Prescription Practices in the Treatment of First-Episode Schizophrenia Spectrum Disorders: Data From the National RAISE-ETP Study
Abstract
Objective:
Treatment guidelines suggest distinctive medication strategies for first-episode and multiepisode patients with schizophrenia. To assess the extent to which community clinicians adjust their usual treatment regimens for first-episode patients, the authors examined prescription patterns and factors associated with prescription choice in a national cohort of early-phase patients.
Method:
Prescription data at study entry were obtained from 404 participants in the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP), a nationwide multisite effectiveness study for patients with first-episode schizophrenia spectrum disorders. Treatment with antipsychotics did not exceed 6 months at study entry.
Results:
The authors identified 159 patients (39.4% of the sample) who might benefit from changes in their psychotropic prescriptions. Of these, 8.8% received prescriptions for recommended antipsychotics at higher than recommended dosages; 32.1% received prescriptions for olanzapine (often at high dosages), 23.3% for more than one antipsychotic, 36.5% for an antipsychotic and also an antidepressant without a clear indication, 10.1% for psychotropic medications without an antipsychotic, and 1.2% for stimulants. Multivariate analysis showed evidence for sex, age, and insurance status effects on prescription practices. Racial and ethnic effects consistent with effects reported in previous studies of multiepisode patients were found in univariate analyses. Despite some regional variations in prescription practices, no region consistently had different practices from the others. Diagnosis had limited and inconsistent effects.
Conclusions:
Besides prescriber education, policy makers may need to consider not only patient factors but also service delivery factors in efforts to improve prescription practices for first-episode schizophrenia patients.
Research supports different medication treatment approaches for first-episode and multiepisode schizophrenia (1), and recent schizophrenia treatment practice guidelines (2–6) include specific recommendations for first-episode patients. Since the incidence of schizophrenia is low (7), most clinicians’ experience outside of specialty centers is heavily weighted toward the treatment of multiepisode patients. The extent to which community clinicians adjust their usual treatment regimens for first-episode patients is unknown.
The Early Treatment Program (ETP) study, a nationwide comparative effectiveness trial that is part of the National Institute of Mental Health’s Recovery After an Initial Schizophrenia Episode (RAISE) Project (RAISE-ETP), provided the basis for the first national report of U.S. community mental health center medication treatments for the crucial early phase of schizophrenia. In the present study, we addressed two questions: What are the medication treatments currently used in community settings? What factors are associated with choice of medication strategy?
Method
Study Overview
RAISE-ETP compares NAVIGATE—a coordinated specialty care treatment program for first-episode psychosis that includes medical management guided by a decision support system, individual therapy, family psychoeducation, and supported employment and education—and community care in which treatment is determined by clinician choice. RAISE-ETP has been conducted under the guidance of the respective institutional review boards for the coordinating center and the study sites.
The study design prioritized enhancing the generalizability of findings to community settings. Inclusion and exclusion criteria were chosen to allow broad inclusion of different patient subgroups. Inclusion criteria were age between 15 and 40 years; diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, psychosis not otherwise specified, or brief psychotic disorder; beginning first treatment for psychosis (defined as having taken antipsychotic medications cumulatively for no more than 6 months); and ability to participate in research assessments in English. Exclusion criteria were history of more than one discrete psychotic episode; diagnosis of bipolar disorder, psychotic depression, substance-induced psychotic disorder, or current psychotic disorder due to a general medical condition; presence of a current neurological disorder that would affect diagnosis or prognosis; history of clinically significant head trauma; and history of any serious medical conditions that would significantly impair assessment, functioning, or treatment. All participants provided written informed consent (or written assent for those under age 18, along with written consent by a parent or guardian).
We employed site randomization to facilitate participation by sites without previous research experience, to eliminate potential treatment strategy “spillover” effects and to enhance study acceptability by patients, who would not need to agree to individual randomization. Thirty-four sites in 21 states were selected after a national search. All were community treatment centers with no preexisting first-episode program. By design, sites were located in diverse settings, ranging from large urban to rural settings. Seventeen sites were randomized to deliver the NAVIGATE treatment and 17 to community care.
The RAISE-ETP data analyzed in this study were collected between July 2010 and July 2012.
Assessments
Site staff obtained medication data using all available sources of information, including direct interview with patients and their families (if available) and record review. We present data on psychotropic medications being prescribed (even if not actually taken) to patients at study entry, before any influence on treatment by study guidelines or procedures. Diagnoses were determined using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID) (8), administered by masked remote assessors via two-way video. The assessors’ information sources were the patients themselves and a structured summary of each patient’s symptoms and treatment history, which was provided to the assessor prior to the SCID interview. Tobacco smoking status was assessed with the Fagerström Test for Nicotine Dependence (9). Data on recent substance use were obtained by clinic staff using record review and direct interview of patients and their families (if available).
