.00, p=0.047), while those whose mothers received the combination treatment or bupropion monotherapy reported no significant change in maternal care and affection over time. The overall week-by-treatment interaction (F=2.99, df=2, 164, p=0.05) suggested that changes in child-reported maternal care and affection over time differed between treatment groups. This was confirmed in pairwise comparisons, with significant differences observed between children of mothers receiving the combination treatment compared with those whose mothers received escitalopram monotherapy (beta=−0.26, SE=0.12; t=−2.14, p=0.03) and between those whose mothers received bupropion monotherapy compared with those whose mothers received escitalopram monotherapy (beta=−0.26, SE=0.13; t=−2.04, p=0.04), while there were no significant differences between children of mothers who received combination treatment compared with those whose mothers received bupropion monotherapy (see Table S5 in the online data supplement).
There were no significant differential medication side effects or dosing patterns in mothers. The mean daily doses were 23.8 mg for escitalopram monotherapy, 244.8 mg for bupropion monotherapy, and 24.3 mg of escitalopram and 314.3 mg of bupropion for the combination treatment.
There was no significant difference in the percentage of children at baseline or in the past who received psychiatric treatment by maternal treatment. There were, however, significant differences in this measure during the study, with 31.5%, 20.6%, and 8.7% of children whose mothers were receiving the combination treatment, bupropion monotherapy, and escitalopram monotherapy, respectively (p<0.02), receiving psychiatric treatment. These findings suggest that the greater improvement among children whose mothers were receiving escitalopram monotherapy could not be explained by the children having received treatment; the children of mothers in the escitalopram monotherapy group received the least amount of treatment.
Gerra et al. (25), in a reanalysis of the adult study, examined negative affectivity—which included three domains of aversive moods: guilt, hostility/irritability, and fear/anxiety—as a moderator of treatment response. This approach was based on the suggestion by Nutt et al. (23) and Stahl et al. (24) that high negative affectivity is a result of serotonin deficiency and should respond to antidepressants that enhance serotonin neurotransmission—such as escitalopram, as opposed to bupropion. This finding was confirmed in the full adult sample, and we tested it in relationship to the mothers and children. We found that children of mothers with low negative affectivity exhibited a significant decrease in depressive symptoms (CDI scores) over time regardless of mothers’ treatment assignment (see Figure S4 in the online data supplement). For children of mothers with high negative affectivity, only those whose mothers received escitalopram monotherapy exhibited a decrease in CDI scores (see Figure S5 in the data supplement).
Further investigation showed that for children of mothers with high baseline negative affectivity, the overall week-by-treatment interaction was significant (F=4.84, df=2, 113, p<0.01), implying that CDI changes over time differed between treatment groups. Individual beta values showed that only children of mothers treated with escitalopram monotherapy (beta=−0.33, SE=0.09, p=0.001) showed a significant reduction in CDI scores over time, whereas children of mothers with high negative affectivity at baseline who received the combination treatment or bupropion monotherapy showed no significant changes over time in CDI scores. However, a formal test of a three-way interaction of differential effects of maternal treatment on children’s CDI scores by maternal baseline negative affectivity did not reach statistical significance, possibly because of lack of statistical power.
Discussion
Depressed mothers receiving escitalopram monotherapy, bupropion monotherapy, or combination treatment with both medications had a high remission rate overall (67%) and a significant reduction in symptoms over 12 weeks; there were no statistically significant differences between treatment groups. Our main hypothesis that mothers receiving the combination treatment would have an earlier onset and higher rate of remission than those receiving either of the monotherapies and that the outcome in their children would follow was not confirmed and could not explain the findings in children. However, there were significant treatment effects in the children. Children whose mothers received escitalopram monotherapy showed significantly greater improvement in symptoms and functioning as compared with those whose mothers received the other treatments. Furthermore, improvement in mothers’ depressive symptoms was significantly related to improvements in children’s depressive symptoms over the 12 weeks only in children whose mothers received escitalopram monotherapy.
