Psychiatric Disorders From Childhood to Adulthood in 22q11.2 Deletion Syndrome: Results From the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome
Abstract
Objective
Chromosome 22q11.2 deletion syndrome is a neurogenetic disorder associated with high rates of schizophrenia and other psychiatric conditions. The authors report what is to their knowledge the first large-scale collaborative study of rates and sex distributions of psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome. The associations among psychopathology, intellect, and functioning were examined in a subgroup of participants.
Method
The 1,402 participants with 22q11.2 deletion syndrome, ages 6–68 years, were assessed for psychiatric disorders with validated diagnostic instruments. Data on intelligence and adaptive functioning were available for 183 participants ages 6 to 24 years.
Results
Attention deficit hyperactivity disorder (ADHD) was the most frequent disorder in children (37.10%) and was overrepresented in males. Anxiety disorders were more prevalent than mood disorders at all ages, but especially in children and adolescents. Anxiety and unipolar mood disorders were overrepresented in females. Psychotic disorders were present in 41% of adults over age 25. Males did not predominate in psychotic or autism spectrum disorders. Hierarchical regressions in the subgroup revealed that daily living skills were predicted by the presence of anxiety disorders. Psychopathology was not associated with communication or socialization skills.
Conclusions
To the authors’ knowledge, this is the largest study of psychiatric morbidity in 22q11.2 deletion syndrome. It validates previous findings that this condition is one of the strongest risk factors for psychosis. Anxiety and developmental disorders were also prevalent. These results highlight the need to monitor and reduce the long-term burden of psychopathology in 22q11.2 deletion syndrome.
Chromosome 22q11.2 deletion syndrome, also known as velocardiofacial or DiGeorge syndrome, is a neurogenetic condition affecting 1 in 2,000–4,000 live births and caused in most cases by a hemizygous 3-megabase microdeletion on the long arm of chromosome 22. Its phenotypic expression is highly variable and ranges from severe life-threatening conditions to only a few features. Frequently associated medical conditions include conotruncal cardiac anomalies, palatal anomalies (including velopharyngeal insufficiency), hypoparathyroidism/hypocalcemia, and subtle dysmorphic facial features (1). The neurocognitive profile is also highly variable, both between individuals and during the course of development (2). From infancy onward, motor delays (often with hypotonia) and speech or language deficits are commonly observed (3). During the preschool and primary school ages, learning difficulties are very common. The majority of patients with 22q11.2 deletion syndrome have an intellectual level that falls in the borderline range (IQ, 70–84), and about one-third have mild to moderate intellectual disability (3, 4). More severe levels of intellectual disability are uncommon in children and adolescents (4) but are more frequent in adults (5).
Psychiatric phenotypes of the syndrome have been described worldwide, and affected individuals have substantially greater rates of various psychiatric disorders than are found in the general population. Early studies focused on adults and consistently showed a greater than average prevalence (23%−43%) of schizophrenia spectrum disorders (6–12). Therefore, 22q11.2 deletion syndrome is currently recognized as a genetic model for understanding the development of schizophrenia.
Studies of children and adolescents with 22q11.2 deletion syndrome also show a heightened frequency of schizophrenia spectrum features and disorders, indicating that psychosis is a clinical characteristic of this syndrome even in pediatric groups (8, 12–25). These reports also highlight the high frequencies of anxiety disorders (40%−76%) and mood disorders (9%−35%), especially major depressive disorder, relative to the general population. Disorders typically diagnosed during childhood and adolescence have also been noted: attention deficit hyperactivity disorder (ADHD, 12%−68%), oppositional defiant disorder (9%−43%), and autism spectrum disorders (14%−50%). Other psychiatric disorders, such as substance use disorders or conduct disorder, have been less studied but do not appear to be unusually common in this population.
Despite elucidating several important aspects of the 22q11.2 deletion syndrome psychiatric phenotype, this body of evidence is inconsistent regarding the frequency of several disorders, probably because of the small number of subjects in most studies, differences in ascertainment sources, and cultural biases. Furthermore, we know of no study that has investigated the patterns of comorbidity across diagnostic categories and developmental stages in this population. Small numbers of subjects have largely precluded the examination of multiple age groups within the same study. To our knowledge, this was performed only in our earlier cross-sectional study on psychiatric morbidity in 22q11.2 deletion syndrome, which appears to be the largest to date (8). That two-center collaboration revealed that the prevalence of schizophrenia spectrum disorders linearly increased with age, while the frequency of mood disorders peaked in early adulthood. That study also demonstrated stability in the frequency of anxiety disorders and a linear decrease with age in the rates of ADHD and oppositional defiant disorder.
In the general population, the base rates for most disorders vary by sex. However, sex differences in the rates of psychiatric disorders remain largely unknown in 22q11.2 deletion syndrome. Preliminary data suggest that the sex distributions of some disorders may differ in this population from those typically observed (8, 24, 26). Finally, little is known about the impact of psychopathology on outcome in 22q11.2 deletion syndrome. Several studies indicated that schizophrenia spectrum disorders were associated with poorer outcome, whereas findings were less consistent for mood and anxiety disorders (22, 27, 28).
The International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome has gathered cohorts from North America, Europe, Australia, and the Middle East. Although the studies were developed independently, the participating sites have collaborated, sharing knowledge and data (1, 29). Here we report on the prevalence of psychiatric disorders across the lifespan in 1,402 individuals with 22q11.2 deletion syndrome. Patterns of comorbidity across diagnostic categories were also explored. Another goal was to identify whether the sex distributions of psychiatric disorders are similar to the pattern observed in the general population. Finally, we examined the association of psychiatric disorders, intellectual functioning, and adaptive functioning in a subgroup of individuals.