Statistical Analysis
Frequencies and percentages were used to describe prescribing patterns. Potential correlates for the analyses of prescribing patterns were chosen on the basis of the following findings and reasoning. Patient sex (10), race (11–13), and ethnicity (14) have been shown to be associated with antipsychotic response or prescription patterns in multiepisode patients. Cigarette smoking decreases blood levels of some psychotropic medications (15). Age was included because some medications have adolescent indications. Previous depressive or anxiety symptoms should influence antidepressant prescription. Prescription may differ among the various schizophrenia spectrum disorders or by the presence of concurrent substance use. Insurance status influences access to particular medications and also treatment settings. Our data from 21 states allowed us to examine regional variations in prescription. We examined these factors in relation to nine key prescription practices: antipsychotic prescription, prescription of more than one antipsychotic, long-acting injectable antipsychotic prescription, first-generation antipsychotic prescription, risperidone prescription and dosage, olanzapine prescription and dosage, and antidepressant prescription.
We adopted a Bayesian perspective for the correlate analyses. Bayesian analyses do not require correction for multiple comparisons (16). Bayesian “credible intervals” are similar to confidence intervals in classical analyses, but in the Bayesian framework, the interval contains the true population parameter. Bayesian analyses do not generate p values. Instead, the posterior probability of being a risk factor (PPRF), also sometimes referred to as the selected percentage, is the probability that a variable is associated with an outcome. The larger the PPRF, the stronger the evidence for an association. We present PPRF evidence classifications (adapted from references 17 and 18): <50% indicates a lack of evidence for an association with outcomes, 50%–75% indicates some evidence, 75%–95% indicates positive evidence, 95%–99% indicates strong evidence, and >99% indicates very strong evidence. Studies comparing results from classical and Bayesian analyses (18) provide additional context for PPRF interpretation. These studies show that variables that are not significant in classical analyses have Bayesian PPRF values less (often much less) than 50%.
For all analyses, a weakly informative prior distribution was constructed by first scaling all nonbinary variables to have a mean of zero and a standard deviation of 0.5, and then placing an independent distribution from the Student t family of prior distributions (specifically, a Cauchy distribution centered at zero and 2.5 scale) on the coefficients (19). This prior has the advantage of always giving solutions even when there is complete separation in the logistic regression (20, 21). Univariate analysis was done using the bayesglm function in the arm package in R; the sim function was used to obtain simulates of the posterior distribution of each coefficient in the general linear model, and 95% credible intervals were obtained based on the 2.5 and 97.5 percentiles of the posterior distribution. Multivariate analyses employed Bayesian model averaging, a Bayesian solution to the problem of inference in the presence of multiple competing models (18). The bic.glm function from the R package BMA was primarily used for these analyses.
Results
Study Participants
The sample included 404 patients (see the flow diagram in Figure S1 in the data supplement that accompanies the online edition of this article). Community centers normally do not conduct outreach; new patients arrive through referral from inpatient units, other clinicians, or self-referral. This pattern mostly held for the study; 335 (83%) patients came from these sources, and 88 (17%) from outreach activities (e.g., educating the professional community about RAISE-ETP; educational efforts with potential patients and their families through articles in the local press; information booths at community events). As shown in Table 1, most patients had a psychiatric hospitalization before study entry. Patients were relatively young on average, with a mean age of 23.6 years. The majority were male, and the sample had diverse racial backgrounds. Approximately half met DSM-IV criteria for schizophrenia; the next most common diagnoses were schizophreniform disorder and schizoaffective disorder. Consistent with early-phase illness, the mean duration of cumulative lifetime antipsychotic treatment was only 46.7 days (SD=45.8).
| Characteristic | Mean or N | |
|---|---|---|
| Mean | SD | |
| Age (years) | 23.6 | 5.1 |
| N | % | |
| Male | 293 | 72.5 |
| Race | ||
| Caucasian | 218 | 54.0 |
| African American | 151 | 37.4 |
| American Indian | 22 | 5.4 |
| Asian | 12 | 3.0 |
| Pacific Islander | 1 | 0.2 |
| Hispanic ethnicity | 73 | 18.1 |
| Had a psychiatric hospitalization prior to enrollment | 316 | 78.2 |
| Diagnosis at study entry | ||
| Schizophrenia | 214 | 53.0 |
| Schizophreniform disorder (provisional) | 57 | 14.1 |
| Schizophreniform disorder (definite) | 10 | 2.5 |
| Schizoaffective disorder, bipolar type | 24 | 5.9 |
| Schizoaffective disorder, depressive type | 57 | 14.1 |
| Brief psychotic disorder | 2 | 0.5 |
| Psychotic disorder not otherwise specified | 40 | 9.9 |
| Currently using substances at study entry | ||
| Cigarettesa | 207 | 51.4 |
| Alcohol | 113 | 28.0 |
| Marijuana | 96 | 23.8 |
| Other drugs of abusea | 10 | 2.5 |
| Regional location of treatment | ||
| North | 69 | 17.1 |
| South | 89 | 22.0 |
| Midwest | 154 | 38.1 |
| West | 92 | 22.8 |
| Insurance statusb | ||
| Private or private and public | 82 | 20.4 |
| Public only | 127 | 31.7 |
| No insurance | 173 | 43.1 |
| Insurance status not known by patient | 19 | 4.7 |
TABLE 1. Characteristics of Patients Assessed in a Study of Prescription Practices in First-Episode Schizophrenia Spectrum Disorders (N=404)
The frequencies of prescriptions in the major medication classes are listed in Table 2.
