Emotion Dysregulation in Attention Deficit Hyperactivity Disorder
Abstract
Although it has long been recognized that many individuals with attention deficit hyperactivity disorder (ADHD) also have difficulties with emotion regulation, no consensus has been reached on how to conceptualize this clinically challenging domain. The authors examine the current literature using both quantitative and qualitative methods. Three key findings emerge. First, emotion dysregulation is prevalent in ADHD throughout the lifespan and is a major contributor to impairment. Second, emotion dysregulation in ADHD may arise from deficits in orienting toward, recognizing, and/or allocating attention to emotional stimuli; these deficits implicate dysfunction within a striato-amygdalo-medial prefrontal cortical network. Third, while current treatments for ADHD often also ameliorate emotion dysregulation, a focus on this combination of symptoms reframes clinical questions and could stimulate novel therapeutic approaches. The authors then consider three models to explain the overlap between emotion dysregulation and ADHD: emotion dysregulation and ADHD are correlated but distinct dimensions; emotion dysregulation is a core diagnostic feature of ADHD; and the combination constitutes a nosological entity distinct from both ADHD and emotion dysregulation alone. The differing predictions from each model can guide research on the much-neglected population of patients with ADHD and emotion dysregulation.
It has long been recognized that emotion dysregulation is common in individuals with neurodevelopmental disorders, including attention deficit hyperactivity disorder (ADHD). Indeed, in the early conceptualization of ADHD as reflecting “minimal brain damage,” emotion dysregulation was placed along with inattention among the cardinal symptoms (1). Only with the publication of DSM-III did emotional symptoms became an “associated feature” rather than a diagnostic criterion of ADHD. Renewed interest in this area makes timely a review of the overlap of emotion dysregulation with ADHD, focusing on prevalence, pathophysiology, and treatment.
In line with previous theorists, we define emotion regulation as an individual’s ability to modify an emotional state so as to promote adaptive, goal-oriented behaviors (2). It encompasses the processes that allow the individual to select, attend to, and appraise emotionally arousing stimuli, and to do so flexibly. These processes trigger behavioral and physiological responses that can be modulated in line with goals. Emotion dysregulation arises when these adaptive processes are impaired, leading to behavior that defeats the individual’s interests. It encompasses 1) emotional expressions and experiences that are excessive in relation to social norms and are context inappropriate; 2) rapid, poorly controlled shifts in emotion (lability); and 3) the anomalous allocation of attention to emotional stimuli. Here, we focus on the clinical expression of emotion dysregulation as irritability, which is often linked with reactive aggression and temper outbursts (3–5).
Emotion dysregulation is a dimensional trait that is not unique to ADHD; rather, it undercuts the traditional divide between internalizing and externalizing diagnoses and, indeed, may partly explain their high correlation (6). For example, a study that contrasted 105 irritable, emotionally dysregulated children with ADHD and 395 nonirritable children with ADHD found higher rates not only of oppositional defiant disorder but also of depression and dysthymia in the group with irritability (7).
Emotion dysregulation is also not synonymous with any single DSM-5 disorder. For example, of the three symptom clusters in oppositional defiant disorder—angry/irritable mood, defiant behavior, and vindictiveness—only the first plausibly reflects dysregulated emotions (8). In its extreme form, emotion dysregulation is likely to emerge as a major etiological factor behind the frequent, severe temper outbursts and irritability of the new DSM-5 diagnosis of disruptive mood dysregulation disorder. However, emotion dysregulation is a dimensional entity, not a categorical diagnosis, and here we consider the full spectrum of emotion dysregulation within ADHD, not just extremes. Thus, we include individuals with emotion dysregulation who do not meet criteria for any DSM diagnoses beyond ADHD.
We focus on emotion dysregulation itself, rather than on diagnoses that may include emotion dysregulation and be comorbid with ADHD, because it is a simpler symptom construct that is familiar to clinicians and, consistent with the Research Domain Criteria initiative, may be more readily tied to underlying neurobiological mechanisms.
Method
We conducted a literature search for relevant articles published before January 1, 2013 (details of the search are available in the data supplement that accompanies the online edition of this article). We summarized data quantitatively where possible. Meta-analyses were possible for studies of aggressive behavior (9–20), emotion recognition (12, 21–36), and delay aversion/reward valuation (35, 37–50). As all the outcomes included in the meta-analysis were continuous, we calculated standardized mean differences. We used a random-effects model to generate a pooled effect size and confidence intervals with the inverse variance method. The remaining studies are reviewed qualitatively under the headings of prevalence, pathophysiology, and treatment.
Results
Prevalence
Childhood.
Most epidemiological research has focused on children and has found a strong association between ADHD and emotion dysregulation (35, 51–56) (Table 1). A population study of 5,326 youths (51) found mood lability in 38% of children with ADHD, ten times the population rate. Elevated rates were observed in children without comorbid ADHD, and similar rates were seen in children with noncomorbid oppositional defiant disorder. Research on the Child Behavior Checklist “dysregulation profile” based on parent-reported problems with mood and aggression in youths who also have attention problems shows community rates of 1%−5%, compatible with high rates of emotion dysregulation among those likely to have ADHD (62). Clinic-based studies in youths with ADHD similarly report prevalence estimates of emotion dysregulation between 24% and 50%.
| Study | Participants | Definition of “Emotion Dysregulation” | Impairment Criterion | Findings |
|---|---|---|---|---|
| Children and adolescents | ||||
| Stringaris and Goodman (51) | Population based; N=5326 | Parent and self-report of emotional lability | Severity ratings of symptoms occurring “a lot” | Parent rating of impairing emotional lability: ADHD alone, 38% (RRR=12 compared with unaffected); ODD alone, 42% (RRR=14.7); self-report: ADHD alone, 27% (RRR=6.9); ODD alone, 14% (RRR=3.0) |
| Sobanski et al. (52) | Family based; ADHD, N=216; siblings, N=142 | Conners emotional lability index, parent and teacher ratings of unpredictable mood changes, temper tantrums; tearfulness; low frustration tolerance | 3 SD above population norms | 25% of ADHD probands had emotional lability >3 SD above population norms |
| Anastopoulos et al. (53) | Family based; ADHD, N=216; siblings, N=142 | Conners emotional lability index (see above) | Above 65th percentile of population norms | Elevated levels: ADHD, 47%; unaffected, 15% |
| Spencer et al. (54) | Clinic based; ADHD, N=197; controls, N=224 | Parent report of “dysregulation profile” based on Child Behavior Checklist subscales of attention problems, anxiety/depression, and aggression | Scores 1–2 SD above norms (above 2 SD, considered bipolar phenotype and excluded) | ADHD, 44% with dysregulation profile; controls, 2% |
| Sjöwall et al. (35) | Clinic based; ADHD, N=102; controls, N=102 | Parent report of child’s ability to regulate specific emotions | Not given | ADHD showed significant impairment compared with controls in regulating all emotions |
| Strine et al. (55) | Population based; history of ADHD, N=512; no history of ADHD, N=8,169 | Strength and Difficulties Questionnaire, parent report of emotional and conduct problems, including: often loses temper, often unhappy (also clingy, fearful, somatic complaints, and having worries) | Parent rating of each symptom’s impact | Emotional problems: history of ADHD, 23%; no history of ADHD, 6.3% |
| Becker et al. (56) | Clinic based; ADHD, N=1,450 | Strength and Difficulties Questionnaire, parent report of emotional problems (see above) | Based on U.K. population norms | 40% of boys and 49% of girls had abnormally high levels of emotional problems |
| Adult studies | ||||
| Able et al. (57) | Population based (N=21,000); diagnosed ADHD, N=198; likely ADHD (based on self-report scale), N=752; controls, N=199 | Self-report of tendency to become angry, disagree, or be critical of others; self-report of degree to which others evoke feelings of anger | Not given | Both diagnosed and likely ADHD subjects more likely to express anger, to engage in conflict, and to have been the target of anger or intimidating behavior |
| Barkley and Fischer (58) | Clinic based; ADHD, N=55; controls, N=75 | Self-report of items reflecting emotional impulsivity (taken from the Behavior Rating of Executive Functioning) | Symptom occurs “often” | Impatient: ADHD, 72%; controls, 3%; quick to anger: ADHD, 65%; controls, 6%; easily frustrated: ADHD, 85%; controls, 7%; emotionally overexcitable: ADHD, 70%; controls, 6%; easily excitable: ADHD, 73%; controls, 14% |
| Reimherr et al. (59) | Clinic based; ADHD, N=536 (enrolled in treatment trials) | Self-report of items from the Wender-Reimherr Adult Attention Disorder Rating Scale: irritability and outbursts; short, unpredictable mood shifts; emotional overreactivity | 2 SD above population norms | 32% met criteria for emotion dysregulation |
| Reimherr et al. (60) | Clinic based; ADHD, N=47 (enrolled in treatment trial) | Wender-Reimherr Adult Attention Disorder Rating Scale (see above) | 2 SD above population norms | 78% met criteria for emotion dysregulation |
| Surman et al. (61) | Clinic based; ADHD, N=206; controls, N=123 | Barkley’s self-report scale: quick to anger, loses temper, argumentative, angry; easily frustrated, touchy; overreactive emotionally, easily excited | Above 95th percentile on population norms | Met criteria for emotion dysregulation: ADHD, 55%; controls, 3% |
Reactive aggression may reflect emotion dysregulation (5). Our meta-analysis found consistent elevation in measures of aggressive behavior in ADHD compared with non-ADHD populations, associated with a large effect size (1.92, 95% CI=0.95–2.89) (Figure 1A). In the general population, the correlation is higher between aggression and hyperactivity-impulsivity (0.60–0.83) than between aggression and inattention (0.20–0.56) (6). In clinical populations, emotion dysregulation is commonly associated with either symptom domain (52, 56).
