Gray Matter Abnormalities in Childhood Maltreatment: A Voxel-Wise Meta-Analysis
Abstract
Objective
Childhood maltreatment acts as a severe stressor that produces a cascade of physiological and neurobiological changes that lead to enduring alterations in brain structure. However, structural neuroimaging findings have been inconsistent. The authors conducted a meta-analysis of published whole-brain voxel-based morphometry studies in childhood maltreatment to elucidate the most robust volumetric gray matter abnormalities relative to comparison subjects to date.
Method
Twelve data sets were included, comprising 331 individuals (56 children/adolescents and 275 adults) with a history of childhood maltreatment and 362 comparison subjects (56 children/adolescents and 306 adults). Anisotropic effect size-signed differential mapping, a voxel-based meta-analytic method, was used to examine regions of smaller and larger gray matter volumes in maltreated individuals relative to comparison subjects.
Results
Relative to comparison subjects, individuals exposed to childhood maltreatment exhibited significantly smaller gray matter volumes in the right orbitofrontal/superior temporal gyrus extending to the amygdala, insula, and parahippocampal and middle temporal gyri and in the left inferior frontal and postcentral gyri. They had larger gray matter volumes in the right superior frontal and left middle occipital gyri. Deficits in the right orbitofrontal-temporal-limbic and left inferior frontal regions remained in a subgroup analysis of unmedicated participants. Abnormalities in the left postcentral and middle occipital gyri were found only in older maltreated individuals relative to age-matched comparison subjects.
Conclusions
The findings demonstrate that the most consistent gray matter abnormalities in individuals exposed to childhood maltreatment are in relatively late-developing ventrolateral prefrontal-limbic-temporal regions that are known to mediate late-developing functions of affect and cognitive control, which are typically compromised in this population.
Individual differences in social, behavioral, and cognitive functioning result from a combination of genetic and environmental influences on brain development. Development of the brain, a highly plastic organ, is regulated by genes but sculpted by environmental experiences (1). Animal studies have shown that environmental factors have an important impact on different aspects of brain development, including the number of neurons, glial cells, dendrites, and synapses; myelination; and neurotransmitter and growth factor activity, all of which underlie behavior (2).
There is an increasing interest in understanding the effects of early environmental adversity on the developing brain. Childhood maltreatment, which may include physical, sexual, and emotional abuse as well as neglect, is common in the United Kingdom, with prevalence rates of 6.9% for severe physical abuse, 4.8% for sexual abuse, and 9.8% for severe emotional and physical neglect (3). Childhood maltreatment has been associated with a host of neurocognitive consequences, such as low academic performance and IQ and deficits in emotion and reward processing, attention, and inhibitory control (4). Large-scale epidemiological studies have reported that childhood adversities, including childhood maltreatment, are significantly associated with first onsets of a wide range of psychiatric disorders over the life course, notably mood, anxiety, and substance use disorders (5, 6).
The human brain continues its development during childhood through processes of synaptic remodeling, activity-dependent myelination, and programmed cell death, which affect the organization of both gray and white matter (7). Neural plasticity due to experience is substantial, with gray matter being less heritable and more affected by early environment than white matter (8). For instance, children from low-income households have smaller and slower growth trajectories in parietal and frontal gray matter volumes than children from middle- and high-income households despite there being no difference at birth, and these trajectories are related to greater behavior problems (9). Also, early stress and exposure to traumatic events has been shown to adversely affect the nature and trajectory of normal brain development (4), particularly in late-developing frontal, temporal, and basal ganglia structures (10, 11).
A better understanding of the neurobiological consequences of childhood maltreatment will indirectly inform our understanding of how early-ife adversities can lead to the emergence of psychiatric conditions. It may also lead to heightened awareness of maltreatment’s biological consequences to brain development and lead to better prevention strategies and targeted treatment to reverse the experience-induced neurobiological abnormalities in those affected.
Modern neuroimaging methods such as MRI have revealed smaller volumes in several brain regions in individuals exposed to childhood maltreatment relative to unexposed comparison subjects, including the prefrontal cortex, hippocampus, amygdala, anterior cingulate cortex, corpus callosum, and cerebellum (12), suggesting that fronto-limbic areas may be the most compromised. However, the majority of studies have used a region-of-interest analysis approach, testing predominantly for frontal and limbic abnormalities (13–17).