Method
Participants
Participants were recruited across 15 sites (see Table 1). They were included if they had a genetically confirmed 22q11.2 microdeletion, were between the ages of 6 and 70 years, and underwent a comprehensive structured psychiatric assessment using a validated instrument. The following exclusion criteria were applied: normal or inconclusive genetic testing (N=2), missing demographic information (N=64), age less than 6 years (N=9), nonstructured clinical psychiatric assessment (N=146), assessment for a single disorder (e.g., autism spectrum disorder) (N=27), and assessment with self- or parent-report questionnaire (N=342).
| Children Under Age 18 | ||||||
|---|---|---|---|---|---|---|
| Site (and Abbreviation) | Principal Investigators | N | Age Range (years) | N | % | Psychiatric Instruments Useda |
| Toronto (TOR) | A.S. Bassett, E.W.C. Chow | 216 | 7–68 | 61 | 28.24 | SCID (N=156), K-SADS (N=60), ADOS (if autism was suspected) (N=15) |
| Cardiff and Dublin (CAR/DU) | M.B.M. van den Bree, M. Owen, K.C. Murphy | 184 | 6–63 | 106 | 57.61 | CAPA (N=101), SCAN (N=83) |
| Syracuse, N.Y. (SYR) | W.R. Kates | 122 | 9–47 | 59 | 48.36 | K-SADS (N=96), SCID (N=26), ADI-R (N=109), adaptive and intellectual functioning data (N=87) |
| Philadelphia (PHL) | D.M. McDonald-McGinn, E.H. Zackai, R.E. Gur | 116 | 8–47 | 70 | 60.34 | K-SADS (N=116), SCID (mood and psychosis modules) (N=116), adaptive and intellectual functioning data (N=1) |
| Geneva (GVA) | S. Eliez | 114 | 6–44 | 88 | 77.19 | K-SADS psychosis supplement (N=114), DICA (N=88), SCID (N=26), adaptive and intellectual functioning data (N=90) |
| Utrecht (UCHT) | J. Vorstman | 110 | 9–26 | 81 | 73.64 | K-SADS (mood and psychosis modules) (N=110), ADI-R (N=110) |
| Tel Aviv (TA) | D. Gothelf | 105 | 6–55 | 58 | 55.24 | K-SADS (N=105), SCID (N=45), adaptive and intellectual functioning data (N=5) |
| Maastricht (MST) | T. Van Amelsvoort | 100 | 14–60 | 4 | 4.00 | MINI (N=74), Mini PAS-ADD (N=26) |
| Durham, N.C. (DRM) | V. Shashi | 85 | 6–19 | 82 | 96.47 | CDISC (N=85) |
| Los Angeles (LA) | C.E. Bearden | 68 | 6–61 | 51 | 75.00 | CDISC (N=51), SCID (N=49), ADI-R/ADOS (N=68) |
| Rome (RO) | M. Armando | 59 | 6–24 | 48 | 81.36 | K-SADS (N=59), ADI-R/ADOS (N=59) |
| London (LDN) | K.C. Murphy, D.G. Murphy | 49 | 6–16 | 49 | 100.00 | CAPA (N=49) |
| Atlanta (ATL) | O. Ousley | 29 | 14–29 | 16 | 55.17 | K-SADS (N=4), SCID (N=25), ADI-R/ADOS (N=29) |
| Newcastle, Australia (NCS) | L.E. Campbell | 25 | 12–21 | 13 | 52.00 | SCID (N=25), K-SADS (N=25) |
| Davis, Calif. (DVS) | T.J. Simon | 20 | 12–21 | 16 | 80.00 | K-SADS (N=20) |
| Total | 1,402 | 6–68 | 802 | 57.20 | ||
The final study group consisted of 1,402 participants ages 6–68 years (mean=18.78, SD=10.66) (Figure 1). Females were overrepresented (53.00% versus 47.00%, p=0.04), owing to a higher proportion among adults (N=600, 56.00% versus 44.00%, p=0.05), which was not the case in children and adolescents (N=802; 51.00% versus 49.00%, p=0.81). The study group was divided into five age groups: children (age 6–12 years, mean=9.50, SD=1.92; N=456), adolescents (age 13–17 years, mean=14.97, SD=1.37; N=346); emerging adults (age 18–25 years, mean=20.72, SD=2.18; N=323), young adults (age 26–35 years, mean=29.95, SD=2.95; N=150), and mature adults (age ≥36 years, mean=44.41, SD=7.13; N=127).
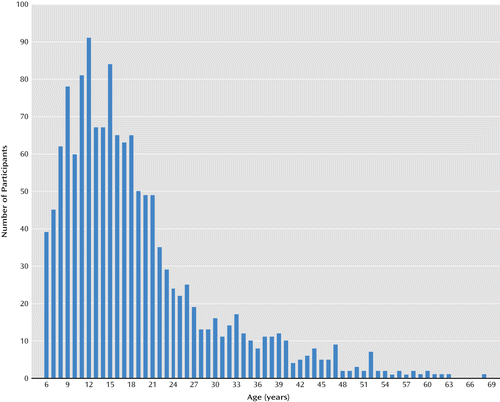
The ascertainment sources were heterogeneous: 40.22% of the patients were referred from nonmedical sources (28.10% through family association, 3.92% by relatives) or self-referred (8.20%), and 44.30% were referred by medical sources (department of genetics, 32.03%; psychiatry, 4.42%; cardiology, 5.49%; pediatrics, 2.35%). Referrals from departments of psychiatry were more frequent with increasing age (χ2=105.83, df=4, p<0.001).
Each site received approval from its local ethics committee (institutional review board). Each participant and his or her legal caregiver, where appropriate, gave written informed consent for study participation.
Psychiatric Diagnostic Assessment
Assessments were performed by using well-validated instruments widely used in psychiatric research (Table 1). Current diagnoses compatible with DSM-IV-TR diagnostic criteria were obtained. As participants’ age and assessment instruments varied, not all disorders were assessed for each cohort (see Table S1 in the data supplement accompanying the online version of this article). Schizophrenia spectrum, anxiety, and mood disorders were assessed in 1,292 participants (92.15% of the study group), and these findings were used for comorbidity analyses.
Intellectual and Adaptive Functioning
Measures of intellectual and adaptive functioning were available in a subgroup of 183 participants ages 6–24 years (mean=14.25, SD=4.55), representing 13.05% of the total group (Table 1). About half of the patients in this subgroup were female (N=93, 50.82%).
Intellectual functioning was assessed by using the Wechsler scales. As different versions of the scales were used, the full-scale IQ was used as a global measure of intellectual functioning. One child was assessed with the Wechsler Preschool and Primary Scale of Intelligence (WIPPSI-R); 123 individuals were assessed with the Wechsler Intelligence Scale for Children, either the WISC-R (N=1) or WISC-III (N=122); 56 were assessed with the Wechsler Adult Intelligence Scale (WAIS-III); and two were assessed with the Wechsler Abbreviated Scale of Intelligence (WASI). Adaptive functioning was assessed by using the Vineland Adaptive Behavior Scales, a semistructured interview conducted with the caregiver(s) of the participant. This scale provides age-adjusted standardized scores (mean=100, SD=15) in the domains of communication, daily living skills, and socialization (40).