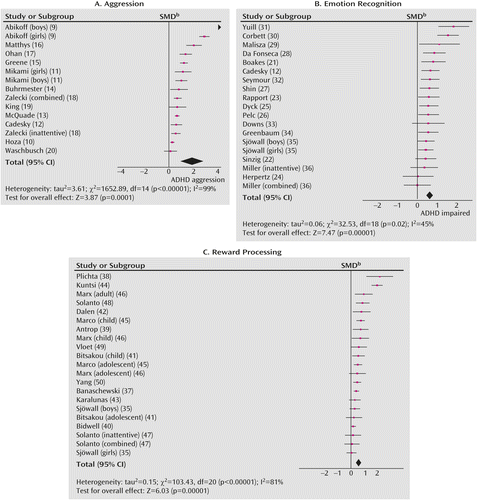
a In panel A, more aggressive behavior is seen in the ADHD groups (the effect size for boys in the Abikoff study [9] was 14). In panel B, emotion recognition deficits are seen in ADHD. In panel C, reward processing is measured by the tendency to prefer immediate small rewards over larger delayed ones; the ADHD participants show a tendency to prefer immediate, small rewards. Further details are provided in the online data supplement.
b SMD=standardized mean difference, inverse variance, random effects, with 95% confidence intervals.
Notably, behaviors reflecting emotion dysregulation can be reliably provoked among those with ADHD using paradigms that induce frustration (see Table S1 in the online data supplement). Children with ADHD show more negative affect and temper outbursts than do comparison subjects during challenging tasks. This consistency among studies is also notable, as different paradigms and behavioral measures were employed.
Infancy and early childhood.
Modest correlations (0.10–0.37) are reported between difficult infantile temperamental characteristics, such as being fussy, angry, or difficult to control, and ADHD arising later in childhood (63–67) (Figure 2; see also Table S2 in the online data supplement). A longitudinal study of 7,140 children found that while temperamental emotionality at age 3 predicted comorbid ADHD and internalizing disorders at age 7, activity level predicted comorbid ADHD and oppositional defiant disorder (68). A second longitudinal study found that infants who developed hyperactive symptoms alone did not differ temperamentally from typical infants, whereas those who ultimately developed both ADHD and aggressive symptoms were uncooperative and irritable from infancy onward (69). In short, a difficult early temperament with prominent negative emotionality is modestly linked with later ADHD combined with emotion dysregulation.
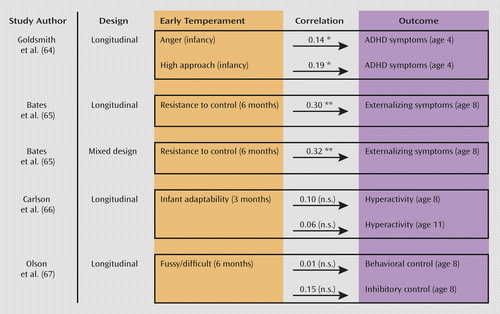
a n.s.=not significant.
*p<0.05. **p<0.01.
Longitudinal studies.
Most studies following children with ADHD into adulthood have focused on DSM-IV diagnoses, without considering emotion dysregulation per se, and have found elevated rates of adult disruptive and antisocial disorders and, less consistently, mood and anxiety disorders (70). One study defined emotion dysregulation as a moderate elevation (one to two standard deviations above the mean) on the combined Child Behavior Checklist subscales for attention problems, aggressive behavior, and anxious/depressed (71). In that study, such emotion dysregulation in 79 children with ADHD was associated 4 years later with more psychiatric comorbidities, greater social impairment, and ADHD persistence, compared with 98 children with ADHD without emotion dysregulation and 204 children without ADHD. A population-based study of 2,076 children (72) found that those matching the Child Behavior Checklist dysregulation profile had higher rates of anxiety disorders and disruptive behavior disorders in adulthood compared with those who did not match the dysregulation profile (72).
Adult studies.
Earlier concepts of adult ADHD included emotion dysregulation as a defining feature (73). This model has been supported to some extent by recent clinic-based studies reporting impairing emotion dysregulation in 34%−70% of adults with ADHD (57–61), although population-based studies are needed (Table 1). Aggressive behaviors are also prominent. In a population study contrasting 950 adults with diagnosed or likely ADHD and 20,000 unaffected adults (57), the ADHD group had higher self-ratings of interpersonal conflict and negative, conflictual social ties. Other cross-sectional studies have compared adults whose childhood ADHD has remitted and those whose ADHD has persisted. In one such comparison (58), 55 adults with persistent ADHD showed higher rates of emotion dysregulation (42%−72%, depending on specific symptoms) than 80 adults with remitted ADHD (23%−45%), although both groups differed from healthy subjects. This suggests a degree of developmental coherence: as symptoms of ADHD improve, so may emotion dysregulation.
Impairment.
The combination of ADHD and emotion dysregulation represents a major source of impairment. In a study of 1,500 children (74), emotional problems were found to have a greater impact than hyperactivity and inattention on well-being and self-esteem. Individuals with ADHD and emotion dysregulation were significantly more impaired in peer relationships, family life, occupational attainment, and academic performance than those with ADHD alone (75), and this result held after controlling for comorbid disorders, including oppositional defiant disorder (76).
In summary, emotion dysregulation is found in some 25%−45% of children and 30%−70% of adults with ADHD. It represents a major source of impairment and presages a poor clinical outcome.
Pathophysiology
Several psychological and neural processes may underpin the overlap between ADHD and emotion dysregulation. Recent models describe both “bottom-up” processes that support or influence emotion regulation and “top-down” processes, such as the allocation of attention to emotionally arousing stimuli (77, 78). Most studies reviewed in this section have either excluded individuals with comorbid diagnoses (including oppositional defiant disorder) or controlled for comorbidities, ensuring that the anomalies pertain to ADHD rather than to other disorders (Table 2).