Examining previously defined regions of interest limits the search to regions hypothesized a priori, thereby providing a biased and inappropriately constrained characterization of anatomy (18). Hence, studies are increasingly using whole-brain analysis and have reported gray matter volume deficits in areas similar to those identified by region-of-interest studies in maltreated individuals, such as the prefrontal cortex (including the dorsolateral prefrontal, orbitofrontal, and medial prefrontal cortices) and the temporal and anterior cingulate cortices, as well as other areas not commonly examined in region-of-interest studies, such as the thalamus, the insula, and the parietal and occipital cortices (19–25). Only one region-of-interest study (26), on intimate partner violence, reported an association between smaller occipital gray matter volume and childhood maltreatment. Whole-brain-analysis studies have also reported larger gray matter volumes for some areas identified by region-of-interest studies in maltreated individuals, such as the cerebellum and the prefrontal, posterior cingulate, and superior temporal cortices, as well as areas not commonly examined in region-of-interest studies, such as the occipital and parahippocampal gyri (13, 21). In addition, similar to region-of-interest studies that, with the exception of one study (14), found no basal ganglia deficits (16, 27), only two whole-brain-analysis studies reported basal ganglia deficits in healthy individuals exposed to childhood maltreatment (22, 23). Abnormalities in limbic areas have also been observed, but mostly in region-of-interest studies. Thus, abnormalities of the amygdala and the glucocorticoid receptor-rich hippocampus have commonly been found in region-of-interest studies of childhood maltreatment (15, 16, 28–32), but only two whole-brain-analysis studies have reported deficits in the hippocampus (31, 33) and none have reported deficits in the amygdala.
Given this variability in the literature, our aim in this preliminary meta-analysis of whole-brain voxel-based morphometry studies of structural abnormalities in childhood maltreatment was to determine which areas are most consistently affected in these maltreated individuals across studies that used whole-brain imaging analyses.
Method
Study Selection
Using PubMed, ScienceDirect, Web of Knowledge, and Scopus, we conducted a comprehensive literature search of studies published up to January 2014 that used whole-brain morphometric comparisons between individuals exposed to childhood maltreatment and unexposed comparison subjects. The search terms were “childhood maltreatment,” “child abuse,” and “early stress” or “childhood adversities” plus “structural gray matter,” “voxel-based morphometry,” or “whole-brain.” Studies that used fewer than 10 patients were excluded. In some cases, we obtained from the study authors additional details essential for the meta-analysis (i.e., peak coordinates) that were not included in the original publications. In our analyses, we followed the guidelines from the Meta-Analysis of Observational Studies in Epidemiology group (34).
Comparison of Regional Gray Matter Volume
Regional differences in gray matter volume between individuals exposed to childhood maltreatment and unexposed comparison subjects were analyzed using the Anisotropic Effect Size version of the Signed Differential Mapping (Anisotropic ES-SDM) software package (www.sdmproject.com), which employs a voxel-based meta-analytic approach that is based on, and improves on, other existing methods (35, 36). Anisotropic ES-SDM uses the reported peak coordinates and effect sizes to recreate, based on the spatial correlation between neighboring voxels, brain maps of the effect size of the volume differences between individuals exposed to childhood maltreatment and comparison subjects, rather than just assessing the probability or likelihood of a peak, and accounts for sample size and variance as well as between-study heterogeneity. These unique features make SDM an optimal method for comparing two groups without biasing the results toward those brain regions that show more interstudy heterogeneity.
The SDM methods have been described in detail elsewhere (35, 36) and are briefly summarized here. First, peak coordinates and effect sizes (derived, for example, from t values) of gray matter differences between maltreated individuals and comparison subjects were extracted from each data set. Notably, those peaks that did not appear statistically significant at the whole-brain level were excluded; that is, while different studies may employ different thresholds, we ensured that the same statistical threshold throughout the brain was used in each study. This was intended to avoid biases toward liberally thresholded brain regions, which is common for regions of interest. Second, a standard Montreal Neurological Institute map of the differences in gray matter was separately recreated for each study by means of an anisotropic Gaussian kernel, which assigns higher effect sizes to the voxels more correlated with peaks. This anisotropic kernel has been found to optimize the recreation of the effect size maps, and at the same time it is robust because it does not depend on a full width at half maximum (36). Third, a map of the effect size variance was derived for each study from its effect size map and its sample size. Fourth, the mean map was obtained by voxel-wise calculation of the random-effects mean of the study maps, weighted by the sample size and variance of each study and the between-study heterogeneity.
In addition, a jackknife sensitivity analysis was conducted to assess the reproducibility of the results by iteratively repeating the same analysis, excluding one data set at a time to establish whether the results remained significant (37). Similarly, a heterogeneity analysis was conducted to determine whether there was significant unexplained between-study variability within the results (35). Finally, we conducted a subgroup analysis on studies that used unmedicated participants only, as well as meta-regression analyses with age and gender as regressors (37). Statistical significance was determined using standard randomization tests, thus creating null distributions from which p values can be directly obtained (35).