| Medication Class | N | % |
|---|---|---|
| No medication | 48 | 11.9 |
| Only medications for general medical conditions | 3 | 0.7 |
| Antipsychotics | 337 | 83.4 |
| Antidepressants | 129 | 31.9 |
| Mood stabilizers | 37 | 9.2 |
| Antianxiety agents | 42 | 10.4 |
| Sedative-hypnotics | 20 | 5.0 |
| Opioid analgesics | 7 | 1.7 |
| Opioid replacement addiction medications | 2 | 0.5 |
| Stimulants | 5 | 1.2 |
| Non-stimulant ADHD medication | 1 | 0.2 |
| α2-Adrenergic agonist | 3 | 0.7 |
TABLE 2. Frequency of Prescription of Major Medication Classes for Patients Assessed in a Study of Prescription Practices in First-Episode Schizophrenia Spectrum Disorders (N=404)a
Most patients received prescriptions for antipsychotics, and approximately one-third of patients received prescriptions for antidepressants.
Patients Without Antipsychotic Prescriptions at Study Entry
Fifty-one patients (12.6%) did not have prescriptions for any psychotropic medications at study entry. Sixteen of them (31.4%) had taken antipsychotics in the past, and 24 of them (47.1%) had a past psychiatric inpatient admission. Sixteen patients had prescriptions for psychotropic medications but not antipsychotics; 14 of these had prescriptions for antidepressants, one for clonazepam, and one for clonidine.
Antipsychotic Prescribing Patterns
Of the 337 patients who had prescriptions for an antipsychotic, only 40 (11.9%) received a first-generation agent, including those who received both a first-generation and a second-generation antipsychotic. Long-acting injectable antipsychotics were prescribed for 32 (9.5%) of the patients who received antipsychotics. Of these, 17 had prescriptions for paliperidone palmitate (53.1% of long-acting prescriptions), 11 for haloperidol decanoate (34.4%), three for risperidone microspheres (9.4%), and one for fluphenazine decanoate (3.1%).
Antipsychotic monotherapy was by far the most common pattern. Three hundred (89.0%) of the 337 patients who received prescriptions for antipsychotics had prescriptions for only one antipsychotic (either in single or multiple formulations), while 35 patients (10.4%) had prescriptions for two different antipsychotics and two (0.6%) had prescriptions for three different antipsychotics.
Prescriptions for antipsychotic monotherapy.
As shown in Table 3, risperidone accounted for about one-third of the 300 prescriptions for antipsychotic monotherapy. The next most commonly prescribed antipsychotic was olanzapine (17.0% of prescriptions), followed by aripiprazole, paliperidone, and quetiapine, each accounting for around 10% of prescriptions.
| Prescriptionsa | Dosageb (mg/day or mg/month) | Dosage Exceeding 2009 PORT Recommendationsc for: | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Medication | N | % | Prescriptions for Only Oral or Only Long-Acting Formulations (N) | Median | Mean | SD | Range | Multiepisode Patients (N) | First-Episode Patients (N) |
| Risperidone | 109 | 36.3 | |||||||
| Orald: 107 | 3 | 2.9 | 1.5 | 0.25–7.0 | 0 | 8 | |||
| Long-acting: 1 | 75 | 75 | N/A | N/A | |||||
| Olanzapine | 51 | 17.0 | Orale: 51 | 15 | 16.5 | 7.8 | 2.5–40 | 8 | 22 |
| Aripiprazole | 35 | 11.7 | Oralf: 35 | 10 | 10.0 | 4.8 | 2–20 | 0 | N/A |
| Paliperidone | 30 | 10.0 | |||||||
| Oral: 17 | 6 | 5.8 | 2.2 | 3–9 | 0 | N/A | |||
| Long-actingf: 13 | 136.5 | 149.5 | 43.5 | 117–234 | N/A | N/A | |||
| Quetiapine | 28 | 9.2 | Oralf: 28 | 300 | 309.7 | 192.7 | 20–800 | 1 | N/A |
| Haloperidol | 21 | 7 | |||||||
| Oralf: 12 | 10 | 11.3 | 7.3 | 3–30 | 3g | N/A | |||
| Long-acting: 4 | 75 | 81.2 | 37.5 | 50–125 | N/A | N/A | |||
| Ziprasidone | 12 | 4.0 | Oralh: 12 | 80 | 102.2 | 57.8 | 40–200 | 1 | N/A |
| Lurasidone | 4 | 1.3 | Oralf: 4 | 40 | 66.7 | 46.2 | 40–120 | N/A | N/A |
| Asenapine | 2 | 0.7 | Oral: 2 | 10 | 10 | 10 | N/A | N/A | |
| Clozapine | 2 | 0.7 | Oral: 2 | 250 | 250 | 70.7 | 200–300 | 0 | N/A |
| Thiothixene | 2 | 0.7 | Oral: 2 | 5 | 5 | 5 | 0 | N/A | |
| Chlorpromazine | 1 | 0.3 | Oral: 1 | N/A | |||||
| Fluphenazine | 1 | 0.3 | Oral: 1 | 15 | 15 | 1g | N/A | ||
| Loxapine | 1 | 0.3 | Oral: 1 | 40 | 40 | 0 | N/A | ||
| Perphenazine | 1 | 0.3 | Oral: 1 | 4 | 4 | 0 | N/A | ||
TABLE 3. Patients Who Received Prescriptions for a Single Antipsychotic Agent in a Study of Prescription Practices in First-Episode Schizophrenia Spectrum Disorders (N=300)
First-episode schizophrenia treatment guidelines emphasize using low-dosage strategies. As shown in Table 3, relatively few patients received prescriptions for antipsychotic dosages higher than the suggested 2009 Schizophrenia Patient Outcomes Research Team (PORT) (4) upper dosing limit for multiepisode patients. High-dosage strategies were found for only certain medications, notably olanzapine; 44.9% of olanzapine prescriptions were for dosages higher than specific PORT guidelines for first-episode treatment, compared with only 7.8% of risperidone prescriptions.