| Study | Participants | Task | Behavioral results | fMRI results |
|---|---|---|---|---|
| Emotion perception and recognition | ||||
| Brotman et al. (79) | ADHD with no comorbidity, N=18; severe mood dysregulation, N=29 (24 with ADHD); bipolar affective disorder, N=43 (20 with ADHD); healthy, N=37 | Rating of fear, nose width, and passive viewing of neutral, fearful, happy, and angry faces | Rating of fear in neutral faces: severe mood dysregulation = bipolar > healthy; ADHD did not differ from any group | Left amygdala activity during fear ratings: ADHD > healthy = bipolar > severe mood dysregulation |
| Marsh et al. (80) | ADHD with no comorbidity, N=12; callous-unemotional traits, N=12; healthy, N=12 | Gender judgments on fearful, neutral, and angry faces | No group differences in accuracy; ADHD had slower reaction times | Amygdala activity in ADHD during fear processing did not differ from healthy |
| Posner et al. (81) | ADHD, N=15 (mix of medication naive and receiving psychostimulants; some had ODD, although the number is unclear); healthy, N=15 | Subliminal presentation of fearful face followed by supraliminal presentation of neutral expression on the same face; postscan face memory test | No group differences | Greater activity in medication-naive ADHD in amygdala and stronger functional connectivity with lateral prefrontal cortex (BA47) |
| Herpertz et al. (24) | ADHD without comorbidity, N=13; conduct disorder, N=22 (16 with ADHD); healthy, N=22 | Passive viewing of negative, positive, and neutral scenes | Subjects with conduct disorder rated emotional pictures as less arousing than did other groups | Increased left amygdala activation in conduct disorder with ADHD, not ADHD alone; ADHD alone had decreased insula activation to negative faces |
| Schlochtermeier et al. (82) | Adults treated in childhood for ADHD with no comorbidity, N=10; adults with childhood ADHD, medication naive, N=10; healthy, N=10 | Rating of positive and negative pictures | Adults with ADHD treated in childhood rated neutral pictures as more pleasant than medication naive and healthy subjects | Decreased ventral striatum and subgenual cingulate activation in medication-naive adults with history of ADHD; ADHD treated in childhood did not differ from healthy |
| Malisza et al. (29) | ADHD, N=9; autism, N=9; healthy, N=9 | View happy and angry faces and respond to happy | Accuracy: autism < ADHD = healthy | ADHD had less fusiform, temporal poles activity than healthy; ADHD showed same amygdala activity as healthy; autism showed less amygdala activity than other two groups |
| Reward processing | ||||
| Ströhle et al. (83) | Adult ADHD without comorbidity, N=10; controls, N=10 | Monetary incentive delay | No group differences | Decreased ventral striatum activation in ADHD during reward anticipation, and increased orbitofrontal activation during reward receipt |
| Plichta et al. (38) | Adult ADHD without comorbidity, N=14; controls, N=12 | Delayed discounting task (choose between immediate small and delayed large rewards) | No group differences | Decreased ventral striatum activation in ADHD during processing of both immediate and delayed rewards; within subjects, delayed reward in ADHD associated with increased activity of amygdala and caudate |
| Scheres et al. (84) | Adolescent ADHD, N=11; controls, N=11 | Monetary incentive delay | No group differences | Decreased ventral striatal activity in ADHD during reward anticipation |
| Stoy et al. (85) | Adult ADHD, N=24 (analyzed as remitted versus persistent, and as history of childhood treatment with psychostimulants versus medication naive); controls, N=12 | Monetary incentive delay | No group differences | Decreased insula activation during outcome of loss avoidance in medication-naive adults compared with other groups |
| Rubia et al. (86) | Childhood ADHD on and off psychostimulants, N=13 (1 with comorbid ODD); healthy, N=13 | Rewarded continuous performance task | No difference between medicated ADHD and healthy; trend to worse performance in unmedicated ADHD | Unmedicated ADHD showed orbitofrontal hyperactivation during reward receipt, normalized by psychostimulants |
| Control of attention to emotional stimuli | ||||
| Passarotti et al. (87) | Adolescent ADHD without comorbidity (N=14); bipolar disorder, N=23; healthy, N=19 | Working memory task using angry, happy, and neutral faces | Accuracy: healthy > ADHD > bipolar | ADHD compared with healthy: decreased prefrontal and striatal activation to angry faces, increased to happy; ADHD compared with bipolar: similar cortical anomalies, more prominent subcortical anomalies in bipolar |
| Passarotti et al. (88) | Adolescent ADHD without comorbidity (N=15); bipolar disorder, N=17; healthy, N=15 | Emotional Stroop test | Bipolar and ADHD slower than healthy; more interference from positive distractors in bipolar and from negative distractors in ADHD | For negative versus neutral words: gradient of ventrolateral prefrontal cortical activation: ADHD < healthy < bipolar; both ADHD and bipolar showed more dorsolateral prefrontal and parietal activation than healthy |
| Posner et al. (89) | Adolescent ADHD, on and off psychostimulants, N=15; healthy, N=15 | Emotional Stroop test | Medication-free ADHD showed medial prefrontal hyperactivity with positive and hypoactivity with negative distractors; normalized on psychostimulants | |
Bottom-up psychological mechanisms.
Two basic processes affect emotion regulation: orienting to emotionally salient stimuli, and the evaluation of signals for reward. In order for emotion to be regulated, posterior attention systems must both detect salient stimuli and signal that control is needed (77, 90). Evidence suggests anomalies in early orienting to emotional stimuli in ADHD. In healthy individuals, affectively charged stimuli receive enhanced early sensory encoding, detectable by electrophysiological markers. Two studies (91, 92) found that this effect is reduced in adults with ADHD when viewing positive, but not negative stimuli; this would be expected to cause overperception of negative stimuli. The studies further linked these early processing deficits with self-rated emotional lability. Additionally, whereas the startle reflex is typically accentuated by precursive positive stimuli and attenuated by negative stimuli, this effect is lost in adults with ADHD, which constitutes further evidence of abnormal early processing of emotional stimuli in ADHD (93). Likewise, the rapid and accurate recognition of emotions in human faces or voices is central to well-regulated behavior; emotional misperception is linked with aberrant emotional responses, and misperception can itself result from emotion dysregulation (94, 95). Studies of emotion labeling have found moderate impairments in ADHD, with our meta-analysis producing an effect size of 0.65 (95% CI=0.48–0.81) (Figure 1B).
The evaluation of emotionally salient stimuli has also been studied in relation to the evaluation of signals for potential reward. A preference for immediate small rewards over larger delayed ones, even when such choice defeats one’s own goals and desires, is held to be a hallmark of impulsivity, reflecting an aversion to delayed reward (96, 97). Our meta-analysis found that ADHD was also moderately associated with this preference (effect size, 0.6, 95% CI=0.40–0.79), albeit with considerable heterogeneity in results (35, 37) (Figure 1C). This style of reward processing can be construed as a contributor to emotion dysregulation, as it may reflect anomalous activity in limbic regions that are pivotal in emotion processing. Equally, the preference for immediate small rewards might also reflect failures in top-down regulatory mechanisms, such as the ability to hold longer-term goals in mind or to exert cognitive control to suppress the arousing value of immediate incentives (78, 98, 99). Thus, anomalies in reward evaluation provide further, albeit indirect, evidence for dysregulation of emotion systems in ADHD.
Top-down regulatory processes.
Parasympathetic response is considered one gauge of regulatory functioning (100). In typically developing children, autonomic nervous system function tracks the valence of emotional stimuli and task demands, with greater top-down regulatory activity when stimuli are negative rather than positive (100). In children with ADHD, this ability to adjust top-down regulation in response to different emotional stimuli was partially lost, based on physiological indicators of regulation.
Another way to assess the recruitment of regulatory resources is to consider the allocation of attention itself to emotional stimuli. Just as emotion regulation requires the ability to recruit autonomic responses, it also relies on the ability to direct attention toward or away from emotional stimuli so as to maintain emotional homeostasis or maintain focus on a goal (101). This ability can be assessed by incorporating an affective dimension in a cognitive paradigm. For example, in the emotional Stroop task, individuals must deflect attention away from the emotional properties, such as emotional expression, and attend to a nonemotional feature, such as eye color. This manipulation exacerbates the performance deficits already evident in ADHD, suggesting that performance drops off more steeply than it does in typical individuals under emotional challenge (87, 89).
Finally, given that ADHD is associated with poor higher-order cognitive control even in the absence of emotionally salient stimuli, what role does poor cognitive control play in emotion dysregulation in ADHD? Evidence suggests a modest connection but not isomorphism. For example, in 49 boys with and without ADHD, cognitive control, indexed by response inhibition, accounted for 11% of the variance in “dysregulated” behavior during a frustrating task (102). A larger study of 424 children with ADHD and their siblings (37) found that while a range of neuropsychological variables correlated with emotional lability, this link was not direct but was mediated almost entirely by the severity of ADHD symptoms.
In summary, emotion dysregulation in ADHD may arise from deficits at multiple levels. At the most basic level, there are anomalies in orienting to emotional stimuli and reward valuation. This is combined with failings in top-down psychological processes, such as the allocation of attention to emotional stimuli. Meanwhile, deficits in cognitive processes, including working memory and response inhibition, may contribute to emotion dysregulation, but by themselves they do not seem to explain its presence in ADHD.
Neural mechanisms.
It is useful to distinguish between regions mediating bottom-up responses to emotional stimuli—specifically the amygdala, ventral striatum, and orbitofrontal cortex—and top-down cortical regions controlling the allocation of attentional resources in emotionally arousing contexts (77, 103). In ADHD, functional imaging studies have yielded disparate findings, possibly because of differences in tasks and sample characteristics and limited power to detect effects in smaller studies, but nonetheless some themes emerge (Table 2 and Figure 3).