Statistical Analyses
Prevalences of psychiatric disorders were compared in the five age groups by means of chi-square tests. Disorders typically diagnosed during childhood (i.e., ADHD, oppositional defiant disorder, conduct disorder, and autism spectrum disorders) were examined in a limited number of adults, and so adults were combined into a single group for these analyses. Binomial tests were used to identify sex differences in the rates of psychiatric disorders. Odds ratios were obtained to investigate patterns of comorbidity. Finally, the associations among psychiatric disorders, intellectual functioning, and adaptive functioning were examined by using analyses of covariance, Pearson correlations, and hierarchical regressions. For hierarchical regressions, one model was computed for each Vineland domain: age and full-scale IQ were entered in the first block and the presence of schizophrenia spectrum, anxiety, or mood disorders was entered in the second block. All statistical analyses were performed with SPSS version 19 (IBM, Armonk, N.Y.), except for calculation of odds ratios, which was performed by using the Medcalc online calculator (MedCalc Software, Ostend, Belgium; http://www.medcalc.org/).
Results
Prevalence of Psychiatric Disorders
Disorders typically diagnosed in childhood.
The rates of disorders typically diagnosed during childhood were examined in children, adolescents, and the combined adult groups (Table 2, bottom). A higher percentage of children than adolescents met the diagnostic criteria for ADHD. Similarly, the prevalence was higher in adolescents than in adults. A description of the ADHD subtype was available for 79.05% of the individuals with ADHD (200 of 253): 63.00% met the diagnostic criteria for the inattentive subtype, 6.50% for the hyperactive-impulsive subtype, and 30.50% for the combined subtype. ADHD was more prevalent in males (60.87%) than females (13.13%) (binomial test, p=0.001).
| Diagnosis | Children and Adolescents | Adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Children (6–12 Years) | Adolescents (13–17 Years) | Emerging Adults (18–25 Years) | Young Adults (26–35 Years) | Mature Adults (≥36 Years) | ||||||
| N | % | N | % | N | % | N | % | N | % | |
| Any schizophrenia spectrum disordera | 9/456 | 1.97 | 35/346 | 10.12 | 76/323 | 23.53 | 62/150 | 41.33 | 53/127 | 41.73 |
| Schizophrenia | 1/456 | 0.22 | 13/342 | 3.80 | 36/291 | 12.37 | 37/132 | 28.03 | 32/106 | 30.19 |
| Schizoaffective disorder | 0/456 | 0.00 | 3/342 | 0.88 | 5/291 | 1.72 | 10/132 | 7.58 | 4/106 | 3.77 |
| Schizophreniform disorder | 0/259 | 0.00 | 1/289 | 0.34 | 3/285 | 1.05 | 0/127 | 0.00 | 0/89 | 0.00 |
| Brief psychotic disorder | 0/259 | 0.00 | 4/289 | 1.38 | 1/288 | 0.35 | 1/131 | 0.76 | 0/106 | 0.00 |
| Psychotic disorder not otherwise specified | 8/456 | 1.75 | 14/346 | 4.05 | 29/323 | 8.98 | 14/150 | 9.33 | 17/126 | 13.49 |
| Delusional disorder | 0/456 | 0.00 | 0/346 | 0.00 | 2/323 | 0.62 | 0/150 | 0.00 | 0/127 | 0.00 |
| Any anxiety disorderb | 155/435 | 35.63 | 97/286 | 33.92 | 71/295 | 24.07 | 37/149 | 24.83 | 35/127 | 27.56 |
| Separation anxiety disorderc | 25/395 | 6.33 | 4/259 | 1.54 | 2/113 | 1.77 | 0/28 | 0.00 | 0/20 | 0.00 |
| Specific phobiad | 95/433 | 21.94 | 48/282 | 17.02 | 19/263 | 7.22 | 5/131 | 3.82 | 3/106 | 2.83 |
| Social phobiae | 45/435 | 10.34 | 28/286 | 9.79 | 14/295 | 4.75 | 4/149 | 2.68 | 1/127 | 0.79 |
| Panic disorderf | 4/333 | 1.20 | 2/231 | 0.87 | 17/270 | 6.30 | 12/137 | 8.76 | 17/118 | 14.41 |
| Posttraumatic stress disorder | 1/274 | 0.36 | 3/222 | 1.35 | 2/240 | 0.83 | 0/109 | 0.00 | 2/73 | 2.74 |
| Obsessive-compulsive disorder | 24/435 | 5.52 | 17/286 | 5.94 | 15/295 | 5.08 | 8/149 | 5.37 | 8/127 | 6.30 |
| Generalized anxiety disorder | 36/435 | 8.28 | 30/286 | 10.49 | 29/295 | 9.83 | 18/148 | 12.16 | 14/127 | 11.02 |
| Anxiety disorder not otherwise specified | 1/435 | 0.23 | 1/286 | 0.34 | 2/295 | 0.68 | 1/149 | 0.67 | 0/127 | 0.00 |
| Any mood disorder | 15/456 | 3.29 | 41/346 | 11.85 | 59/323 | 18.27 | 22/150 | 14.67 | 26/127 | 20.47 |
| Major depressive disorderg | 10/456 | 2.19 | 31/346 | 8.96 | 35/323 | 10.84 | 18/150 | 12.00 | 20/127 | 15.75 |
| Dysthymiah | 5/456 | 1.10 | 8/346 | 2.31 | 16/320 | 5.00 | 2/145 | 1.38 | 1/110 | 0.91 |
| Bipolar disorder or (hypo)manic episode in children | 0/318 | 0.00 | 2/317 | 0.32 | 6/320 | 1.88 | 3/150 | 2.00 | 5/127 | 3.94 |
| Mood disorder not otherwise specified | 0/456 | 0.00 | 4/346 | 1.16 | 7/323 | 2.17 | 0/150 | 0.00 | 2/127 | 1.57 |
| Substance-related disorder (substance abuse and dependence) | 0/300 | 0.00 | 1/221 | 0.45 | 7/278 | 2.52 | 9/142 | 6.34 | 5/110 | 4.55 |
| Children (6–12 Years) | Adolescents (13–17 Years) | Adults (≥18 years) | ||||||||
| N | % | N | % | N | % | |||||
| ADHDi | 161/434 | 37.10 | 63/264 | 23.86 | 29/186 | 15.59 | ||||
| Autism spectrum disordersj | 12/94 | 12.77 | 43/162 | 26.54 | 47/292 | 16.10 | ||||
| Any disruptive disorderk | 57/400 | 14.25 | 25/229 | 10.92 | 9/127 | 7.09 | ||||
| Oppositional defiant disorder | 57/400 | 14.25 | 25/229 | 14.79 | 7/115 | 6.09 | ||||
| Conduct disorder | 0/316 | 0.00 | 0/180 | 0.00 | 2/138 | 1.45 | ||||
Rates of disruptive disorders (oppositional defiant disorder and conduct disorder) remained stable with age, ranging between about 7% and 14% of cases depending on the age group. The majority of participants with a disruptive disorder (89 of 91) were diagnosed with oppositional defiant disorder. Disruptive disorders were more frequent in males (63.74%) than females (36.26%) (binomial test, p=0.02).