Prescriptions for two or more antipsychotics.
Prescriptions for multiple antipsychotics included combinations of 13 different antipsychotics. The most commonly prescribed agents were risperidone, which was prescribed to 16 (43.2%) of the 37 patients who received multiple agents; quetiapine, prescribed to 13 patients (35.1%); olanzapine, prescribed to 10 patients (27.0%); aripiprazole, prescribed to nine patients (24.3%); and haloperidol, prescribed to eight patients (21.6%).
Medications for motor side effects.
We lack data on perceived indications for prescriptions, but anticholinergic medications and beta-blockers are usually prescribed for motor side effects in patients taking antipsychotics. Seventy-one (21.1%) of the 337 patients who received antipsychotics had concurrent prescriptions for an anticholinergic medication, and seven (2.1%) for a beta-blocker. Antianxiety agents can be prescribed for motor side effects as well as for anxiety, or for both. Thirty-nine (11.6%) of the 337 patients who received prescriptions for antipsychotics also received prescriptions for antianxiety agents.
Antidepressants prescribed with antipsychotics.
A total of 115 patients had prescriptions for both an antidepressant and an antipsychotic. Only 57 (49.6%) of these patients had any SCID interview documentation of lifetime depression (i.e., major depressive disorder, depressive disorder not otherwise specified, or schizoaffective disorder, depressive type) or anxiety (i.e., panic disorder, social phobia, obsessive-compulsive disorder, posttraumatic stress disorder, generalized anxiety disorder, anxiety due to a medical condition, or anxiety disorder not otherwise specified) that might broadly be considered justification for antidepressant treatment. Although not recommended by treatment guidelines, antidepressants are sometimes prescribed for negative symptoms. However, only six (10.3%) of the remaining 58 patients with prescriptions for antidepressants had any prominent negative symptoms according to the SCID interview.
Factors Associated With Prescribing Patterns
These analyses are summarized here and in Figures 1 and 2 (factors associated with prescription patterns) and Figures 3 and 4 (factors associated with dosing patterns for oral risperidone or olanzapine), and are presented in detail in Tables S1 and S2 in the online data supplement.
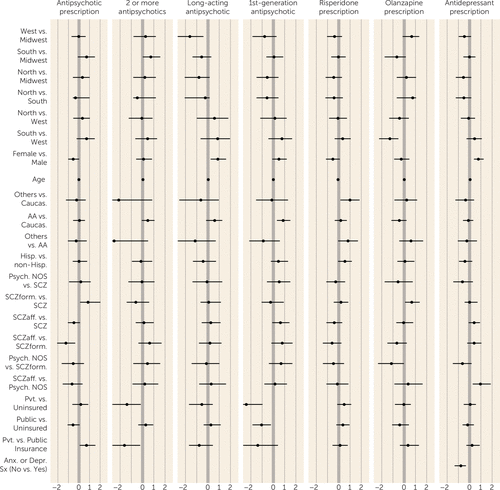
FIGURE 1. Factors Associated With Prescription Patterns in First-Episode Schizophrenia Spectrum Disorders: Univariate Analysesa
a The plot shows log odds ratios, with 95% credible intervals. Antipsychotic prescription=prescription of one or more antipsychotics versus no antipsychotic prescribed; 2 or more antipsychotics=prescription for two or more antipsychotics (among patients who received prescriptions for antipsychotics; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Long-acting antipsychotic=prescription for a long-acting antipsychotic or for a long-acting antipsychotic plus an oral antipsychotic (among patients who received prescriptions for antipsychotics); 1st-generation antipsychotic=prescription for a first-generation antipsychotic or both a first- and a second-generation antipsychotic (among patients who received prescriptions for antipsychotics); Risperidone prescription=prescription for risperidone (among patients with prescriptions for only one antipsychotic; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Olanzapine prescription=prescription for olanzapine (among patients with prescriptions for only one antipsychotic; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Antidepressant prescription=prescription for one or more antidepressants; Caucas.=Caucasian; AA=African American; Others=racial categories other than Caucasian and African American; Hisp.=Hispanic ethnicity; non-Hisp.=not of Hispanic ethnicity; Psych. NOS=psychosis not otherwise specified; SCZ=schizophrenia; SCZform=schizophreniform disorder; SCzaff=schizoaffective disorder; Pvt.=private; Anx. or Depr. Sx (No vs. Yes)=presence of anxiety or depressive symptoms (not present versus present).