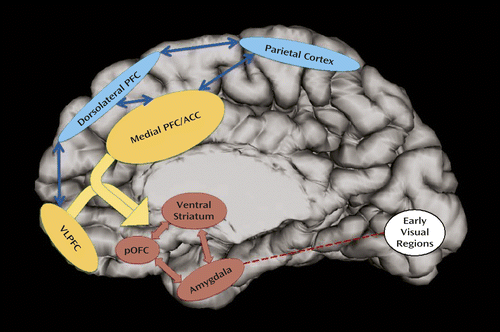
a The circuitry that underpins deficits in early orienting to emotional stimuli and their perception is shown in red. Regions that interface between emotional and cognitive circuits, allocating attention to emotional stimuli, are shown in yellow. Circuitry implicated in cognitive control, motor planning, and attention is shown in blue. ACC=anterior cingulate cortex; pOFC=posterior orbitofrontal cortex; PFC=prefrontal cortex; VLPFC=ventrolateral prefrontal cortex.
Amygdala activation during emotion processing in ADHD has received some research attention. The larger studies find amygdala hyperactivation in ADHD, during both the subliminal perception of fearful expressions and while subjects rated their fear of neutral faces, although results are mixed (24, 29, 79–82) (Table 2; see also the online data supplement). Amygdala hyperactivation has also been reported in ADHD during the processing of delayed rewards, perhaps consistent with the delay aversion found in some behavioral studies (38, 83–85). Deficits in early processing of visual emotional stimuli and in the modulation of the startle reflex, described above, also suggest amygdala dysfunction in ADHD. These functional deficits align with reports of amygdala structural abnormalities in ADHD, including surface morphology and dopamine receptor density (104).
The orbitofrontal cortex, which has rich interconnections with the amygdala, the thalamus, and multiple cortical regions, is pivotal in emotion regulation and reward representations (77, 105). Some data suggest orbitofrontal anatomic anomalies (106) and abnormal activation during the anticipation and receipt of rewards in ADHD. There is also decreased connectivity between the amygdala and the orbitofrontal cortex, reflected in a loss of the typical correlation between the volumes of these structures (104).
The ventral striatum is the third important hub in the bottom-up circuitry, partly by virtue of its role in mediating positive affect and reward processing (107). Functional neuroimaging studies find reduced ventral striatum responsiveness in ADHD during the anticipation (and receipt) of rewards, thus contributing to aversion to delay (Table 2). By examining brain activity at rest in ADHD, two groups have reported both increased functional connectivity between the ventral striatum and the orbitofrontal/ventromedial prefrontal cortex and decreased connectivity between these regions and cortical attentional control regions (108, 109). Thus, evidence suggests dysfunction in a network encompassing the amygdala, ventral striatum, and orbitofrontal cortex that processes emotional stimuli and is implicated in emotion regulation.
With regard to cortical regions, in healthy subjects the addition of an emotional dimension to cognitive tasks usually boosts top-down prefrontal cortical activation (particularly in ventrolateral, medial prefrontal, and anterior cingulate cortical regions) and diminishes subcortical activity (77). These patterns are partly lost in ADHD. Specifically, when negative stimuli are added to a working memory task, performance deficits in ADHD are associated with hypoactivation in prefrontal control regions, including the ventrolateral, orbitofrontal, and medial prefrontal cortices. However, when positive stimuli are used, ADHD patients show hyperactivation in these regions (87). Similarly, two independent studies using the emotional Stroop task (88, 89) found hypoactivation in ADHD in the right medial and ventrolateral prefrontal cortex while processing negative distractors but hyperactivation in the left medial prefrontal cortex while processing positive distractors. Such work represents the first stage in charting the neural basis of dysregulated attentional control in ADHD in the presence of emotional stimuli.
In summary, emotion dysregulation in ADHD implicates dysfunction in the amygdala, ventral striatum, and orbitofrontal cortex, which could be regarded as the bottom-up contributor. Regions at the interface of cognition and emotion (the medial and ventrolateral prefrontal cortex) may underpin the abnormal allocation of attention to emotional stimuli and could thus be regarded as the major top-down contributor to emotion dysregulation in ADHD (Figure 2). Higher cortical centers involved in motor control (supplementary motor areas, motor cortex), monitoring for salient stimuli (temporoparietal junction, frontal operculum), and shifting attention flexibly (frontal eye fields, intraparietal sulcus) may play a less direct role (110). The exact balance of symptoms stemming from ADHD and emotion dysregulation in an individual may depend on the degree to which each neural network or level is compromised. We predict that dysfunction at the cortical nexus between cognition and emotion (the medial and ventrolateral prefrontal cortex) is strongly associated with symptoms of both ADHD and emotion dysregulation. If, however, an individual has dysfunction that is more focused in higher, more lateral prefrontal/parietal cortical regions, then in that individual symptoms of ADHD such as inattention would predominate over emotion dysregulation. Conversely, an individual with predominantly (para)limbic dysfunction may exhibit mainly symptoms stemming from emotion dysregulation.
Etiological factors.
It has been proposed that the combination of ADHD and emotion dysregulation defines a distinct genetic group. In support of this view, one group found that the siblings of probands with both ADHD and emotion dysregulation also had significantly elevated rates of this combination, although this has not been replicated (52, 111, 112). The Child Behavior Checklist-defined dysregulation profile is highly heritable (67%) (113), and studies have suggested candidate genes (114).
Among possible environmental factors, high levels of parental criticism and hostility have been linked both with the development of conduct problems in children with ADHD and with the development of childhood ADHD in preschoolers with behavioral problems (115, 116). A plausible hypothesis is that failures of parental emotion regulation, reflected by high expressed hostility, contribute to the development of emotion dysregulation in children with ADHD.
Treatment
The management of emotion dysregulation in ADHD presents formidable therapeutic challenges, partly because clinical trials in ADHD either fail to assess change in emotion regulation or do so as a secondary outcome measure. Psychostimulants are highly effective in treating oppositional defiant disorder comorbid with ADHD (meta-analyses are available at http://www.nice.org.uk/CG72). However, evidence for psychostimulant efficacy in treating emotion dysregulation in ADHD is more limited (Table 3). A review found that two randomized placebo-controlled studies in children with ADHD reported that psychostimulants reduced emotional lability and irritability (127). In adults, several studies found that the beneficial effects of psychostimulants on emotion dysregulation parallel the improvement seen in hyperactivity and impulsivity. However, two randomized controlled trials comparing amphetamine and placebo found no beneficial medication impact on a broad range of emotional problems, and some studies have found that amphetamine preparations increase irritability and lability (127).