The prevalence of autism spectrum disorders (autistic disorder, Asperger's syndrome, and pervasive developmental disorder not otherwise specified) differed among age groups and was highest among adolescents. There was no significant gender difference (p=0.77).
Mood and anxiety disorders.
The frequency of major depressive disorder significantly increased with age, whereas dysthymia peaked in emerging adults (Table 2). Unipolar disorders (major depressive disorder and dysthymia) were overrepresented in females (61.18%) (binomial test, p=0.007). This difference was significant in adults (69.79% of females; binomial test, p<0.001) but not in children and adolescents (46.43% of females; p=0.69). Although the prevalence of bipolar disorders increased over the age groups, absolute numbers were small even in adults.
Anxiety disorders were common at all age groups (30.57% of the total group, 395 of 1,292) but were more frequent in children and adolescents than in the three adult groups. Across the entire study group, anxiety disorders were more frequent in females (57.22%) (binomial test, p=0.005). This difference was significant in adults (61.54% of females, binomial test, p=0.007) but not in children and adolescents (54.76% of females, p=0.15). Among the 395 participants with an anxiety disorder, 69.11% met the diagnostic criteria for one anxiety disorder, 22.03% for two, and 9.11% for three or more disorders. The rates of obsessive-compulsive disorder and generalized anxiety disorder were similar across age groups. The prevalences of specific phobia, social phobia, and separation anxiety disorder decreased with age. Panic disorder significantly increased with age. Posttraumatic stress disorder was rarely diagnosed (0.87% overall, eight of 918 participants).
Substance-related disorders.
The prevalence of substance-related disorders was low in this group (Table 2). Alcohol abuse or dependence was diagnosed in 13 of the 22 subjects with reported substance use disorders; drug use disorders were rare.
Schizophrenia spectrum disorders.
Schizophrenia spectrum disorders included the following disorders from the “schizophrenia and other psychotic disorders” section of the DSM-IV-TR: schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, brief psychotic disorder, and psychotic disorder not otherwise specified. Mood disorders with psychotic features (e.g., bipolar disorder with psychotic features) were not included in this category.
The prevalence of schizophrenia spectrum disorders significantly increased over the five age groups (Table 2). The prevalence of schizophrenia spectrum disorders was 23.53% in emerging adults and approximately 41% in the two groups over 25 years of age (Table 2, Figure 2). Findings were similar for schizophrenia and schizoaffective disorder combined (χ2=215.94, df=4, p<0.001), where the prevalence was 14.09% in emerging adults, 35.61% in young adults, and 33.96% in mature adults. There was no sex difference in the prevalence of psychotic disorders (p=0.24).
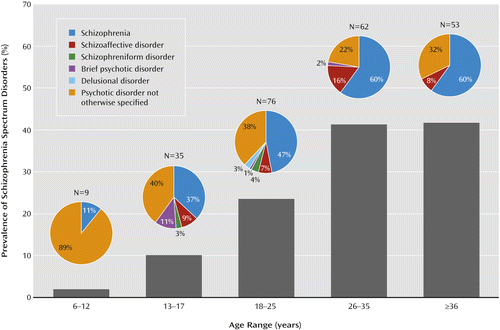
a Among the 235 subjects with schizophrenia spectrum disorders, the prevalence of a schizophrenia diagnosis increased significantly over the age groups (χ2=12.54, df=4, p=0.01), whereas the diagnosis of psychotic disorder not otherwise specified decreased (χ2=17.17, df=4, p=0.002).
The distributions of the different forms of psychotic disorders varied across age groups (Figure 2). Among the 235 subjects diagnosed with a psychotic disorder, the prevalence of a diagnosis of schizophrenia increased over the five age groups, whereas the diagnosis of psychotic disorder not otherwise specified decreased. The prevalence of schizoaffective disorder was not significantly different among age groups. Other psychotic disorders were rarely diagnosed.
Comorbidity
The patterns of comorbidity among schizophrenia spectrum, anxiety, and mood disorders revealed a significant association between mood and anxiety disorders in that the presence of an anxiety disorder increased the likelihood of a mood disorder, with an odds ratio of 2.50 (95% confidence interval [CI]: 1.75–3.57; p<0.001). Having an anxiety disorder also increased the likelihood of having comorbid diagnoses of both a mood disorder and a schizophrenia spectrum disorder (odds ratio: 6.07, 95% CI: 2.15–17.15; p<0.001). Similarly, being diagnosed with a mood disorder significantly increased the probability of having comorbid diagnoses of both an anxiety disorder and a schizophrenia spectrum disorder (odds ratio: 2.73, 95% CI: 1.43–5.22; p=0.002).
Associations Among Intellectual Functioning, Adaptive Functioning, and Psychiatric Disorders
Measures of intellectual and adaptive functioning were available for a subgroup of 183 participants ages 6–24 years (mean=14.25, SD=4.55; 50.82% female [N=93]), representing 13.05% of the total group.