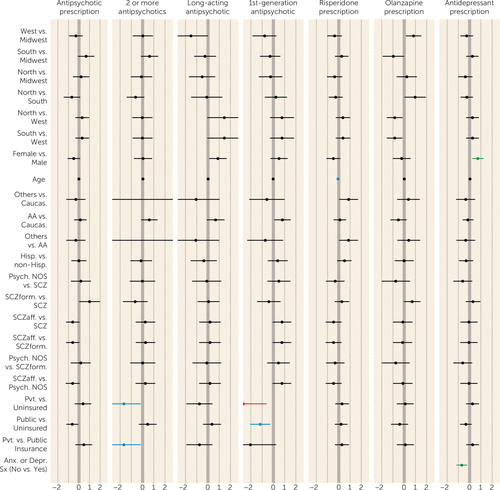
FIGURE 2. Factors Associated With Prescription Patterns in First-Episode Schizophrenia Spectrum Disorders: Multivariate Analysesa
a The plot shows log odds ratios, with 95% credible intervals. Odds ratios in blue are from multivariate analyses with a probability of being a risk factor (PPRF) of ≥50% but <75% (some evidence of association); those in green are from multivariate analyses with a PPRF of ≥75% but <95% (positive evidence of association); those in red are from multivariate analyses with a PPRF of ≥95% but <99% (strong evidence of association). Antipsychotic prescription=prescription of one or more antipsychotics versus no antipsychotic prescribed; 2 or more antipsychotics=prescription for two or more antipsychotics (among patients who received antipsychotics; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Long-acting antipsychotic=prescription for a long-acting antipsychotic or for a long-acting antipsychotic plus an oral antipsychotic (among patients who received antipsychotics); 1st-generation antipsychotic=prescription for a first-generation antipsychotic or both a first- and a second-generation antipsychotic (among patients who received antipsychotics); Risperidone prescription=prescription for risperidone (among patients with prescriptions for only one antipsychotic; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Olanzapine prescription=prescription for olanzapine (among patients with prescriptions for only one antipsychotic; multiple formulations of the same antipsychotic were counted as a single antipsychotic); Antidepressant prescription=prescription for one or more antidepressants; Caucas.=Caucasian; AA=African American; Others=racial categories other than Caucasian and African American; Hisp.=Hispanic ethnicity; non-Hisp.=not of Hispanic ethnicity; Psych. NOS=psychosis not otherwise specified; SCZ=schizophrenia; SCZform=schizophreniform disorder; SCzaff=schizoaffective disorder; Pvt.=private; Anx. or Depr. Sx (No vs. Yes)=presence of anxiety or depressive symptoms (not present versus present).
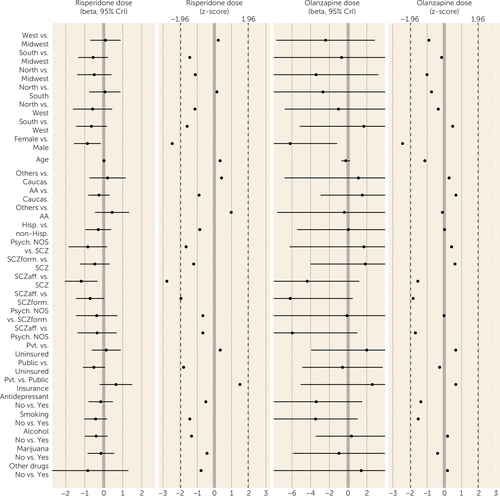
FIGURE 3. Factors Associated With Dosing Patterns for Oral Risperidone or Olanzapine in First-Episode Schizophrenia Spectrum Disorders: Univariate Analysesa
a The plot shows beta and Z scores, with 95% credible intervals. Dosing data (expressed as total daily dose) are from prescriptions requiring patients to take a single antipsychotic solely in an oral formulation. CrI=credible interval; Caucas.=Caucasian; AA=African American; Others=racial categories other than Caucasian and African American; Hisp.=Hispanic ethnicity; non-Hisp.=not of Hispanic ethnicity; Psych. NOS=psychosis not otherwise specified; SCZ=schizophrenia; SCZform=schizophreniform disorder; SCzaff=schizoaffective disorder; Smoking=smoking cigarettes at study entry; alcohol, marijuana, and other drugs=use of these substances at study entry.