| Study | Participants | Measures | Results |
|---|---|---|---|
| Children | |||
| Childress et al. (117) | Lisdexamphetamine versus placebo for 4 weeks, N=283; at baseline 179 had prominent emotional lability | Conners Parent Rating Scale of emotional lability (angry/resentful, losing temper, and irritability); prominent emotional lability defined as having at least one symptom “pretty much” or “very much” | For those with prominent emotional lability, medication was associated with a significant reduction in emotional symptoms; no change in emotionality seen in those with low emotional lability |
| Ahmann et al. (118) | Crossover design; treated with placebo or low-dosage (0.3 mg/kg) and higher-dosage (0.5 mg/kg) methylphenidate; N=234 | Side effect questionnaire including items on dysthymia, euphoria, irritability, and anxiety | Decreased irritability on methylphenidate (odds ratio=0.33, 95% CI=0.18–0.61) |
| Gillberg et al. (119) | Amphetamine versus placebo for 6 months, N=56 | Side effect questionnaire including items on dysthymia, euphoria, irritability, and anxiety | No differences between treated and placebo groups |
| Kratochvil et al. (120) | Atomoxetine versus placebo, for 8 weeks, N=179 | Emotion and Expression Scale for Children | No difference between atomoxetine and placebo |
| Coghill et al. (121) | Crossover methylphenidate 0.3 mg/kg and 0.6 mg/kg versus placebo for 12 weeks, N=75 | Conners’ emotional lability subscale | Significant reduction in emotional lability for both low-dosage (parent-report effect size, 0.46; teacher-report effect size, 0.45) and high-dosage methylphenidate (parent-report effect size, 0.42; teacher-report effect size, 0.79) |
| Herbert et al. (122) | Randomized to waiting list or parent training to boost child’s emotion regulation and socialization, N=31 | Emotion Regulation Checklist | Parent training linked with moderate reduction of child’s emotional lability (effect size, 0.27–0.45). |
| Webster-Stratton et al. (123) | Randomized to waiting list or parenting program boosting positive and consistent parenting style, N=99 | Emotion regulation scale | Moderate effect of intervention on emotion regulation (effect size, 0.25) |
| Adults | |||
| Reimherr et al. (60) | Crossover trial of extended-release methylphenidate versus placebo (4 weeks each arm), N=47 | Wender-Reimherr adult ADHD scale, emotion dysregulation items | Decrease in emotion dysregulation on methylphenidate (effect size, 0.7) |
| Reimherr et al. (59) | Post hoc analyses of trials comparing atomoxetine and placebo; ADHD only, N=359; ADHD and emotion dysregulation, N=170 | Wender-Reimherr adult ADHD scale, emotion dysregulation items | Decrease in emotion dysregulation on atomoxetine (effect size, 0.66) |
| Marchant et al. (124) | Crossover trial of transdermal methylphenidate versus placebo (4 weeks each arm); ADHD alone, N=21; ADHD and emotion dysregulation, N=28; ADHD and oppositional defiant disorder, N=9; ADHD, oppositional defiant disorder, and emotion dysregulation, N=32 | Wender-Reimherr adult ADHD scale, emotion dysregulation items | All groups showed benefit on psychostimulants; trend for those with emotion dysregulation to improve most |
| Rösler et al. (125) | Methylphenidate versus placebo with 24-week double-blind phase, N=363 | Wender-Reimherr adult ADHD scale, emotion dysregulation items | Methylphenidate reduced emotional lability (effect size, 0.28–0.4) |
| Emilsson et al. (126) | Cognitive behavioral therapy and medication versus medication alone, N=54 | Self-report scale of emotional control | Combination group did not show significantly better emotional control at end of intervention but did 3 months following intervention (d=1.12) |
Among individuals with ADHD, psychostimulants also improve emotion recognition (91) and normalize both the startle modulation by affective stimuli (93) and performance on the emotional Stroop task (89). This behavioral normalization is accompanied by normalization of underlying neural activity (89, 91, 93).
Among the non-stimulant treatments, improvement of emotion regulation on atomoxetine paralleled improvement in core symptoms of ADHD among adults (59). Mood stabilizers have yielded mixed results. A trial of lithium for children with severe mood dysregulation, most of whom had ADHD, was negative (128). However, a comparison of behavioral therapy combined with either divalproex or stimulants found that the use of divalproex was more efficacious in children with severe aggressive behavior, most of whom also had ADHD (129). Another study found that among 30 children with ADHD whose aggression did not respond to open-label psychostimulant treatment and behavioral therapy, the addition of divalproex resulted in significantly higher rates of remission compared with placebo (130). The use of atypical antipsychotic medications for the combination of ADHD, emotion dysregulation, and aggression still lacks a clear evidence base.
While cognitive and behavioral psychotherapies have a limited impact on core symptoms of ADHD, there is preliminary evidence that interventions that specifically target emotion dysregulation are efficacious (131, 132). We consider such interventions to be a promising future direction for research.
The current literature suggests the following treatment approach. Psychostimulant treatment of the core symptoms of ADHD is often linked to a beneficial effect on emotion dysregulation and should be considered first-line treatment. Atomoxetine also appears effective for symptoms of ADHD and emotion dysregulation. Use of adjunctive behavioral modification in children is reasonable, as this combination is effective in those with mixed internalizing and externalizing symptoms, many of whom have emotional dysregulation (51, 133). Group-based psychotherapy in adults with ADHD to bolster emotion regulation skills shows promise but requires replication (132). Lacking an evidence base for second-line pharmacological approaches to emotion dysregulation in ADHD, treatment will be guided largely by the presence of comorbid disorders. For example, for patients with ADHD and depression, in whom emotion dysregulation is often prominent, use of serotonin reuptake inhibitors combined with psychostimulants is reasonable (134).
Conceptual Models
Three models have been proposed, whose proponents can be characterized as “lumpers,” who view emotion dysregulation as an integral component of ADHD; “splitters,” who view the combination as defining a distinct entity; and “diplomats,” who view symptoms of ADHD and emotion dysregulation as correlated but ultimately dissociable dimensions (Table 4). The current evidence is insufficient to choose decisively among these models, partly because few studies have been designed to address specifically the question of why emotion dysregulation is so prominent in ADHD. However, this framework generates testable hypotheses that can stimulate future research.
| Phenomenology | Pathophysiology | |||||
|---|---|---|---|---|---|---|
| Model | Correlations Between ADHD and Emotion Dysregulation | Clinical Course | Psychological Basis | Neural Basis | Genetic | Treatment |
| Emotion dysregulation is integral to ADHD | Extremely high | Yoked clinical courses for symptoms of ADHD and emotion dysregulation | Deficits in behavioral inhibition and working memory mediate both core ADHD symptoms and emotion dysregulation | Anomalies confined to fronto-striatal-cerebellar circuits | Same genetic basis for ADHD with emotion dysregulation and ADHD alone | Treatments that improve ADHD will improve emotion dysregulation |
| Combined ADHD and emotion dysregulation defines a distinct entity | ADHD subgroup exists that is high on both symptom domains | Distinct clinical course for ADHD with emotion dysregulation and ADHD alone | Distinct cognitive deficits in ADHD with emotion dysregulation and ADHD alone | Distinct neural bases for ADHD with emotion dysregulation and ADHD alone | Distinct genetic bases for ADHD with emotion dysregulation and ADHD alone | Existing treatments for ADHD may be less effective for ADHD with emotion dysregulation |
| Symptoms of ADHD and emotion dysregulation are correlated but distinct dimensions | Modest | Similar but dissociable clinical courses for symptoms of ADHD and emotion dysregulation | Deficits in emotion processing mediate dysregulation and correlate with deficits mediating core ADHD symptoms | Anomalies extend beyond fronto-striato-cerebellar circuits to (para)limbic regions | Some genes shared between ADHD alone and ADHD with emotion dysregulation | Treating “core” ADHD symptoms benefits emotion dysregulation, but separate treatment may also be needed |
The first model, which harks back to earlier conceptualizations of ADHD, posits that emotion dysregulation is a core defining feature of ADHD that is as central to the disorder as hyperactivity, impulsivity, and inattention (135). Emotion dysregulation is seen as an expression of the same neurocognitive deficits that underpin other symptoms of ADHD, and Occam’s razor dictates that it is unnecessary to invoke additional emotion processing deficits. The model is parsimonious and recognizes the close associations between cognitive and emotional regulation systems. However, as noted above, the overlap between ADHD and emotion dysregulation is far from complete: many ADHD patients do not exhibit impairing levels of emotion dysregulation (55%−75% of children and 30%−70% of adults with ADHD). Additionally, the evidence for widespread (para)limbic dysfunction in ADHD and associated deficits in emotional processes might argue against a reductionist model of emotion dysregulation in ADHD as another expression of purely cortico-striatal-cerebellar dysfunction. This model also predicts that treatments that ameliorate core symptoms would have an almost equal impact on emotion dysregulation, which seems to occur in adulthood but does so less clearly in childhood.
The second model holds that the combination of ADHD and emotion dysregulation defines a distinct entity (111, 112). This model has been generated largely on the basis of genetic findings of familial cosegregation of ADHD and emotion dysregulation, although evidence on this point is mixed (52, 111). This model could imply both a distinct neurocognitive etiology and a distinct clinical course for those with the combination of ADHD and emotion dysregulation, a possibility that warrants further testing.
The third model holds that symptoms of ADHD and emotion dysregulation are distinct but correlated dimensions, each underpinned by partly overlapping but dissociable neurocognitive deficits. This model has much in common with the concept of multiple but overlapping pathways to ADHD (103, 110). It is supported by the significant but modest correlations between symptoms of ADHD and emotion dysregulation reviewed earlier; the symptom domains commonly coexist but are far from completely overlapping. Similarly, modest correlations have been reported between deficits in emotional processes—such as deficits in emotion recognition and frustration tolerance—and the executive dysfunction often held to be a core feature of the disorder (35, 37). Longitudinal data reviewed earlier also suggest modest links between the course of ADHD symptoms and emotion dysregulation in early childhood, and perhaps in adulthood, consistent with a model of correlated but distinct symptom dimensions.