Results
Included Studies and Sample Characteristics
The search yielded 17 studies, five of which were excluded: two of these computed correlations within a maltreated sample only, without a comparison group (22, 23); one study was part of a larger study on family risk for depression that included only four individuals who experienced emotional abuse (29); one genetic study on childhood adversity consisted of 11% of childhood maltreatment cases while most participants had experienced other stressors, such as moving house and death of a parent (38); and one study used a tensor-based morphometry analysis (21). Thus, 12 studies were included in the final meta-analysis, comprising 331 individuals exposed to childhood maltreatment and 362 comparison subjects. Of the 12 studies, nine consisted of adult and three of child/adolescent samples. Overall, the studies included 581 adults (306 comparison subjects) and 112 children/adolescents (56 comparison subjects). Nine of the studies included males and females, and three (19, 31, 39) included only females. All studies excluded participants with substance abuse or medical conditions that could adversely affect growth and development. All except one study (24) included maltreated individuals with psychiatric comorbidities, and eight studies recruited only unmedicated participants. The studies examined various forms of childhood maltreatment, including sexual, physical, and emotional abuse; neglect; witnessed domestic violence; parental verbal abuse; and harsh corporal punishment. No significant differences in age were found between participants exposed to childhood maltreatment and comparison subjects, reflecting the group matching in the original studies. Table 1 summarizes the participants’ demographic and clinical characteristics. All studies had received ethical approval from their respective ethics boards.
| Exposed to Childhood Maltreatment (N=331) | Unexposed Comparison Subjects (N=362) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | N | Mean Age (Years) | % Male | Comorbid Disorders | Maltreatment Typesb | Onset Age (Years) | Duration (years) | Mean IQ | Medications | N | Mean Age (Years) | % Male | Comorbid Disorders | Mean IQ |
| Child/adolescent samples | ||||||||||||||
| Carrion et al. (13) | 24 | 11.0 | 58 | PTSD, 50%; sub-PTSD, 50%; depression, 17%; social phobia, 13%; ADHD, 13%; separation anxiety disorder, 8%; generalized anxiety disorder, 8%; simple phobia, 8% | WV, PA, SA, EA, PN | NR | NR | 90.0 | Stimulants and/or SSRIs, 21% | 24 | 11.0 | 58 | 0 | 105 |
| De Brito et al. (24) | 18 | 12.0 | 61 | 0c | PA, N, SA, EA | 1.9–5 | 2.7–7.3 | 103.7 | 0 | 20 | 12.6 | 50 | 0 | 109.2 |
| Liao et al. (25) | 14 | 17.0 | 50 | Generalized anxiety disorder, 100% | PA, SA, EA, EN, PN | NR | NR | NR | 0 | 12 | 16.7 | 50 | Generalized anxiety disorder, 100% | NR |
| Adult samples | ||||||||||||||
| Tomoda et al. (40) | 23 | 21.7 | 65 | ADHD, 4% | HCP | 3.9 | 8.5 | 119.5 | 0 | 22 | 21.7 | 27 | 0 | 118.7 |
| Tomoda et al. (19) | 23 | 20.2 | 0 | Major depression, 17%; PTSD, 17%; depersonalization disorder, 4% | SA | 2–15 | 4.1 | NR | 0 | 14 | 19.0 | 0 | 0 | NR |
| van Harmelen et al. (41) | 84 | 38.7 | 35 | Major depression, 77%; anxiety disorders, 68% | EN, EA | NR | NR | NR | 0 | 97 | 36.6 | 33 | Major depression, 43%; anxiety disorders, 41% | NR |
| Landré et al. (39) | 17 | 24.9 | 0 | PTSD, 100%; major depression, 47%; suicidal risk, 65%; agoraphobia, 19%; addiction, 6% | SA | NR | NR | NR | 0 | 17 | 24.7 | 0 | 0 | NR |
| Thomaes et al. (31) | 31 | 35.3 | 0 | PTSD, 100%; other anxiety disorders, 70%; major depression, 64%; eating disorders, 8%; other mood disorders, 9%; borderline personality disorder, 33%; cluster C personality disorder, 30% | SA, PA | NR | NR | NR | Fluoxetine, 64%; benzodiazepines, 48% | 28 | 35.2 | 0 | 0 | NR |
| Tomoda et al. (42) | 21 | 21.2 | 43 | mood disorders, 48%; anxiety disorders, 24% | PVA | NR | NR | 119 | 0 | 19 | 21.1 | 37 | 0 | 122.8 |