The mean full-scale IQ was 71.25 (SD=12.13). There were 84 individuals (45.90%) with a full-scale IQ below 70, consistent with intellectual disability. The mean scores for the Vineland domains were approximately two standard deviations below the means of the general population (communication: mean=69.68, SD=14.14; daily living skills: mean=68.95, SD=16.38; socialization: mean=72.35, SD=14.68), corresponding to a moderate degree of impairment. Pearson correlations between full-scale IQ and the Vineland domains revealed significant and positive associations (p<0.001 in all cases). Intellectual functioning explained between 7.6% and 14.4% of the variance in adaptive functioning. Age was not significantly associated with intellectual functioning (r=0.06, p=0.42). Age was, however, negatively associated with the Vineland communication (r=−0.20, p=0.006) and socialization (r=−0.25, p=0.001) domains, and it was positively associated with the daily living skills domain (r=0.15, p<0.05).
One-way analyses of covariance with age as a covariate revealed that individuals with a schizophrenia spectrum disorder (N=10) had a significantly lower full-scale IQ than those without a schizophrenia spectrum disorder (F=14.06, df=1, 180, p<0.001). In contrast, individuals with an anxiety (N=74) or a mood (N=17) disorder did not differ from those without an anxiety or mood disorder, respectively, on full-scale IQ (p>0.05 in both cases).
Hierarchical regressions revealed that communication and socialization skills were significantly predicted by age and full-scale IQ (Table 3). Daily living skills were significantly predicted by full-scale IQ and the presence of an anxiety disorder. The presence of a schizophrenia spectrum disorder had a nearly significant effect.
| Hierarchical Regression Parameter | |||||||
|---|---|---|---|---|---|---|---|
| Model | Coefficient | ||||||
| Vineland Adaptive Behavior Scales Domain and Independent Variable | R2 | F (df=5, 177) | b | SE b | β | t | p |
| Communication domain | 0.26 | 12.23 | |||||
| Agea | –0.72 | 0.21 | –0.24 | –3.37 | 0.001 | ||
| Intellectual functioning (full-scale IQ)a | 0.44 | 0.08 | 0.39 | 5.73 | <0.001 | ||
| Presence of an anxiety disorder | –3.21 | 1.93 | –0.11 | –1.67 | 0.10 | ||
| Presence of a mood disorder | –0.38 | 3.18 | –0.01 | –0.12 | 0.91 | ||
| Presence of a schizophrenia spectrum disorder | –7.45 | 4.15 | –0.12 | –1.80 | 0.08 | ||
| Socialization domain | 0.17 | 7.16 | |||||
| Agea | –0.79 | 0.22 | –0.26 | –3.56 | <0.001 | ||
| Intellectual functioning (full-scale IQ)a | 0.33 | 0.08 | 0.29 | 4.06 | <0.001 | ||
| Presence of an anxiety disorder | –1.44 | 2.02 | –0.05 | –0.71 | 0.48 | ||
| Presence of a mood disorder | –2.40 | 3.34 | –0.05 | –0.72 | 0.48 | ||
| Presence of a schizophrenia spectrum disorder | –2.82 | 4.35 | –0.05 | –0.65 | 0.52 | ||
| Daily living skills domain | 0.14 | 5.60 | |||||
| Age | 0.40 | 0.27 | 0.11 | 1.48 | 0.15 | ||
| Intellectual functioning (full-scale IQ)a | 0.29 | 0.10 | 0.22 | 2.97 | 0.003 | ||
| Presence of an anxiety disordera | –5.23 | 2.42 | –0.16 | –2.16 | 0.04 | ||
| Presence of a mood disorder | 1.55 | 4.00 | 0.03 | 0.39 | 0.70 | ||
| Presence of a schizophrenia spectrum disorderb | –10.01 | 5.22 | –0.14 | –1.92 | 0.06 | ||
Discussion
The present study is an international collaborative investigation of 22q11.2 deletion syndrome, a genetic syndrome widely recognized as a model for schizophrenia. The inclusion of more than 1,400 participants makes it, to the best of our knowledge, the largest study to date on the frequency of psychiatric disorders throughout the lifespan.
Disorders Typically Diagnosed in Childhood
ADHD was diagnosed in 37.10% of children and was the most common diagnosis at this age. Although ADHD was less frequent in adults (15.59%), the rate was still higher than for adults in the general population (4.40%) (41). Consistent with findings in a recent longitudinal study (42), this suggests that ADHD persists until adulthood in many individuals with 22q11.2 deletion syndrome. In most cases the inattentive subtype prevailed, confirming findings from previous studies (43). This pattern contrasts with data from the general population and individuals with intellectual disability, which indicate a predominance of the combined subtype (44). It is still debated in the literature whether predominantly inattentive ADHD is a separate disorder, rather than a subtype of ADHD. Indeed, some data point toward specific neurobiological and environmental factors leading to the development of predominantly inattentive ADHD (45).
The prevalence of oppositional defiant disorder was slightly higher than in the general population but was similar to what has been described in youth with intellectual disability (46, 47). In our experience, individuals diagnosed with oppositional defiant disorder show a higher degree of impairment within the family, whereas they are often described as introverted by people outside the family. Future investigators may wish to compare these results with teacher reports of behavioral difficulties. Compared with oppositional defiant disorder, conduct disorder was rarely diagnosed, suggesting that severe externalizing disorders are underrepresented in 22q11.2 deletion syndrome.
Autism spectrum disorders were frequent in all age groups but peaked during adolescence. This was unexpected, as the prevalence is typically lower in adolescents than in younger groups from the general population. Several factors could explain this result. First, the prevalence of autism spectrum disorders was based on a subgroup of participants (N=548, 39% of the study group), as appropriate diagnostic instruments were used in seven cohorts only. This may have increased the variability of the results among the age groups. Second, the rate of autism spectrum disorders was highly variable among the cohorts, ranging from 0% (Rome cohort) to 80% (Maastricht cohort assessed with the Mini PAS-ADD). In the adolescent group, 72% of the participants diagnosed with autism spectrum disorders belonged to the Utrecht cohort, a group with a high rate of autism spectrum disorders (54%). The variation may be due to differences in ascertainment referral, intellectual functioning, and/or assessment tools. Indeed, only three cohorts based the diagnosis on both the Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule, which are the gold standards for the assessment of autism spectrum disorders. Third, it may be the case that at least a subgroup of adolescents with 22q11.2 deletion syndrome display a symptom pattern that resembles autism and is characterized in part by social deficits (48). This would be in agreement with previous findings from dimensional studies that showed impaired social functioning in children and adolescents with 22q11.2 deletion syndrome (22, 49). Also, a decrease in social functioning during adolescence may be an early marker for the development of psychosis in this population, as it is in the general population (50). Future studies are needed to better delineate the social phenotype in 22q11.2 deletion syndrome and its association with the onset of psychosis.