Future Research Directions
Phenomenology and Pathophysiology
Refinement of the phenotype is needed, as emotion dysregulation in individuals with ADHD is likely to have a number of clinically important components, such as irritability and mood lability (4, 51). It will be important to operationalize each component, develop consensus measurement techniques, and conduct longitudinal studies to define how the developmental trajectories of the components interact with each other and with the dimensions of ADHD. Pathophysiological studies should include individuals lying along the spectrum of emotion regulation abilities, but perhaps oversample those most in clinical need, lying at the extreme of dysregulation. Such work would allow direct links to be made between emotion dysregulation in ADHD and the underlying neural anomalies—a link that has been made in relatively few studies.
Functional imaging studies should include a broad range of tasks of emotion regulation, defining the neural bases of the ability to reinterpret the meaning of emotional stimuli, the adaptive suppression of ongoing emotional responses, and the ability to employ strategies such as distancing oneself from emotionally arousing materials (77). We predict that emotionally dysregulated individuals with ADHD would lose the coordinated increase in medial prefrontal/anterior cingulate cortex activation and altered amygdala activation that underpins many forms of emotion regulation.
To what extent do individuals with ADHD develop emotion dysregulation for reasons different from those of individuals with other disorders? Could ADHD-specific symptoms or cognitive aberrations be related to emotion dysregulation? “Mind wandering” is one candidate cognitive mechanism. It is typically measured as interference in tasks of cognitive control and appears to be related to a failure to deactivate the so-called default mode network of the brain, a deficit also found in ADHD (136). Notably, mind wandering appears to lead to transient dysphoric mood and vice versa (137). Testing the links between attentional lapses and emotion dysregulation and the possible mediating role of the default mode network may be a promising research avenue.
There is evidence in ADHD of an altered structural and functional maturation of the prefrontal cortical regions that support top-down emotion regulation (138). Could disrupted developmental trajectories be particularly pronounced among those with both ADHD and impaired emotion regulation? Our model predicts a disruption of white matter tracts such as the uncinate fasciculus that connect limbic regions, including the amygdala, hippocampus, and orbitofrontal cortex; these tracts can be assessed using diffusion tensor imaging.
Behavioral genetic data on twins could parse out the degree to which ADHD and emotion dysregulation share genetic or environmental risk factors. This could be achieved by reanalysis of existing data sets that include measures of irritability and other relevant traits. Studies of environmental risk factors have focused on familial characteristics but could also define the characteristics of a child’s peer group that confer vulnerability to emotion dysregulation.
Treatment
Is a two-pronged pharmacological approach targeting both the symptoms of ADHD and those of emotion dysregulation more effective than use of psychostimulants alone? This question is being examined in children with severe mood dysregulation (most of whom have ADHD) in trials that use both psychostimulants and selective serotonin reuptake inhibitors (SSRIs) (clinicaltrials.gov identifiers NCT00794040, NCT01714310). The use of SSRIs is grounded in preclinical studies showing that the modulation of serotonergic tone affects the processing of emotionally charged stimuli and clinical studies showing efficacy in promoting emotion regulation in other disorders (139).
Other agents show promise. Postsynaptic alpha-2 adrenoceptor agonists such as guanfacine treat core symptoms of ADHD and oppositionality (140). In healthy adults, guanfacine reverses the bias to respond less accurately to negative compared with positive emotional stimuli, partly by boosting activation of the left dorsolateral prefrontal cortex (141). Given the interactions between the lateral prefrontal cortex and the ventral/medial prefrontal cortical regions linked to emotion regulation, guanfacine emerges as a potential emotion regulator in ADHD. Modafinil, which inhibits dopamine and norepinephrine transporters and decreases GABA, also shows promise as a treatment for ADHD (142). In healthy adults, modafinil decreases amygdala activation during the viewing of fearful stimuli and boosts prefrontal cortical activation during executive functions (143). Again, this profile points to a possible benefit for ADHD and associated emotion dysregulation. Dietary interventions can be considered, as there appears to be benefit from omega-3 fatty acid supplementation in ADHD (131). Given that low levels of omega-3 fatty acids are associated with electrophysiological anomalies during emotion processing in ADHD (144), might emotion dysregulation in ADHD also benefit from supplementation?
Which psychotherapies are promising? Cognitive therapy can help individuals with ADHD recognize and label emotions accurately, challenge emotions that are not context appropriate, and cope with intense negative emotional reactions (132). These skills have been augmented with mindfulness training that promotes a nonjudgmental, present-centered focused awareness of emotions. This approach, derived partly from dialectical behavior therapy for disorders with prominent emotion dysregulation, such as borderline personality disorder, is currently being assessed in adults with ADHD (73). Improving executive functions such as working memory and planning abilities helps core ADHD symptoms in adults, but future studies should also ask if these cognitive interventions also improve emotion regulation (132). Similarly, it has been argued that parent-led games can boost a preschooler’s executive skills and might prevent later ADHD (145, 146). Might such early intervention also promote emotion regulation?
What of interventions that target not just the individual with ADHD but also the individual’s social context? For example, there is a strong rationale for family-based interventions to decrease negative family dynamics and thus perhaps enhance emotion regulation in both the parent and the child with ADHD (115). A novel approach leverages a child’s peer group as a therapeutic ally (147). Children with ADHD often form cliques with disruptive others, and classroom interventions might promote alliances with less disruptive children who can perhaps better model emotion regulation.
Conclusions
Since Still described the “morbid excitability” of children with ADHD (148), the presence of emotion dysregulation in ADHD has been well recognized. Recent advances in the behavioral, neuroimaging, and genomic sciences hold the promise that our renewed focus on this overlap will result in an understanding of the underlying pathophysiological mechanisms and stimulate novel treatment approaches.
1 Clements SD: Minimal Brain Dysfunction in Children: Terminology and Identification: Phase One of a Three-Phase Project. Washington, DC, US Department of Health, Education, and Welfare, 1966Google Scholar
2 Thompson RA: Emotion Regulation: A Theme in Search of Definition. Monographs of the Society for Research in Child Development, vol 59, issue 2/3, Feb 1994, pp 25–52Google Scholar
3 : Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry 2011; 168:129–142Link, Google Scholar
4 : Irritability in children and adolescents: a challenge for DSM-5. Eur Child Adolesc Psychiatry 2011; 20:61–66Crossref, Medline, Google Scholar
5 Dodge KA, Pepler D, Rubin K: The structure and function of reactive and proactive aggression, in The Development and Treatment of Childhood Aggression. Edited by Pepler DJ, Rubin KH. Hillsdale, NJ, Lawrence Erlbaum, 1991, pp 201–218Google Scholar
6 : ASEBA School Age Forms and Profiles. Burlington, Vt, ASEBA, 2001Google Scholar
7 : Attention deficit hyperactivity disorder characteristics, II: clinical correlates of irritable mood. J Affect Disord 2013; 145:70–76Crossref, Medline, Google Scholar
8 : Three dimensions of oppositionality in youth. J Child Psychol Psychiatry 2009; 50:216–223Crossref, Medline, Google Scholar
9 : Observed classroom behavior of children with ADHD: relationship to gender and comorbidity. J Abnorm Child Psychol 2002; 30:349–359Crossref, Medline, Google Scholar
10 ;
11 : Influence of anxiety on the social functioning of children with and without ADHD. J Atten Disord 2011; 15:473–484Crossref, Medline, Google Scholar
12 : Beyond words: how do children with ADHD and/or conduct problems process nonverbal information about affect? J Am Acad Child Adolesc Psychiatry 2000; 39:1160–1167Crossref, Medline, Google Scholar
13 : Perceived social acceptance and peer status differentially predict adjustment in youth with and without ADHD. J Atten Disord (Epub ahead of print, April 3, 2012)Google Scholar
14 : Prosocial behavior in hyperactive boys: effects of stimulant medication and comparison with normal boys. J Abnorm Child Psychol 1992; 20:103–121Crossref, Medline, Google Scholar
15 : Toward a new psychometric definition of social disability in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1996; 35:571–578Crossref, Medline, Google Scholar
16 : Deficient social problem-solving in boys with ODD/CD, with ADHD, and with both disorders. J Am Acad Child Adolesc Psychiatry 1999; 38:311–321Crossref, Medline, Google Scholar