| Tomoda et al. (20) | 22 | 21.8 | 27 | Major depression, 41%; anxiety disorders, 32%; PTSD, 18%; eating disorders, 9%; personality disorders, 5% | WDV | NR | 9.8 | 120.2 | 0 | 30 | 21.6 | 27 | 0 | 123.6 |
| Chaney et al. (33) | 30 | 41.7 | 57 | Major depression, 67%; healthy control, 33% | PA, SA, EA, EN, PN | NR | NR | NR | SSRIs, 32%, venlafaxine or mirtazapine, 12% | 53 | 36.3 | 32 | Major depression, 32%; healthy control, 68% | NR |
| Sheffield et al. (43) | 24 | 41.7 | 33 | Psychotic disorders, 100%; anxiety disorders, 46%; PTSD, 29%; OCD, 17%; panic disorder, 8%; eating disorder, 8%; generalized anxiety disorder, 4% | SA | NR | NR | 94.7 | Chlorpromazine, 93% | 26 | 38.2 | 50 | 0 | 103.7 |
Regional Differences in Gray Matter Volume
Data were obtained from all 12 studies included in the meta-analysis. As shown in Table 2 and Figure 1, individuals exposed to childhood maltreatment, relative to unexposed comparison subjects, had significantly smaller gray matter volumes in the right orbitofrontal/superior temporal gyrus extending to the amygdala, insula, and parahippocampal and middle temporal gyri and in the left inferior frontal, left postcentral, and right middle temporal gyri. They had larger gray matter volumes in the right superior frontal and left middle occipital gyri. However, the larger volumes in these regions should be interpreted with caution, as they were driven by one study (33).
| Local Maxima | |||||
|---|---|---|---|---|---|
| Cluster Peak (Size) | Cluster Breakdown (Brodmann’s Area; Size) | Region (Brodmann’s Area) | MNI Coordinates | SDM Value | pb |
| Participants with childhood maltreatment < comparison subjects | |||||
| Right orbitofrontal/ superior temporal gyrus (506 voxels) | Right superior temporal gyrus (BA 38; 283 voxels) | Right superior temporal gyrus (BA 38) | 32, 12, –26 | –1.556 | 0.0005 |
| Right inferior orbitofrontal gyrus (BA 47; 67) | Right parahippocampal gyrus (BA 36) | 30, –6, –30 | –1.219 | 0.004 | |
| Right insula (37 voxels) | |||||
| Right middle temporal gyrus (BA 21; 31 voxels) | |||||
| Right amygdala (18 voxels) | |||||
| Right parahippocampal gyrus (BA 36; 11 voxels) | |||||
| Left inferior frontal gyrus (131 voxels) | Left inferior frontal gyrus (BA 44/45; 80 voxels) | Left inferior frontal gyrus (BA 45) | –44, 18, 12 | –1.384 | 0.002 |
| Left inferior frontal gyrus (BA 45; 49 voxels) | Left inferior frontal gyrus (BA 44) | 52, 12, 6 | –1.27 | 0.003 | |
| Left postcentral gyrus (625 voxels) | Left postcentral gyrus (BA 43/4/3/2; 482 voxels) | Left postcentral gyrus (BA 43) | –60, –10, 20 | –1.555 | 0.0005 |
| Left precentral gyrus (BA 4/3/5; 139 voxels) | Left postcentral gyrus (BA 43) | –60, –12, 30 | –1.555 | 0.0005 | |
| Left postcentral gyrus (BA 43) | –58, –14, 26 | –1.555 | 0.0005 | ||
| Right middle temporal gyrus (61 voxels) | Right middle temporal gyrus (BA 21/22; 60 voxels) | (BA 21) | 60, –34, 0 | –1.285 | 0.003 |
| Participants with childhood maltreatment > comparison subjects | |||||
| Right superior frontal gyrus (106 voxels) | Right superior frontal gyrus (BA 9; 44 voxels) | Right superior frontal gyrus (BA 9) | 16, 68, 18 | 1.128 | 0.0003 |
| Right medial superior frontal gyrus (BA 10; 62 voxels) | |||||
| Left middle occipital gyrus (162 voxels) | Left middle occipital gyrus (BA 18; 108 voxels) | Left middle occipital gyrus (BA 18) | –28, –66, 32 | 1.139 | 0.0002 |
| Left inferior parietal gyrus (BA 40; 20 voxels) | Left middle occipital gyrus (BA 18) | –30, –60, 34 | 1.139 | 0.0002 | |
| Left angular gyrus (BA 39; 14 voxels) | |||||
| Left superior occipital gyrus (BA 19; 7 voxels) | |||||
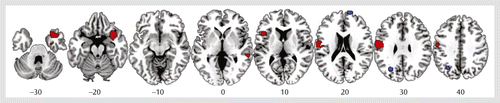
a Slices are shown in axial view and marked with the z coordinate as distance in millimeters from the anterior-posterior commissure. The right side of the image corresponds to the right side of the brain. Smaller volumes are indicated in red and larger volumes in blue.