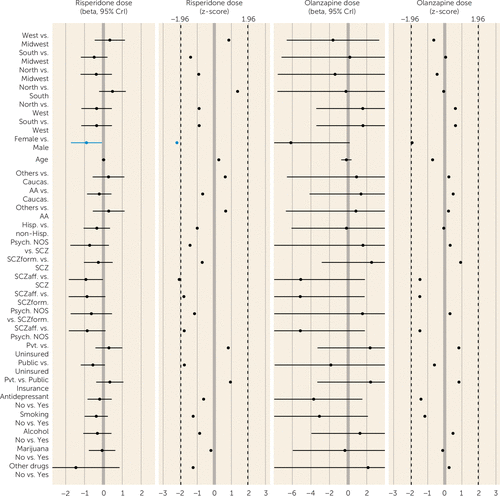
FIGURE 4. Factors Associated With Dosing Patterns for Oral Risperidone or Olanzapine in First-Episode Schizophrenia Spectrum Disorders: Multivariate Analysesa
a The plot shows beta and Z scores, with 95% credible intervals. Dosing data (expressed as total daily dose) are from prescriptions requiring patients to take a single antipsychotic solely in an oral formulation. Doses in blue are from multivariate analyses with a probability of being a risk factor (PPRF) of ≥50% but <75% (some evidence of association). CrI=credible interval; Caucas.=Caucasian; AA=African American; Others=racial categories other than Caucasian and African American; Hisp.=Hispanic ethnicity; non-Hisp.=not of Hispanic ethnicity; Psych. NOS=psychosis not otherwise specified; SCZ=schizophrenia; SCZform=schizophreniform disorder; SCzaff=schizoaffective disorder; Smoking=smoking cigarettes at study entry; alcohol, marijuana, and other drugs=use of these substances at study entry.
For antipsychotic prescription in general, univariate but not multivariate analyses (all PPRF values <21.2%; all lacking evidence) suggested that women and patients with public (compared with private) insurance were less likely to receive an antipsychotic, and patients with schizophreniform disorder (compared with schizophrenia or schizoaffective disorder) were more likely to receive an antipsychotic. Prescriptions for two or more antipsychotics were more likely for patients at southern (compared with midwestern) sites in univariate but not multivariate analyses (PPRF=4.6%; lacking evidence). In multivariate analyses, patients with private insurance were less likely to receive prescriptions for more than one antipsychotic than either patients with public insurance (PPRF=52.9%; some evidence) or those with no insurance (PPRF=51.9%; some evidence). Prescription of long-acting injectable antipsychotics was more frequent for women and for patients at midwestern (compared with western) sites in univariate but not multivariate analyses (PPRF values, 40.2% and 20.9%, respectively; both lacking evidence).
In multivariate analyses, prescription of first-generation antipsychotics was more common for uninsured patients (compared with those with private insurance [PPRF=96.2%; strong evidence] or public insurance [PPRF=56.4%; some evidence]). African Americans were more likely than Caucasians to receive prescriptions for first-generation antipsychotics in univariate but not multivariate analyses (PPRF=23.1%; lacking evidence).
For second-generation antipsychotics, multivariate analyses revealed that younger patients (PPRF=66.4%; some evidence) were more likely to receive prescriptions for risperidone, and univariate analyses showed that Hispanics (PPRF=17.5%; lacking evidence) and other racial groups (PPRF=17.5%; lacking evidence) (compared with Caucasians) were more likely to receive prescriptions for risperidone. Women received lower risperidone dosages than men (PPRF=52.2%; some evidence). Univariate but not multivariate analyses (all PPRF values <17%; all lacking evidence) showed that patients with psychosis not otherwise specified or schizoaffective disorder (compared with those with schizophrenia) as well as those with public insurance (compared with those with no insurance) received lower dosages of risperidone. Olanzapine prescription in univariate but not multivariate analyses (all PPRF values <24%; all lacking evidence) was more likely among patients at western (compared with southern) sites and among those with schizophreniform disorder (compared with psychosis not otherwise specified or schizophrenia). The mean olanzapine dosage was lower for women than men and for those with schizoaffective disorder (compared with psychosis not otherwise specified) in univariate but not multivariate analyses (PPRF values, 47.7% and 9.7%, respectively; all lacking evidence).
Antidepressant prescription was more likely among women (PPRF=83.5%; positive evidence) and among patients with depression or anxiety symptoms (PPRF=87.1%; positive evidence); older patients and those with schizoaffective disorder (compared with psychosis not otherwise specified) were more likely to receive antidepressants in univariate analyses only (PPRF=23.8% and 1.6%, respectively; all lacking evidence).