We undertook a number of exploratory analyses to understand the findings. Mothers in the escitalopram monotherapy group, unlike those in the other treatment groups, showed improvement in self-reported parental functioning. They reported being better able to talk to and listen to their children. These findings were paralleled in the children’s reports that their mothers in the escitalopram group as compared with those in the other two treatment groups were more caring over the 12 weeks. These differential treatment effects could not be explained by differential dosage of maternal medications or side effects or by the child’s receiving psychiatric treatment. However, maternal negative affectivity at baseline moderated the effects of escitalopram monotherapy.
We do not know why children did better when their mothers received escitalopram monotherapy as compared with bupropion monotherapy or combination treatment, or why the results for escitalopram monotherapy were not similar to those for combination treatment. There is a small advantage (6%) of SSRIs as compared with bupropion in the treatment of anxious depression (35, 36). Negative affectivity, which captures high levels of stress, irritability, and anxiety, may be similar to DSM-5 major depression with anxious distress. This subgroup may be better treated with a medication like escitalopram that enhances serotonergic neurotransmission as compared with bupropion, which enhances dopaminergic neurotransmission. The results for the combination treatment group might suggest that avoiding bupropion’s effects is important. The findings do not imply that treatment of depressed mothers with escitalopram is better for their children than other SSRIs or psychotherapy (12).
The effects we observed of improved parenting on children are comparable to those of Garber et al. (11), who showed that the relationship between parent’s and child’s depressive symptoms was partially mediated by improvement in parental acceptance and care. Both studies suggest that reduction in maternal symptoms results in changes in parenting behavior, which in turn may be related to symptom reductions in their children. Our findings are similar to those of Swartz et al. (12), who found that the decrease in the children’s depressive symptoms (as measured with the CDI) but not impairment (as measured with the CIS) was mediated by improvement in the mothers’ depressive symptoms.
This study has some limitations. Treatment of mothers was limited to two medications or their combination; the study did not use psychotherapy or a placebo arm. It also did not use a longitudinal control sample of children of nondepressed mothers (11). Fathers were not included in the study. While controlled clinical trials are needed to determine the effects of maternal treatment, differential treatment dropout could confound analyses in a randomized study. Our low attrition of mothers and children is a strength. The sample size, while the largest of this type of study, was still too small to fully carry out the exploratory analyses. In addition, multiple ancillary analyses were performed without statistical adjustments for multiplicity, and consequently results from these exploratory analyses must be interpreted with caution. There were fewer married mothers in the bupropion group, but marital status was controlled for in all analyses and was explained by site differences (fewer married women in New York as compared with Ottawa). The differential effect of maternal treatment with escitalopram on child outcomes was observed in both cities. The negative affectivity measurement is based on unproven approximations and requires replication. Finally, the maximum medication dosage of escitalopram in this study was higher than the 20 mg/day currently recommended by the FDA. The average maternal daily dose over 12 weeks was about 24 mg, and the average maximum daily dose at last interview was 30 mg. However, dosage was not related to outcome in the mothers or in their children.
Clinically, medication for depression may not show differential effects on standard symptom measures used in clinical trials. More subtle behavioral effects may be captured by measures of parental functioning that could have differential effects on children. A similar conclusion regarding behavioral outcome was reached by a National Institute of Mental Health panel examining the data from the Sequenced Treatment Alternatives to Relieve Depression study (37). Personalizing the treatment of depressed mothers may be enhanced by assessing parental behavior and monitoring the impact on children. Concomitant targeted interventions that enhance parenting may be useful. Medications that reduce symptoms of high anxious distress and irritability may be required. These findings also highlight the importance of active treatment of depressed mothers, which may help them and their children.