Reliability and Subgroup Analyses
Jackknife sensitivity analyses revealed that the deficits in the right orbitofrontal/superior temporal gyrus were highly robust, as they were replicable in all 12 studies; abnormalities in the left postcentral, left middle occipital, and right superior frontal gyri were highly replicable, as they remained significant in 11 combinations of studies, and smaller volume of the left inferior frontal gyrus remained significant in 10 combinations of studies. (Details of the analysis are provided in Table S1 in the online data supplement.)
Analysis of heterogeneity showed that there was significant unexplained between-study variability in the right orbitofrontal/superior temporal, left inferior frontal, and postcentral gyri.
In the subgroup analysis of unmedicated participants, the deficits in the right orbitofrontal/superior temporal, left inferior frontal, and right middle temporal gyri remained, and no regions were enhanced in volume.
Meta-Regression Analyses: Effects of Age and Gender
Information on both age and gender was available for all 12 data sets. Using a stringent threshold of p<0.0005 to minimize spurious findings, age was negatively correlated with left postcentral occipital gray matter volume (x=−56, y=−10, z=26; SDM value=−2.15, p=0.00005; 255 voxels) and positively correlated with left middle occipital gray matter volume (x=−14, y=−94, z=14; SDM value=1.79, p=0.00007; 368 voxels). Smaller postcentral and larger middle occipital gray matter volumes were found in older but not younger maltreated individuals relative to age-matched comparison subjects. There were no significant gender differences.
Discussion
To our knowledge, this is the first preliminary meta-analysis of whole-brain voxel-based morphometry studies in childhood maltreatment. Maltreated individuals, relative to comparison subjects, exhibited significantly smaller gray matter volumes in the right orbitofrontal/superior temporal gyrus extending to the amygdala, insula, and parahippocampal and middle temporal gyri and in the left inferior frontal, postcentral, and right middle temporal gyri. They also had larger gray matter volumes in the right superior frontal and left middle occipital gyri. Deficits in the right orbitofrontal-temporo-limbic and left inferior frontal regions remained in the subgroup analysis of unmedicated participants. Age was negatively correlated with left postcentral and positively correlated with left middle occipital gray matter volumes.
These whole-brain meta-analysis findings highlight the detrimental effects of childhood maltreatment on several brain regions, including the ventral prefrontal, temporal, and limbic regions, consistent with previous region-of-interest and whole-brain-analysis structural imaging studies. Although many of the previous whole-brain-analysis studies did not directly report abnormalities in the amygdala and hippocampus, four of the included studies (24, 31, 33, 43) reported clusters that included the right amygdala/parahippocampal gyrus, although their peaks, as in this study, were located in nearby regions.
The findings thus demonstrate that childhood maltreatment is associated with abnormalities in the right orbitofrontal-temporo-limbic regions that form the paralimbic system, which is known to be implicated in affect and motivational processing and the self-regulation of social-emotional behaviors (44–46). Maltreated individuals also exhibited deficits in the left inferior frontal gyrus, which is part of the ventral attention system and a key area of cognitive control (47), mediating saliency detection, action selection, switching, inhibition, and sustained attention (48–50).
The abnormalities in the paralimbic network of affect control in the maltreated individuals could possibly be related to the typical development of common psychiatric comorbidities, particularly depression and posttraumatic stress disorder (PTSD), which have also been associated with gray matter abnormalities in these orbitofrontal and limbic regions (51, 52).
The meta-analytic association between childhood maltreatment and structural abnormalities in these regions is further underpinned by findings of direct correlations between severity or duration of maltreatment and brain volumetric abnormalities in these regions in individual studies. For instance, left inferior prefrontal volume was negatively correlated with sexual abuse severity (43). Amygdala volumes were inversely associated with time spent in institutions (15) and positively associated with age at adoption (16) in severely deprived children/adolescents. Hippocampal volumes were negatively correlated with duration (53) and severity (30) of childhood maltreatment. Left and right occipital volumes were negatively correlated with the duration of the childhood sexual abuse that occurred before age 12 (19). Furthermore, large-sample studies using whole-brain regression analysis in healthy adolescents and adults also reported a correlation between childhood maltreatment exposure and smaller corticostriatal-limbic gray matter volumes (22, 23).