Patients Who Might Benefit From Prescription Modifications
Analyses of which patients might benefit from prescription modifications excluded those who had not received prescriptions for psychotropic medications at baseline, as some of these patients would not be expected to have prescriptions (e.g., those who were initiating psychiatric treatment at study entry). A total of 159 patients (39.4% of the sample) met criteria for potential benefit. Of these, 14 (8.8%) had prescriptions for recommended antipsychotics at higher-than-recommended dosages, 51 (32.1%) had prescriptions for olanzapine (often at high dosages), 37 (23.3%) had prescriptions for more than one antipsychotic, 58 (36.5%) had prescriptions for an antipsychotic but also an antidepressant without a clear indication, 16 (10.1%) had prescriptions for psychotropic medications without an antipsychotic, and five (1.2%) had prescriptions for stimulants.
Discussion
To our knowledge, this is the first report of psychotropic medication prescription patterns for people with first-episode schizophrenia spectrum disorders in U.S. community mental health settings. A Finnish national discharge registry study by Tiihonen et al. (22) provides an international comparison. Prescription rates for long-acting injectables and for multiple antipsychotics were similar in the two studies, and risperidone was the most commonly used oral antipsychotic in both countries, followed by olanzapine. Clozapine use was much higher in Finland. This may reflect different treatment practices, or it may be that more of the Finnish patients failed to respond to other antipsychotics during outpatient treatment before cohort identification based on their first hospitalization.
Practice guidelines with specific first-episode recommendations and first-episode research data (2–6) support 1) the need for antipsychotic treatment, 2) the use of low antipsychotic dosing, and 3) the need to minimize side effects, especially metabolic ones, during early-phase treatment. Did community clinicians follow these core principles? The need for antipsychotic treatment was widely recognized. Only 16 patients did not receive prescriptions for antipsychotics who clearly had been evaluated for psychiatric problems, as evidenced by the prescription of a psychotropic agent. Another 35 patients did not receive prescriptions for any psychotropic agents; how many of these patients had recently seen a prescribing clinician who did not recognize a need for psychotropic agents is unknown.
Antipsychotic prescriptions were mostly concordant with recommendations. An exception was the relatively common use of olanzapine (17% of antipsychotic prescriptions). Given olanzapine’s more frequent adverse metabolic side effects (23), especially with first-episode patients (24), PORT guidelines recommend that this agent not be used for first-episode treatment. Strikingly, olanzapine was much more frequently prescribed at higher-than-recommended dosages than other antipsychotics. We considered the possibility that this agent was prescribed for patients who had not improved with other antipsychotics, but the data do not support this. The mean duration of antipsychotic treatment for patients with olanzapine prescriptions (56.2 days [95% CI=45.7, 66.7]) was similar to that for other antipsychotics (e.g., 57.8 days [95% CI=44.3, 71.3] for paliperidone). The minimizing of side effects requires optimizing all medications, not just antipsychotics. Of note, antidepressants were prescribed for about one-third of patients, but only about half of these patients had clear symptom indications for antidepressants.
Our univariate analyses identified specific factors associated with particular prescription practices. Prescription may be influenced by several factors. People designing practice improvement efforts may wish to focus on factors identified by the multivariate analyses, given that these are less biased when confounding factors are present. Both analysis types are informative in different contexts, and we include both in our discussion.
Among demographic variables, we found that women in our sample received lower antipsychotic dosages, as has been reported for multiepisode patients (10). Women were also more likely to receive prescriptions for long-acting injectable antipsychotics (univariate analyses only) and, even controlling for depressive and anxiety symptoms, for antidepressants. In multiepisode studies, African Americans have been found to be more likely to receive prescriptions for first-generation antipsychotics (12, 13), and Hispanics to receive prescriptions for risperidone (25); our univariate results suggest that these patterns may also apply to first-episode patients. Younger patients were more likely to receive prescriptions for risperidone, possibly because of this agent's U.S. Food and Drug Administration indication for adolescent treatment.
Among variables related to service delivery, we found some univariate regional differences in prescription practices; the regions that differed varied across prescription practices, with no region consistently having different practices from the other regions. Insurance status effects were highly consistent. Private insurance was associated with better medication prescription: a higher likelihood of receiving a prescription for an antipsychotic and a lower likelihood of receiving two or more antipsychotics or receiving a first-generation antipsychotic—a medication choice discouraged by some (3) but not all (4) first-episode guidelines.
Diagnosis had no effect on prescription of more than one antipsychotic or prescription of long-acting injectables, first-generation antipsychotics, or risperidone. The univariate association between schizoaffective diagnoses and antidepressant prescription is consistent with the mood symptoms required for the diagnosis; the basis for the univariate association between schizophreniform disorder and olanzapine prescription is unclear. Diagnostic associations were not consistent across analyses of risperidone and olanzapine dosing. Finally, smoking and substance use were not associated with risperidone or olanzapine dosing.