Altogether, these results indicate that 22q11.2 deletion syndrome is characterized by social deficits and attentional disturbances without prominent conduct disorder. This pattern of deficits has been previously described as part of the core neuropsychiatric phenotype of 22q11.2 deletion syndrome (51).
Mood and Anxiety Disorders
Anxiety disorders were more prevalent than mood disorders at all ages, especially in pediatric age groups. Furthermore, mood and anxiety disorders had a distinct developmental trajectory, consistent with findings in previous studies (6, 8). As in the general population, mood and anxiety disorders were common comorbidities.
Previous studies have suggested that mood disorders emerging in young adults may be related to difficulties achieving social integration and independence and/or deviant trajectories of brain maturation during adolescence in areas underlying mood regulation (8, 52). The prevalence of bipolar disorders does not appear to be higher in individuals with 22q11.2 deletion syndrome than in the general population, a pattern consistent with findings in previous smaller studies (6, 8, 14, 15, 19). It does contrast with very early reports of the psychiatric phenotype in 22q11.2 deletion syndrome, which indicated bipolar disorders in small cohorts (7, 53). It is possible that some individuals with 22q11.2 deletion syndrome experience periods of mood lability without meeting criteria for a full manic or hypomanic episode.
Anxiety disorders were especially prevalent among children and adolescents. Our data highlighted the severity of anxiety in at least some individuals, as one-third of the participants with an anxiety disorder qualified for multiple anxiety disorders. Generalized anxiety disorder, specific phobia, and social phobia were the most frequent anxiety disorders during childhood and adolescence. Whereas the rate of specific phobia is similar to that in individuals with intellectual disability, social phobia and generalized anxiety disorder appear to be overrepresented in 22q11.2 deletion syndrome (46). This finding, combined with the high rate of autism spectrum disorders, indicates that difficulties in the social domain may represent key characteristics of the syndrome (51). A novel finding relates to the high rate of panic disorder among adults (14% in mature adults). Some authors have suggested that panic disorder may be associated with the development of schizophrenia in the general population (54). This warrants further investigation in 22q11.2 deletion syndrome.
Schizophrenia Spectrum Disorders
The results are in accordance with previous studies showing that a 22q11.2 deletion is the strongest known molecular risk factor for schizophrenia. Indeed, the rates of psychotic disorders in the present study are some of the highest reported to date. The combined rate of schizophrenia and schizoaffective disorder was commensurate with previous findings (8, 9, 14–16, 18, 24), although it is still higher than for individuals with 22q11.2 deletion syndrome ascertained in a nonpsychiatric setting (6).
The 10% of adolescents studied who qualified for a diagnosis of a psychotic disorder indicates that early-onset psychosis is relatively common in individuals with 22q11.2 deletion syndrome who are referred for psychiatric research studies. Indeed, in a recent study the mean age at onset of psychotic disorders in youths with 22q11.2 deletion syndrome was 17.7 years (55). Psychotic disorder not otherwise specified was a somewhat more common diagnosis in children and adolescents than was schizophrenia. This may be because some young subjects do not meet the duration and/or severity criteria for a schizophrenia diagnosis and/or because of some reluctance to diagnose children with schizophrenia.
Sex Differences
Sex differences in the rates of several psychiatric disorders were comparable to those reported in the general population. Specifically, disruptive disorders and ADHD were more frequent in males than females, as documented in the general population (56, 57). There was a predominance of females among those diagnosed with anxiety and mood disorders as adults. Numerous studies have observed that sex differences in the rate of internalizing disorders emerge around puberty (58), suggesting the possible impact of hormonal changes in the development of affective symptoms in females.
In contrast, the male predominance of autism spectrum disorders and to a lesser extent psychotic disorders in the general population was not observed in 22q11.2 deletion syndrome. This may be related to the strong genetic contribution to the pathogenesis of social deficits and psychosis in 22q11.2 deletion syndrome and deserves investigation in future studies.
Intellectual Functioning, Adaptive Functioning, and Psychiatric Disorders
The associations among these variables were examined in a subgroup of participants with 22q11.2 deletion syndrome (13%). This subgroup did not include adults age 25 or older, preventing the generalizability of the findings to older adults with the syndrome (28). The results showed that the presence of schizophrenia spectrum disorders was associated with lower intellectual functioning, whereas anxiety and mood disorders were not. This is consistent with findings from the general population showing that schizophrenia spectrum disorders are associated with broad cognitive impairments (59).
On average, adaptive functioning scores were comparable to full-scale IQ in this subgroup (approximately two standard deviations below the means for the general population) and were significantly associated with the intellectual level. However, intellectual functioning explained only a small proportion of variance in adaptive functioning in this group of youths. This is consistent with a finding of no significant effect of IQ on adaptive functioning in a study of 78 children (25) but contrasts with the strong association between intellect and adaptive functioning in 100 adults with 22q11.2 deletion syndrome (28). The current study and these two previous studies also indicate that other factors contribute to adaptive functioning in this population. We found that older age was significantly associated with poorer socialization and communication skills, but psychotic, anxiety, or mood disorders did not affect socialization or communication. Longitudinal studies would be needed to determine if there is functional decline in some individuals and whether these deficits may precede the onset of psychiatric disorder. Other pathways could also lead to social and communication impairments in 22q11.2 deletion syndrome. Indeed, it has been shown that some cerebral regions involved in the development of social competence are altered and have an atypical developmental trajectory in 22q11.2 deletion syndrome (52). It is also possible that children with this condition may be socially motivated but fail to interact in a rewarding manner, leading to a more socially avoidant style and a reduction of social motivation and communication. This may explain the higher degree of social impairment during adolescence and adulthood.
Contrary to the results observed for communication and socialization skills, we found that, when the effects of age and full-scale IQ were controlled for, the presence of anxiety disorders was associated with poorer daily living skills and schizophrenia spectrum disorders had a nearly significant relationship, whereas mood disorders were not related to daily living skills. These relationships are consistent with previous findings and highlight the need for reducing the burden associated with psychiatric morbidity in 22q11.2 deletion syndrome (27, 28).