17 : Positive illusions of social competence in girls with and without ADHD. J Abnorm Child Psychol 2011; 39:527–539Crossref, Medline, Google Scholar
18 : Overt and relational aggression in girls with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol 2004; 33:125–137Crossref, Medline, Google Scholar
19 : Subtypes of aggression in children with attention deficit hyperactivity disorder: medication effects and comparison with typical children. J Clin Child Adolesc Psychol 2009; 38:619–629Crossref, Medline, Google Scholar
20 : Reactive aggression in boys with disruptive behavior disorders: behavior, physiology, and affect. J Abnorm Child Psychol 2002; 30:641–656Crossref, Medline, Google Scholar
21 : Facial affect interpretation in boys with attention deficit/hyperactivity disorder. Child Neuropsychol 2008; 14:82–96Crossref, Medline, Google Scholar
22 : Do hyperactivity, impulsivity, and inattention have an impact on the ability of facial affect recognition in children with autism and ADHD? Eur Child Adolesc Psychiatry 2008; 17:63–72Crossref, Medline, Google Scholar
23 : Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology 2002; 16:102–110Crossref, Medline, Google Scholar
24 : Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry 2008; 49:781–791Crossref, Medline, Google Scholar
25 : Do autism spectrum disorders differ from each other and from non-spectrum disorders on emotion recognition tests? Eur Child Adolesc Psychiatry 2001; 10:105–116Crossref, Medline, Google Scholar
26 : Recognition of emotional facial expressions in attention-deficit hyperactivity disorder. Pediatr Neurol 2006; 35:93–97Crossref, Medline, Google Scholar
27 : Visual attention deficits contribute to impaired facial emotion recognition in boys with attention-deficit/hyperactivity disorder. Neuropediatrics 2008; 39:323–327Crossref, Medline, Google Scholar
28 : Emotion understanding in children with ADHD. Child Psychiatry Hum Dev 2009; 40:111–121Crossref, Medline, Google Scholar
29 : Functional magnetic resonance imaging of facial information processing in children with autistic disorder, attention deficit hyperactivity disorder, and typically developing controls. Int J Adolesc Med Health 2011; 23:269–277Crossref, Medline, Google Scholar
30 : Processing affective stimuli in children with attention-deficit hyperactivity disorder. Child Neuropsychol 2000; 6:144–155Crossref, Medline, Google Scholar
31 : Selective difficulty in recognising facial expressions of emotion in boys with ADHD: general performance impairments or specific problems in social cognition? Eur Child Adolesc Psychiatry 2007; 16:398–404Crossref, Medline, Google Scholar
32 : Emotional face identification in youths with primary bipolar disorder or primary attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2013; 52:537–46.e3Crossref, Medline, Google Scholar
33 : Emotional understanding, cooperation, and social behavior in high-functioning children with autism. J Autism Dev Disord 2004; 34:625–635Crossref, Medline, Google Scholar
34 : Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: a comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res 2009; 33:1656–1670Crossref, Medline, Google Scholar
35 : Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. J Child Psychol Psychiatry 2013; 54:619–627Crossref, Medline, Google Scholar
36 : Affect recognition in adults with ADHD. J Atten Disord 2011; 15:452–460Crossref, Medline, Google Scholar
37 : Neuropsychological correlates of emotional lability in children with ADHD. J Child Psychol Psychiatry 2012; 53:1139–1148Crossref, Medline, Google Scholar
38 : Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2009; 65:7–14Crossref, Medline, Google Scholar
39 : ADHD and delay aversion: the influence of non-temporal stimulation on choice for delayed rewards. J Child Psychol Psychiatry 2006; 47:1152–1158Crossref, Medline, Google Scholar
40 : Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 62:991–998Crossref, Medline, Google Scholar
41 : Delay aversion in attention deficit/hyperactivity disorder: an empirical investigation of the broader phenotype. Neuropsychologia 2009; 47:446–456Crossref, Medline, Google Scholar
42 : Inhibitory deficits, delay aversion, and preschool AD/HD: implications for the dual pathway model. Neural Plast 2004; 11:1–11Crossref, Medline, Google Scholar
43 : Examining relationships between executive functioning and delay aversion in attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol 2011; 40:837–847Crossref, Medline, Google Scholar
44 : Psychological mechanisms in hyperactivity, I: response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry 2001; 42:199–210Crossref, Medline, Google Scholar
45 : Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology 2009; 23:367–380Crossref, Medline, Google Scholar
46 : Cross-sectional evaluation of cognitive functioning in children, adolescents, and young adults with ADHD. J Neural Transm 2010; 117:403–419Crossref, Medline, Google Scholar
47 : Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. J Abnorm Child Psychol 2007; 35:729–744Crossref, Medline, Google Scholar
48 : The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH Multimodal Treatment Study of AD/HD. J Abnorm Child Psychol 2001; 29:215–228Crossref, Medline, Google Scholar
49 : Neurocognitive performance in children with ADHD and OCD. J Abnorm Child Psychol 2010; 38:961–969Crossref, Medline, Google Scholar
50 : Cool and hot executive functions in medication-naive attention deficit hyperactivity disorder children. Psychol Med 2011; 41:2593–2602Crossref, Medline, Google Scholar
51 : Mood lability and psychopathology in youth. Psychol Med 2009; 39:1237–1245Crossref, Medline, Google Scholar
52 : Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry 2010; 51:915–923Crossref, Medline, Google Scholar
53 : Self-regulation of emotion, functional impairment, and comorbidity among children with AD/HD. J Atten Disord 2011; 15:583–592Crossref, Medline, Google Scholar
54 : Toward defining deficient emotional self-regulation in children with attention-deficit/hyperactivity disorder using the Child Behavior Checklist: a controlled study. Postgrad Med 2011; 123:50–59Crossref, Medline, Google Scholar
55 : Emotional and behavioral difficulties and impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis 2006; 3:A52Medline, Google Scholar
56 ;
57 : Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med 2007; 37:97–107Crossref, Medline, Google Scholar
58 : The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry 2010; 49:503–513Crossref, Medline, Google Scholar
59 : Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005; 58:125–131Crossref, Medline, Google Scholar
60 : A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007; 68:93–101Crossref, Medline, Google Scholar
61 : Understanding deficient emotional self-regulation in adults with attention deficit hyperactivity disorder: a controlled study. Atten Defic Hyperact Disord 2013; 5:273–281Crossref, Medline, Google Scholar
62 : The latent class structure of ADHD is stable across informants. Twin Res Hum Genet 2006; 9:507–522Crossref, Medline, Google Scholar
63 : Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry 2009; 48:404–412Crossref, Medline, Google Scholar
64 Goldsmith H, Lemery K, Essex M: Roles for temperament in the liability to psychopathology in childhood, in Behavior Genetics Principles: Perspectives in Development, Personality, and Psychopathology. Edited by DiLalla LF. Washington, DC, American Psychological Association Press, 2004, pp 9–19Google Scholar
65 : Interaction of temperamental resistance to control and restrictive parenting in the development of externalizing behavior. Dev Psychol 1998; 34:982–995Crossref, Medline, Google Scholar
66 : A developmental investigation of inattentiveness and hyperactivity. Child Dev 1995; 66:37–54Crossref, Medline, Google Scholar
67 : Early developmental precursors of impulsive and inattentive behavior: from infancy to middle childhood. J Child Psychol Psychiatry 2002; 43:435–447Crossref, Medline, Google Scholar
68 : What’s in a disruptive disorder? Temperamental antecedents of oppositional defiant disorder: findings from the Avon Longitudinal Study. J Am Acad Child Adolesc Psychiatry 2010; 49:474–483Medline, Google Scholar
69 : Precursors of hyperactivity and aggression. J Am Acad Child Adolesc Psychiatry 1993; 32:1207–1216Crossref, Medline, Google Scholar
70 : Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry 2012; 69:1295–1303Crossref, Medline, Google Scholar
71 : Longitudinal course of deficient emotional self-regulation CBCL profile in youth with ADHD: prospective controlled study. Neuropsychiatr Dis Treat 2012; 8:267–276Crossref, Medline, Google Scholar
72 : Adult outcomes of childhood dysregulation: a 14-year follow-up study. J Am Acad Child Adolesc Psychiatry 2010; 49:1105–1116Medline, Google Scholar
73 : Attention deficit disorder (“minimal brain dysfunction”) in adults: a replication study of diagnosis and drug treatment. Arch Gen Psychiatry 1981; 38:449–456Crossref, Medline, Google Scholar
74 ;
75 : Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 2010; 46:209–217Crossref, Medline, Google Scholar
76 : The Child Behavior Checklist–Pediatric Bipolar Disorder profile predicts a subsequent diagnosis of bipolar disorder and associated impairments in ADHD youth growing up: a longitudinal analysis. J Clin Psychiatry 2009; 70:732–740Crossref, Medline, Google Scholar
77 : A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13:829, 833–857Crossref, Medline, Google Scholar