1 : Transmission and prevention of mood disorders among children of affectively ill parents: a review. J Am Acad Child Adolesc Psychiatry 2011; 50:1098–1109Crossref, Medline, Google Scholar
2 : Children of treatment-seeking depressed mothers: a comparison with the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) child study. J Am Acad Child Adolesc Psychiatry 2012; 51:1185–1196Crossref, Medline, Google Scholar
3 : Longitudinal study of diagnoses in children of women with unipolar and bipolar affective disorder. Arch Gen Psychiatry 1990; 47:1112–1117Crossref, Medline, Google Scholar
4 : Children of currently depressed mothers: a STAR*D ancillary study. J Clin Psychiatry 2006; 67:126–136Crossref, Medline, Google Scholar
5 : Depressed mothers coming to primary care: maternal reports of problems with their children. J Affect Disord 2004; 78:93–100Crossref, Medline, Google Scholar
6 : Families at high and low risk for depression: a 3-generation study. Arch Gen Psychiatry 2005; 62:29–36Crossref, Medline, Google Scholar
7 : Relative impact of maternal depression and associated risk factors on offspring psychopathology. Br J Psychiatry 2012; 200:124–129Crossref, Medline, Google Scholar
8 : Remissions in maternal depression and child psychopathology: a STAR*D-Child report. JAMA 2006; 295:1389–1398Crossref, Medline, Google Scholar
9 : Children of depressed mothers 1 year after the initiation of maternal treatment: findings from the STAR*D-Child Study. Am J Psychiatry 2008; 165:1136–1147Link, Google Scholar
10 : Children of depressed mothers 1 year after remission of maternal depression: findings from the STAR*D-Child study. Am J Psychiatry 2011; 168:593–602Link, Google Scholar
11 : Remission of depression in parents: links to healthy functioning in their children. Child Dev 2011; 82:226–243Crossref, Medline, Google Scholar
12 : Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. Am J Psychiatry 2008; 165:1155–1162Link, Google Scholar
13 : Effects on children of treating their mothers’ depression: results of a 12-month follow-up. Psychiatr Serv 2012; 63:357–363Link, Google Scholar
14 : Maternal depression and child behaviour problems: randomised placebo-controlled trial of a cognitive-behavioural group intervention. Br J Psychiatry 2003; 183:342–348Crossref, Medline, Google Scholar
15 : Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res 2014; 52:7–14Crossref, Medline, Google Scholar
16 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
17 : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
18 : Hamilton Depression Rating Scale: extracted from regular and change versions of the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 1981; 38:98–103Crossref, Medline, Google Scholar
19 : The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54:573–583Crossref, Medline, Google Scholar
20 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
21 : Social adjustment by self-report in a community sample and in psychiatric outpatients. J Nerv Ment Dis 1978; 166:317–326Crossref, Medline, Google Scholar
22 : Testing the short and screener versions of the Social Adjustment Scale–Self-Report (SAS-SR). Int J Methods Psychiatr Res 2012; 21:52–65Crossref, Medline, Google Scholar
23 : The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol 2007; 21:461–471Crossref, Medline, Google Scholar
24 : A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 2004; 6:159–166Crossref, Medline, Google Scholar
25 : Does negative affectivity predict differential response to an SSRI versus a non-SSRI antidepressant? J Clin Psychiatry 2014; 75:e939–e944Crossref, Medline, Google Scholar
26 : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
27 : The Children’s Depression Inventory Manual. Toronto, Multi-Health Systems, 1992Google Scholar
28 : Symptom-specific measures for disorders usually first diagnosed in infancy, childhood, or adolescence, in Handbook of Psychiatric Measures, 2nd ed. Edited by Rush AJ, First MB, Blacker D. Washington, DC, American Psychiatric Publishing, 2008, pp 333–335Google Scholar
29 : The Columbia Impairment Scale (CIS): pilot findings on a measure of global impairment for children and adolescents. Int J Methods Psychiatr Res 1993; 3:167–176Google Scholar
30 : A parental bonding instrument. Br J Med Psychol 1979; 52:1–10Crossref, Google Scholar
31 : Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22Crossref, Google Scholar
32 : Using SAS PROC mixed to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 1998; 29:323–355Crossref, Google Scholar
33 : Modeling Longitudinal Data. New York, Springer, 2005Google Scholar
34 : Applied Longitudinal Analysis, 2nd ed. Hoboken, NJ, John Wiley & Sons, 2012Google Scholar
35 : Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry 2008; 69:1287–1292Crossref, Medline, Google Scholar
36 : Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res 2008; 42:134–140Crossref, Medline, Google Scholar
37 : Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry 2013; 70:343–350Crossref, Medline, Google Scholar