Suggestions for Clinical Management
The results suggest that in school-age children with 22q11.2 deletion syndrome, special emphasis is needed with regard to the diagnosis and management of attentional deficits, as these can interfere with learning and academic achievement. The presence of attentional deficits should be screened systematically and at regular intervals from the transition to elementary school onward (1). When ADHD is diagnosed, standard management options should be offered. Stimulants, as one of the treatment options, are effective and relatively well tolerated in children with 22q11.2 deletion syndrome (43), despite official warnings by health authorities against its use in individuals with cardiac anomalies. Monitoring of cardiovascular status during treatment should, however, be conducted. Learning may also be maximized by adjusting the child’s school environment (small group teaching, promoting a quiet and highly structured environment).
The emergence of social deficits during adolescence can represent a major source of disability in some individuals with 22q11.2 deletion syndrome. Interventions focusing on appropriate adaptation of social demands, such as introducing “buddies” as partners and mediators in social contacts, and stimulating participation in (structured) social activities should be considered. Future studies are needed to investigate the utility of sociocognitive remediation programs and cognitive-behavioral therapy to improve social skills.
The results also indicate that, as part of anticipatory care, individuals with 22q11.2 deletion syndrome should be screened for anxiety and mood disorders throughout their lifetimes. It is possible that successful management of anxiety and depression may be helpful in preventing or delaying the onset of psychotic disorders and reducing the severity of psychotic symptoms (18, 55). Recommended treatment strategies include effective cognitive-behavioral and pharmacological approaches.
Given the markedly elevated risk for schizophrenia spectrum disorders, individuals with 22q11.2 deletion syndrome should be closely monitored for prodromal and full-blown symptoms of psychosis. Negative symptoms being highly prevalent in adolescents with 22q11.2 deletion syndrome (49), future studies should investigate the usefulness of omega-3 polyunsaturated fatty acids in the reduction of prodromal symptoms (60). Clinically significant psychotic symptoms should be treated in accordance with the most recent recommendations for effective management. Antipsychotic medications are mainstays of treatment for schizophrenia spectrum disorders, as are psychoeducation, rehabilitation, and active stress reduction strategies.
In all cases, careful attention to and treatment of the comorbid medical conditions associated with 22q11.2 deletion syndrome that can mimic psychiatric illness or medication side effects, including hypocalcemia, hypothyroidism, and seizures, will help in optimizing psychiatric management (1).
Limitations and Conclusions
There are a number of limitations to this study. First, it is unknown how representative data on psychiatric research participants, even those in such a large consortium, would be for a true epidemiologic sample of people with 22q11.2 deletion syndrome. Children and adolescents were overrepresented in this study group, likely because most research groups in the field of 22q11.2 deletion syndrome predominantly recruit children and adolescents with the syndrome and because it is known to be particularly underrecognized in adults (1). Indeed, the two oldest age groups of adults studied were about half the size of the three youngest age groups. In addition, this is a cross-sectional study and there is a need for large-scale longitudinal studies to investigate the trajectories of psychiatric disorders from childhood to adulthood. Second, the reported rates of psychiatric disorders were based on different instruments across sites. For the small minority of subjects with severe intellectual disability, use of assessment tools tailored to persons with severe intellectual disability may be needed, in addition to the information from multiple informants and sources (parents, caregivers, and medical records) that is essential to the evaluation of any patient. Furthermore, no cross-site diagnostic reliability assessment was performed, and lack of such a measure could inflate the variability of the results. Third, only results for psychiatric disorders meeting diagnostic criteria were presented. Future studies that include a dimensional examination of symptoms may better capture the full spectrum of psychopathology. Additional studies of at-risk mental states in this population would be particularly relevant. Finally, several important variables could not be taken into account in the results related to prevalence of psychiatric disorders. In particular, intellectual level, the presence of medical conditions, and treatment status were not available for all participants, although these factors, particularly intellectual level, may have significantly influenced the results. Also, information regarding the exact break points of the 22q11.2 deletion for each participant was not available for this study, which prevented examination of the influence of deletion size on the psychiatric phenotype. However, previous studies have shown that the vast majority of individuals with 22q11.2 deletion syndrome have the typical 3-Mb 22q11.2 deletion, that there is no convincing evidence that phenotypic expression is related to the length of the deletion, and that there is no difference in the prevalence of the typical deletion between those with and without major psychiatric disorders, such as schizophrenia (1). The strengths of the study include the collaborative nature of the work, the large number of subjects, and the wide age range of the participants.
In conclusion, this study provides the prevalence of psychiatric disorders from childhood to adulthood in a group of more than 1,400 individuals with 22q11.2 deletion syndrome, a genetic condition associated with one of the highest risks known for developing schizophrenia. Such large-scale studies promise to help improve knowledge about 22q11.2 deletion syndrome and in the future will provide data on key modifiers of psychiatric expression that could be generalizable to all forms of psychiatric illness.