78 : The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249Crossref, Medline, Google Scholar
79 : Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry 2010; 167:61–69Link, Google Scholar
80 : Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008; 165:712–720Link, Google Scholar
81 : Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2011; 50:828–837.e3Crossref, Medline, Google Scholar
82 : Childhood methylphenidate treatment of ADHD and response to affective stimuli. Eur Neuropsychopharmacol 2011; 21:646–654Crossref, Medline, Google Scholar
83 : Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 2008; 39:966–972Crossref, Medline, Google Scholar
84 : Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:720–724Crossref, Medline, Google Scholar
85 : Reward processing in male adults with childhood ADHD: a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology (Berl) 2011; 215:467–481Crossref, Medline, Google Scholar
86 : Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009; 57:640–652Crossref, Medline, Google Scholar
87 : Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:1064–1080Crossref, Medline, Google Scholar
88 : Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc 2010; 16:106–117Crossref, Medline, Google Scholar
89 : The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry Res 2011; 193:151–160Crossref, Medline, Google Scholar
90 : Emotional lability, comorbidity, and impairment in adults with attention-deficit hyperactivity disorder. J Affect Disord 2012; 147:80–86Crossref, Medline, Google Scholar
91 : Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol Psychiatry 2008; 63:917–926Crossref, Medline, Google Scholar
92 : Event-related potentials in adolescents with combined ADHD and CD disorder: a single stimulus paradigm. Brain Cogn 2006; 60:70–75Crossref, Medline, Google Scholar
93 : Methylphenidate normalizes emotional processing in adult patients with attention-deficit/hyperactivity disorder: preliminary findings. Brain Res 2011; 1381:159–166Crossref, Medline, Google Scholar
94 : On the nature of emotion regulation. Child Dev 2004; 75:377–394Crossref, Medline, Google Scholar
95 : Increasing recognition of happiness in ambiguous facial expressions reduces anger and aggressive behavior. Psychol Sci 2013; 24:688–697Crossref, Medline, Google Scholar
96 : A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci 2005; 28:397–419, discussion 419–468Crossref, Medline, Google Scholar
97 : Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psychiatry 2003; 42:1335–1342Crossref, Medline, Google Scholar
98 : Emotion-related regulation: sharpening the definition. Child Dev 2004; 75:334–339Crossref, Medline, Google Scholar
99 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26Crossref, Medline, Google Scholar
100 : Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2013; 52:163–171.e2Crossref, Medline, Google Scholar
101 : The emerging field of emotion regulation: an integrative review. Rev Gen Psychol 1998; 2:271Crossref, Google Scholar
102 : The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol 2004; 33:772–782Crossref, Medline, Google Scholar
103 : An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 2005; 17:785–806Crossref, Medline, Google Scholar
104 : Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63:795–807Crossref, Medline, Google Scholar
105 : Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 2001; 4:95–102Crossref, Medline, Google Scholar
106 : Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med 2001; 31:1425–1435Crossref, Medline, Google Scholar
107 : Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21:RC159Crossref, Medline, Google Scholar
108 : Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry 2012; 71:443–450Crossref, Medline, Google Scholar
109 : Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 2012; 23:33–45Crossref, Medline, Google Scholar
110 : Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 2012; 16:17–26Crossref, Medline, Google Scholar
111 : Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry 2011; 168:617–623Link, Google Scholar
112 : Deficient emotional self-regulation and pediatric attention deficit hyperactivity disorder: a family risk analysis. Psychol Med 2012; 42:639–646Crossref, Medline, Google Scholar
113 : Prevalence and genetic architecture of Child Behavior Checklist-juvenile bipolar disorder. Biol Psychiatry 2005; 58:562–568Crossref, Medline, Google Scholar
114 : Genome-wide association study of the Child Behavior Checklist dysregulation profile. J Am Acad Child Adolesc Psychiatry 2011; 50:807–817.e8Crossref, Medline, Google Scholar
115 : Developmental neuropsychopathology of attention deficit and impulsiveness. Dev Psychopathol 1999; 11:607–628Crossref, Medline, Google Scholar
116 : Applications of the expressed emotion construct to young children with externalizing behavior: stability and prediction over time. J Child Psychol Psychiatry 2000; 41:457–462Crossref, Medline, Google Scholar
117 : The effects of lisdexamfetamine dimesylate on emotional lability in children 6 to 12 years of age with ADHD in a double-blind placebo-controlled trial. J Atten Disord (Epub ahead of print, June 26, 2012)Google Scholar
118 : Placebo-controlled evaluation of Ritalin side effects. Pediatrics 1993; 91:1101–1106Medline, Google Scholar
119 : Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms: a randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry 1997; 54:857–864Crossref, Medline, Google Scholar
120 : Emotional expression during attention-deficit/hyperactivity disorders treatment: initial assessment of treatment effects. J Child Adolesc Psychopharmacol 2007; 17:51–62Crossref, Medline, Google Scholar
121 : The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 62:954–962Crossref, Medline, Google Scholar
122 : A randomized controlled trial of a parent training and emotion socialization program for families of hyperactive preschool-aged children. Behav Ther 2013; 44:302–316Crossref, Medline, Google Scholar
123 : Combining parent and child training for young children with ADHD. J Clin Child Adolesc Psychol 2011; 40:191–203Crossref, Medline, Google Scholar
124 : Methylphenidate transdermal system in adult ADHD and impact on emotional and oppositional symptoms. J Atten Disord 2011; 15:295–304Crossref, Medline, Google Scholar
125 : Twenty-four-week treatment with extended release methylphenidate improves emotional symptoms in adult ADHD. World J Biol Psychiatry 2010; 11:709–718Crossref, Medline, Google Scholar
126 : Cognitive behaviour therapy in medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. BMC Psychiatry 2011; 11:116Crossref, Medline, Google Scholar
127 : Changes in emotions related to medication used to treat ADHD, part I: literature review. J Atten Disord 2011; 15:101–112Crossref, Medline, Google Scholar
128 : Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol 2009; 19:61–73Crossref, Medline, Google Scholar
129 : Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000; 157:818–820Link, Google Scholar
130 : Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry 2009; 166:1392–1401Link, Google Scholar
131 ;
132 : Cognitive behavior therapy for adults with attention-deficit/hyperactivity disorder: a review of recent randomized controlled trials. Curr Psychiatry Rep 2012; 14:561–567Crossref, Medline, Google Scholar
133 : ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry 2001; 40:147–158Crossref, Medline, Google Scholar
134 : A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol 2008; 18:565–571Crossref, Medline, Google Scholar
135 : Deficient emotional self-regulation in children and adults with attention deficit/hhyepractivity disorder. Journal of ADHD and Related Disorders 2010; 1:5–29Google Scholar
136 : Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 2010; 31:904–916Crossref, Medline, Google Scholar
137 : A wandering mind is an unhappy mind. Science 2010; 330:932Crossref, Medline, Google Scholar
138 : Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry 2012; 72:191–197Crossref, Medline, Google Scholar
139 : Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 2008; 12:31–40Crossref, Medline, Google Scholar
140 : Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs 2010; 24:755–768Medline, Google Scholar
141 : Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology (Berl) 2013; 226:261–271Crossref, Medline, Google Scholar
142 : A 9-week, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study to evaluate the efficacy and safety of modafinil as treatment for adults with ADHD. J Atten Disord (Epub ahead of print, May 22, 2012)Google Scholar
143 : Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology 2010; 35:2101–2109Crossref, Medline, Google Scholar
144 : Omega-3 fatty acids are related to abnormal emotion processing in adolescent boys with attention deficit hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 2013; 88:419–429Crossref, Medline, Google Scholar
145 : More than child’s play: the potential benefits of play-based interventions for young children with ADHD. Expert Rev 2012; 12:1165–1167Medline, Google Scholar
146 : An open trial of a metacognitive executive function training for young children with ADHD. J Atten Disord (Epub ahead of print, May 29, 2012)Google Scholar
147 : A randomized trial of a classroom intervention to increase peers’ social inclusion of children with attention-deficit/hyperactivity disorder. J Consult Clin Psychol 2013; 81:100–112Crossref, Medline, Google Scholar
148 : Some abnormal psychical conditions in children: the Goulstonian lectures. Lancet 1902; 1:1008–1011Google Scholar