Therefore, it is likely that the abnormalities we observed here in the orbitofrontal-temporo-limbic regions, which mediate affect control, and in the left inferior frontal gyrus, which mediates cognitive control, underlie the consistently observed neuropsychological deficits associated with childhood maltreatment, such as emotion and reward processing (54, 55), attention, and inhibitory control (56, 57).
This relationship is further supported by functional MRI (fMRI) studies of childhood maltreatment finding abnormal activations in orbitofrontal-limbic regions during affect processing and in inferior frontal regions during response inhibition and attention tasks. For instance, predominantly right amygdala and insula hyperresponsiveness to negative facial expressions has consistently been observed in maltreated children/adolescents (58–60) and adults (61) relative to healthy subjects, together with lower orbitofrontal activation in severely deprived children (62) and healthy adults exposed to childhood physical abuse (63), suggesting a deficit in their emotion-regulation abilities. Also, in a recent large correlational fMRI study of healthy adults, childhood maltreatment scores were strongly correlated with right amygdala and insula responsiveness to fearful/angry (23) and sad (64) faces. Women with sexual abuse-related PTSD exhibited overactivation of the left inferior frontal gyrus, which was absent in healthy subjects, during the processing of trauma-related words (65). In cognitive inhibition tasks, adopted adolescents who experienced childhood maltreatment showed greater activation in the left inferior frontal gyrus than did healthy subjects (66). Finally, resting-state functional connectivity studies have also reported lower prefrontal-limbic connectivity in individuals exposed to childhood maltreatment compared with healthy subjects (67–69), and this lower connectivity has in turn been found to mediate the development of internalizing symptoms (68). Thus, the structural findings of orbitofrontal-limbic and inferior frontal deficits in childhood maltreatment may be associated with the observed functional abnormalities in the same regions during affect and cognitive control, respectively.
Interestingly, the meta-regression analysis showed an age effect on smaller postcentral gray matter volume that was observed only in older maltreated participants. Childhood maltreatment has been associated with abnormal development of the sensory systems that relay adverse sensory experiences. For instance, women who experienced childhood sexual and emotional abuse had thinner left somatosensory cortex surrounding the regions representing the clitoris and the face, respectively, which suggests that the developing brain may adapt to shield the child by sensory gating of abusive experiences (70). Thus, decreased somatosensory volume may represent atrophy due to childhood maltreatment and may not manifest until adulthood, as found in the present meta-analysis.
The human brain is a highly plastic organ that is continually modified by experience and undergoes changes in structure and function across the lifespan. Given that the orbitofrontal, inferior frontal, and superior temporal gyri develop relatively late (by late adolescence) (10, 71, 72), these regions may be more susceptible to impairment in individuals with early adversities. Diffusion tensor imaging studies have shown that the orbitofrontal-temporo-limbic white matter tracts that mediate affect control and the inferior frontal-temporal white matter tracts that mediate complex cognitive functions, such as executive functioning and attention, are late developing, beyond childhood and adolescence, and reach their maturity in mid-adulthood (73, 74). Thus, our meta-analytic finding of an association between childhood maltreatment and gray matter abnormalities in regions that form these late-developing orbitofrontal-temporo-limbic affective and inferior frontal cognitive networks suggests an environmentally triggered disturbance in the normal development of these networks that may underlie the cognitive and emotional problems that develop as a consequence of early adversities. Furthermore, the findings were not confounded by medication, as they survived the subgroup analysis of unmedicated participants. Finally, childhood maltreatment may also affect and delay the normal development of the sensory regions, although the abnormalities may not manifest until adulthood.
Limitations
This meta-analysis has several limitations, some of which are inherent to meta-analyses. First, it was based on peak coordinates and effect sizes from published studies, rather than raw statistical brain maps, and this approach may result in less accurate results (35). Second, different studies used different statistical thresholds. Third, while voxel-wise meta-analytic methods provide excellent control for false positive results, false negative results are more difficult to avoid (35). Fourth, there are some inherent limitations to the voxel-based morphometry method, such as reduced effectiveness in detecting spatially complex and subtle group differences (75). Fifth, we were unable to assess whether age at onset or duration of childhood maltreatment was associated with any of the reported structural changes because the included studies did not report that information.