1 ;
2 : Cognitive development in children with 22q11.2 deletion syndrome. Br J Psychiatry 2012; 200:462–468Crossref, Medline, Google Scholar
3 : Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychol 1999; 5:230–241Crossref, Medline, Google Scholar
4 : Intellectual abilities in a large sample of children with velo-cardio-facial syndrome: an update. J Intellect Disabil Res 2007; 51:666–670Crossref, Medline, Google Scholar
5 : The velo-cardio-facial syndrome: the spectrum of psychiatric problems and cognitive deterioration at adult age. Genet Couns 2009; 20:307–315Medline, Google Scholar
6 : Elevated prevalence of generalized anxiety disorder in adults with 22q11.2 deletion syndrome (letter). Am J Psychiatry 2010; 167:998Link, Google Scholar
7 : Clinical characteristics of schizophrenia associated with velo-cardio-facial syndrome. Schizophr Res 1999; 35:105–112Crossref, Medline, Google Scholar
8 : Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry 2009; 48:1060–1068Crossref, Medline, Google Scholar
9 : High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 1999; 56:940–945Crossref, Medline, Google Scholar
10 : Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis 1994; 182:476–478Crossref, Medline, Google Scholar
11 : Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet 1992; 42:141–142Crossref, Medline, Google Scholar
12 : Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med (Epub ahead of print, Sept 9, 2013)Google Scholar
13 : Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion). J Autism Dev Disord 2007; 37:1776–1786Crossref, Medline, Google Scholar
14 : ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry 2006; 45:596–603Crossref, Medline, Google Scholar
15 : Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry 2010; 49:333–344Medline, Google Scholar
16 : Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br J Psychiatry 2005; 186:115–120Crossref, Medline, Google Scholar
17 : Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry 2002; 51:312–318Crossref, Medline, Google Scholar
18 : Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry 2007; 164:663–669Link, Google Scholar
19 : Affective disorders and other psychiatric diagnoses in children and adolescents with 22q11.2 deletion syndrome. J Affect Disord 2009; 119:177–180Crossref, Medline, Google Scholar
20 : Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet 2007; 144B:27–36Crossref, Medline, Google Scholar
21 : Chromosome 22q11 deletion syndrome (CATCH 22): neuropsychiatric and neuropsychological aspects. Dev Med Child Neurol 2002; 44:44–50Crossref, Medline, Google Scholar
22 : Social skills and associated psychopathology in children with chromosome 22q11.2 deletion syndrome: implications for interventions. J Intellect Disabil Res 2012; 56:865–878Crossref, Medline, Google Scholar
23 : Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res 2010; 118:118–121Crossref, Medline, Google Scholar
24 : The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry 2006; 45:1104–1113Crossref, Medline, Google Scholar
25 : Discordance in diagnoses and treatment of psychiatric disorders in children and adolescents with 22q11.2 deletion syndrome. Asian J Psychiatr 2011; 4:119–124Crossref, Medline, Google Scholar
26 : Sex differences in the behavior of children with the 22q11 deletion syndrome. Psychiatry Res 2009; 166:24–34Crossref, Medline, Google Scholar
27 : An examination of the relationship of anxiety and intelligence to adaptive functioning in children with chromosome 22q11.2 deletion syndrome. J Dev Behav Pediatr 2012; 33:713–720Crossref, Medline, Google Scholar
28 : Functional outcomes of adults with 22q11.2 deletion syndrome. Genet Med 2012; 14:836–843Crossref, Medline, Google Scholar
29 : Enhanced maternal origin of the 22q11.2 deletion in velocardiofacial and DiGeorge syndromes. Am J Hum Genet 2013; 92:439–447; correction, 92:637Crossref, Medline, Google Scholar
30 : The Child and Adolescent Psychiatric Assessment (CAPA). Psychol Med 1995; 25:739–753Crossref, Medline, Google Scholar
31 : SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
32 : Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I). Washington, DC, American Psychiatric Association, 1996Google Scholar
33 : Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 1989; 19:185–212Crossref, Medline, Google Scholar
34 : Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
35 : Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
36 : Diagnostic Interview for Children and Adolescents (DICA). J Am Acad Child Adolesc Psychiatry 2000; 39:59–66Crossref, Medline, Google Scholar
37 : The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 1997; 12:232–241Crossref, Google Scholar
38 : Reliability and validity of the Mini PAS-ADD for assessing psychiatric disorders in adults with intellectual disability. J Intellect Disabil Res 1998; 42:264–272Crossref, Medline, Google Scholar
39 : The Diagnostic Interview Schedule for Children–Revised Version (DISC-R), I: preparation, field testing, interrater reliability, and acceptability. J Am Acad Child Adolesc Psychiatry 1993; 32:643–650Crossref, Medline, Google Scholar
40 : Vineland Adaptive Behavior Scales. Circle Pines, Minn, American Guidance Service, 1984Google Scholar
41 : The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723Link, Google Scholar
42 : The longitudinal course of attention deficit/hyperactivity disorder in velo-cardio-facial syndrome. J Pediatr 2013; 163:187–193.e1Crossref, Medline, Google Scholar
43 : The effect of methylphenidate on prefrontal cognitive functioning, inattention, and hyperactivity in velocardiofacial syndrome. J Child Adolesc Psychopharmacol 2011; 21:589–595Crossref, Medline, Google Scholar
44 : Intellectual disability in children with attention deficit hyperactivity disorder. J Pediatr 2013; 163:890–895.e1Crossref, Medline, Google Scholar
45 : Is the inattentive subtype of ADHD different from the combined/hyperactive subtype? J Atten Disord 2010; 13:649–657Crossref, Medline, Google Scholar
46 : DSM-IV disorders in children with borderline to moderate intellectual disability. I: prevalence and impact. J Am Acad Child Adolesc Psychiatry 2003; 42:915–922Crossref, Medline, Google Scholar
47 : Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry 2012; 69:372–380Crossref, Medline, Google Scholar
48 : Social impairments in chromosome 22q11.2 deletion syndrome (22q11.2DS): autism spectrum disorder or a different endophenotype? J Autism Dev Disord (Epub ahead of print, Sept 18, 2013)Medline, Google Scholar
49 : Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res 2012; 196:277–284Crossref, Medline, Google Scholar
50 : Premorbid adjustment and schizophrenia in individuals with 22q11.2 deletion syndrome. Schizophr Res 2013; 151:221–225Crossref, Medline, Google Scholar
51 : Is there a core neuropsychiatric phenotype in 22q11.2 deletion syndrome? Curr Opin Neurol 2012; 25:131–137Crossref, Medline, Google Scholar
52 : 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci 2010; 11:402–416Crossref, Medline, Google Scholar
53 : Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry 1996; 153:1541–1547Link, Google Scholar
54 : Treatment of comorbid panic disorder and schizophrenia: evidence for a panic psychosis. Psychiatr Ann 2000; 30:29–33Crossref, Google Scholar
55 : Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry 2013; 52:1192–1203.e3Crossref, Medline, Google Scholar
56 : Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. J Child Psychol Psychiatry 2004; 45:609–621Crossref, Medline, Google Scholar
57 : Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics 2010; 125:75–81Crossref, Medline, Google Scholar
58 : Puberty and the emergence of gender differences in psychopathology. J Adolesc Health 2002; 30(4 suppl):49–58Crossref, Medline, Google Scholar
59 : Intellectual functioning in schizophrenia: a marker of neurodevelopmental damage? J Intellect Disabil Res 2004; 48(part 6):519–523Crossref, Medline, Google Scholar
60 : Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders. Arch Gen Psychiatry 2010; 67:146–154Crossref, Medline, Google Scholar