Our data have limitations. Our sample may not be as generalizable as a true epidemiological sample, despite its recruitment from 34 sites in 21 states. Second, most patients’ prescriptions were made at another facility, usually an inpatient unit, prior to referral to our study community centers. Thus, we lack data on the prescribing clinicians’ decision processes, their perceived indications for prescriptions, and the effects of patient preferences. Third, the mean total lifetime duration of antipsychotic prescription in our sample was only 46.7 days. For most patients, past treatment response should not have substantially influenced medication selection, but some patients may have had enough treatment to document unusual responses to medication, which could have led to some treatments’ not conforming to guidelines. Fourth, although our sample was relatively large, our study also had a large number of sites, which prevented us from including individual sites in our analyses. Grouping sites into geographic regions provided a means to examine the uniformity of prescribing patterns nationally, but it cannot provide data on individual site practices.
Despite these limitations, our data have health policy implications. The marked use of second-generation over first-generation antipsychotics in our study may be warranted by evidence of better efficacy (26) and relapse prevention (27) and fewer motor side effects (26) with second-generation antipsychotics with early-phase patients. However, the marked metabolic effects of some second-generation antipsychotics in early-phase patients (28–32) suggests that efforts to increase adherence to recommended physical health monitoring of first-episode patients (e.g., 33) should be strongly encouraged.
Although each questionable medication practice we identified affected only 1.2% to 14.4% of patients, cumulatively 39.4% of patients might have benefited from changes in their psychotropic medication prescriptions. Primary immediate targets for improving first-episode community treatment include discouraging the use of two or more antipsychotics and the prescription of olanzapine, especially at high dosages. Aside from educational efforts for prescribing clinicians, changes in reimbursement models or care delivery may need to be considered to facilitate evidence-based treatment during the crucial early phase of schizophrenia. Patients with private insurance had strikingly lower rates of prescription of two or more antipsychotics than patients with public insurance or no insurance.
A large number of our patients received antidepressants without clear indications for their use. Either prescribers responded to symptoms not detected by our research interviews or they interpreted schizophrenia symptoms as mood or anxiety symptoms. If the latter is true, training to improve clinicians’ ability to diagnose schizophrenia spectrum disorders as distinct from mood or anxiety disorders is warranted, especially for female patients, given our finding that women were more likely to receive prescriptions for antidepressants independent of symptom indications.
Better medication treatment of the initial illness episode raises the possibility of better acute and long-term outcomes. An important first-episode research question is whether promoting more evidence-based care does indeed improve outcomes and, if it does, what level of adherence to evidence-based practice is required.
1 : Pharmacological treatments for first-episode schizophrenia. Schizophr Bull 2005; 31:705–722Crossref, Medline, Google Scholar
2
3 : The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 2007; 68:1751–1762Crossref, Medline, Google Scholar
4 ;
5 ;
6 ;
7 : A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status, and methodology. BMC Med 2004; 2:13Crossref, Medline, Google Scholar
8 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1998Google Scholar
9 : The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86:1119–1127Crossref, Medline, Google Scholar
10 : Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry 2004; 161:1324–1333Link, Google Scholar
11 : Racial disparities in antipsychotic prescription patterns for patients with schizophrenia. Am J Psychiatry 2002; 159:567–572Link, Google Scholar
12 : Outpatient prescriptions for atypical antipsychotics for African Americans, Hispanics, and whites in the United States. Arch Gen Psychiatry 2003; 60:121–128Crossref, Medline, Google Scholar
13 : Variations in use of second-generation antipsychotic medication by race among adult psychiatric patients. Psychiatr Serv 2004; 55:677–684Link, Google Scholar
14 : Changes over time and disparities in schizophrenia treatment quality. Med Care 2009; 47:199–207Crossref, Medline, Google Scholar
15 : Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs 2001; 15:469–494Crossref, Medline, Google Scholar
16 : Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff 2012; 5:189–211Google Scholar
17 : Bayes factors. J Am Stat Assoc 1995; 90:773–795Crossref, Google Scholar
18 : Variable selection and Bayesian model averaging in case-control studies. Stat Med 2001; 20:3215–3230Crossref, Medline, Google Scholar
19 : A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2008; 2:1360–1383Crossref, Google Scholar
20 : On the existence of maximum likelihood estimates in logistic regression models. Biometrika 1984; 71:1–10Crossref, Google Scholar
21 : Partial separation in logistic discrimination. J R Statist Soc B 1989; 51:109–116Google Scholar
22 : A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011; 168:603–609Link, Google Scholar
23 : Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012; 8:114–126Crossref, Google Scholar
24 : Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011; 68:609–616Crossref, Medline, Google Scholar
25 : Patient characteristics and the likelihood of initiation on olanzapine or risperidone among patients with schizophrenia. Schizophr Res 2005; 77:167–177Crossref, Medline, Google Scholar
26 : Efficacy and safety of individual second-generation vs first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2013; 6:1205–1218Crossref, Google Scholar
27 ;
28 ;
29 : Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry 2006; 163:2096–2102Link, Google Scholar
30 : Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 2007; 164:1050–1060Link, Google Scholar
31 ;
32 : Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009; 302:1765–1773Crossref, Medline, Google Scholar
33 : Targeted intervention to improve monitoring of antipsychotic-induced weight gain and metabolic disturbance in first episode psychosis. Aust N Z J Psychiatry 2011; 45:740–748Crossref, Medline, Google Scholar