Among other limitations is the heterogeneity of maltreatment types included in most studies of neglect and sexual, physical, and emotional abuse, which makes it impossible to disentangle the specific effect of each type of maltreatment on the brain. It is plausible that exposure to single types of maltreatment, depending on the nature of the abusive experience, is associated with more specific alterations in regions that are crucial to the perception and processing of the adverse experience, whereas exposure to multiple forms of maltreatment is more commonly associated with morphological alterations in cortico-limbic regions (20, 70). Also, all except one study included maltreated participants with comorbid psychiatric conditions, making it impossible to determine the specific effect of childhood maltreatment independent of psychiatric comorbidities. All studies were cross-sectional, and hence the meta-analytic findings are still correlational. The included studies also differed in their recruitment criteria, with some studies recruiting maltreated participants meeting criteria for specific psychiatric disorders (13, 25, 31, 33, 39, 41, 43) and others recruiting maltreated participants regardless of psychiatric outcome (19, 20, 24, 40, 42); the latter approach is more likely to provide an unbiased perspective of the effects of childhood maltreatment. However, a strength is that all the studies excluded participants with substance abuse and medical conditions that could adversely affect growth and development. Also, it must be noted that there was between-study heterogeneity in nearly all main findings of the meta-analysis. Meta-regression analyses allowed us to explain some of this variability; for example, we found that older but not younger maltreated individuals had smaller postcentral gray matter volumes relative to age-matched comparison subjects. The remaining heterogeneity should be viewed with some caution, because heterogeneity may be supra-estimated in SDM when peaks from the different studies are spatially very close to a voxel, as individual study effect size estimates are either very large (i.e., similar to those of the peaks) or null (i.e., in studies without peaks close to the voxel). Lastly, meta-analytic results may change in the future as more studies using whole-brain-analysis methods are included.
Conclusions
Our meta-analytic findings show that the most consistent structural abnormalities in childhood maltreatment are in right orbitofrontal-temporo-limbic and left inferior frontal regions, which likely underlie the observed deficits in affect and cognitive control. Insights into the neurobiological abnormalities associated with early environmental adversities such as childhood maltreatment are important, as they emphasize the devastating consequences of early environmental adversities on brain development. Hopefully, such findings will aid in future developments to minimize environmental risk in early life and to develop strategies that strengthen resilience as well as treatments to normalize these experience-induced morphological alterations.
1 : The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol 2008; 20:1161–1175Crossref, Medline, Google Scholar
2 : Neural consequences of environmental enrichment. Nat Rev Neurosci 2000; 1:191–198Crossref, Medline, Google Scholar
3 Radford L, Corral S, Bradley C, Fisher H, Bassett C, Howat N, Collishaw S: Child Abuse and Neglect in the UK Today. London, National Society for the Prevention of Cruelty to Children, Sept 2011Google Scholar
4 : Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011; 214:55–70Crossref, Medline, Google Scholar
5 : Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication, I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 2010; 67:113–123Crossref, Medline, Google Scholar
6 : Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 2010; 197:378–385Crossref, Medline, Google Scholar
7 : Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 2006; 82:257–266Crossref, Medline, Google Scholar
8 : Genetic and environmental contributions to neonatal brain structure: a twin study. Hum Brain Mapp 2010; 31:1174–1182Medline, Google Scholar
9 : Family poverty affects the rate of human infant brain growth. PLoS One 2013; 8:e80954Crossref, Medline, Google Scholar
10 : Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 2010; 67:728–734Crossref, Medline, Google Scholar
11 : Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry 2007; 164:647–655Link, Google Scholar
12 : Neuroimaging of child abuse: a critical review. Front Hum Neurosci 2012; 6:52Crossref, Medline, Google Scholar
13 : Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res 2009; 172:226–234Crossref, Medline, Google Scholar
14 : Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med 2006; 36:35–52Crossref, Medline, Google Scholar
15 : Amygdala, hippocampal, and corpus callosum size following severe early institutional deprivation: the English and Romanian adoptees study pilot. J Child Psychol Psychiatry 2009; 50:943–951Crossref, Medline, Google Scholar
16 : Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010; 13:46–61Crossref, Medline, Google Scholar
17 : Childhood abuse is associated with structural impairment in the ventrolateral prefrontal cortex and aggressiveness in patients with borderline personality disorder. Psychiatry Res 2013; 213:18–23Crossref, Medline, Google Scholar
18 : A critique of functional localisers. Neuroimage 2006; 30:1077–1087Crossref, Medline, Google Scholar
19 : Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry 2009; 66:642–648Crossref, Medline, Google Scholar
20 : Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS One 2012; 7:e52528Crossref, Medline, Google Scholar
21 : Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 2010; 30:7466–7472Crossref, Medline, Google Scholar
22 : Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med 2011; 165:1069–1077Crossref, Medline, Google Scholar
23 : Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 2012; 71:286–293Crossref, Medline, Google Scholar
24 : Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 2013; 54:105–112Crossref, Medline, Google Scholar
25 : Childhood maltreatment is associated with larger left thalamic gray matter volume in adolescents with generalized anxiety disorder. PLoS One 2013; 8:e71898Crossref, Medline, Google Scholar
26 : Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 52:1089–1101Crossref, Medline, Google Scholar
27 : Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002; 52:1066–1078Crossref, Medline, Google Scholar
28 : Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008; 20:292–301Link, Google Scholar
29 : Early life adversity is associated with brain changes in subjects at family risk for depression. World J Biol Psychiatry 2012; 13:569–578Crossref, Medline, Google Scholar
30 : Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 2012; 109:E563–E572Crossref, Medline, Google Scholar
31 : Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 2010; 71:1636–1644Crossref, Medline, Google Scholar
32 : Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand 2008; 118:281–290Crossref, Medline, Google Scholar
33 : Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J Psychiatry Neurosci 2014; 39:50–59Crossref, Medline, Google Scholar
34 ;
35 : A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 2012; 27:605–611Crossref, Medline, Google Scholar
36 : Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry 2014; 5:13Crossref, Medline, Google Scholar
37 : Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009; 195:393–402Crossref, Medline, Google Scholar
38 : BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry 2012; 17:597–603Crossref, Medline, Google Scholar
39 : Preserved subcortical volumes and cortical thickness in women with sexual abuse-related PTSD. Psychiatry Res 2010; 183:181–186Crossref, Medline, Google Scholar
40 : Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage 2009; 47(suppl 2):T66–T71Crossref, Medline, Google Scholar
41 : Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry 2010; 68:832–838Crossref, Medline, Google Scholar
42 : Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 2011; 54(suppl 1):S280–S286Crossref, Medline, Google Scholar
43 : Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res 2013; 143:185–191Crossref, Medline, Google Scholar
44 : The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci 2007; 362:787–799Crossref, Medline, Google Scholar
45 : Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 2007; 9:141–151Medline, Google Scholar
46 : Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA 2007; 104:6430–6435Crossref, Medline, Google Scholar
47 : The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 2007; 37:343–360Crossref, Medline, Google Scholar
48 : Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage 2013; 83:690–703Crossref, Medline, Google Scholar
49 : Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 2008; 9:102Crossref, Medline, Google Scholar
50 : Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp 2012; 33:89–104Crossref, Medline, Google Scholar
51 : Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 2009; 30:3719–3735Crossref, Medline, Google Scholar
52 : Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research: past, present, and future. Biol Psychiatry 2006; 60:376–382Crossref, Medline, Google Scholar
53 : Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997; 41:23–32Crossref, Medline, Google Scholar
54 : Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry 2005; 162:291–296Link, Google Scholar
55 : Decision-making deficits among maltreated children. Child Maltreat 2013; 18:184–194Crossref, Medline, Google Scholar
56 : Executive function performance and trauma exposure in a community sample of children. Child Abuse Negl 2009; 33:353–361Crossref, Medline, Google Scholar
57 : Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev 2010; 81:224–236Crossref, Medline, Google Scholar
58 : Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry 2013; 202:269–276Crossref, Medline, Google Scholar
59 : Heightened neural reactivity to threat in child victims of family violence. Curr Biol 2011; 21:R947–R948Crossref, Medline, Google Scholar
60 : Neural substrates for processing task-irrelevant emotional distracters in maltreated adolescents with depressive disorders: a pilot study. J Trauma Stress 2012; 25:198–202Crossref, Medline, Google Scholar
61 : Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res 2011; 45:886–895Crossref, Medline, Google Scholar
62 : Elevated amygdala response to faces following early deprivation. Dev Sci 2011; 14:190–204Crossref, Medline, Google Scholar
63 : Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry 2006; 60:296–301Crossref, Medline, Google Scholar
64 : Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp 2013; 34:2899–2909Crossref, Medline, Google Scholar
65 : Working memory processing of traumatic material in women with posttraumatic stress disorder. J Psychiatry Neurosci 2012; 37:87–94Crossref, Medline, Google Scholar
66 : Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia 2010; 48:3037–3044Crossref, Medline, Google Scholar
67 : Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J Psychiatr Res 2014; 48:47–55Crossref, Medline, Google Scholar
68 : Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 2013; 110:19119–19124Crossref, Medline, Google Scholar
69 : Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp 2014; 35:1154–1166Crossref, Medline, Google Scholar
70 : Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry 2013; 170:616–623Link, Google Scholar
71 : Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 2009; 29:11772–11782Crossref, Medline, Google Scholar
72 : Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 2008; 28:3586–3594Crossref, Medline, Google Scholar
73 : Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 2012; 60:340–352Crossref, Medline, Google Scholar
74 : Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008; 40:1044–1055Crossref, Medline, Google Scholar
75 : Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry 2005; 62:734–741Crossref, Medline, Google Scholar



