Repeated Δ9-Tetrahydrocannabinol Exposure in Adolescent Monkeys: Persistent Effects Selective for Spatial Working Memory
Abstract
Objective
Epidemiological findings suggest that, relative to adults, adolescents are more vulnerable to the adverse persistent effects of cannabis on working memory. However, the potential confounds inherent in human studies preclude direct determination of a cause-and-effect relationship between adolescent cannabis use and heightened susceptibility to persistent working memory impairments. Consequently, the authors examined the effects of repeated exposure to Δ9-tetrahydrocannabinol (THC) on performance of spatial and object working memory tasks in adolescent monkeys.
Method
Seven pairs of male adolescent rhesus monkeys, matched for baseline cognitive performance, received vehicle or THC intravenously 5 days/week for 6 months. Performance on spatial and object memory tasks was assessed 23 or 71 hours after drug administration throughout the study. In addition, acute effects on working memory were also assessed at the beginning and end of the 6-month period.
Results
Relative to the vehicle-exposed control animals, those with repeated THC exposure had a blunted trajectory of accuracy improvements on the spatial working memory task in a delay-dependent manner. Accuracy improvements on the object working memory task did not differ between groups. Relative to the acute effects of THC on working memory at the beginning of the study, neither sensitivity nor tolerance was evident after 6 months of THC exposure.
Conclusions
Because maturation of performance is later for spatial than for object working memory, these findings suggest that persistent effects of THC on cognitive abilities are more evident when exposure coincides with the developmental stage during which the underlying neural circuits are actively maturing.
Working memory impairments are well-established acute effects (i.e., occurring within 6 hours of exposure) of cannabis in adult users (1), and these impairments are associated with altered activation of the prefrontal cortex (2–4). However, persistent effects (i.e., occurring 7 hours to 20 days after exposure) of cannabis on working memory appear less consistently in adult users than in adolescent users (5–8). Initiation of cannabis use during adolescence has also been associated with lower IQ in adulthood (9) and an increased risk for the later appearance of schizophrenia (10), a disorder characterized by working memory impairments (11). Although these studies suggest that adolescents may be more vulnerable to the adverse effects of cannabis on executive functions, the prevalence of cannabis use among youths aged 12 to 17 continues to increase (12).
Studies in monkeys provide the opportunity to control for individual and environmental factors that can affect working memory performance, as well as the ability to examine the effects of repeated THC administration in a controlled manner. We examined in adolescent rhesus monkeys whether 6 months of repeated intravenous THC administration, compared with vehicle administration, would 1) result in persistent adverse effects on performance improvements of spatial versus object working memory tasks and 2) alter sensitivity to the acute effects of THC on these tasks.
Method
Subjects
Experimentally naive Chinese-origin rhesus monkeys (Macaca mulatta), 14–18 months of age, were obtained as a cohort (N=14) and singly housed in cages with environmental enrichment in the same room. Because of group size limitations, only males were studied to minimize sources of variance, such as potential differences due to gender. At a mean age of 23.9 (SD=0.6) months, the animals were placed on a water-regulation regimen and trained to respond to cues on a touch-screen monitor, as previously described (24). None of the monkeys showed evidence of dehydration at any time, and all of the monkeys gained weight (∼0.1 kg/month) throughout the study period. All procedures were conducted in accordance with U.S. Department of Agriculture and National Institutes of Health guidelines and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Working Memory Tasks
Details of the tasks were described previously (21). Briefly, the working memory and control trials for both the spatial and object memory tasks (Figure 1) had delays of 1, 4, 8, and 16 seconds. The spatial memory task was always performed before the object memory task to ensure that floor effects, which could be induced by partial satiation associated with water reinforcement, did not confound the spatial memory measures. In an effort to offset a potential order effect, the object memory task had two reinforcement conditions, distinguished by color. The animals were paired to counterbalance assignments to THC or vehicle (see below); therefore, blue or yellow objects indicated double reinforcement for three pairs of monkeys while red or green objects indicated double reinforcement for the other four pairs of monkeys. Baseline accuracy on the spatial memory and double-reinforcement object memory trials was better than on the single-reinforcement object memory trials, demonstrating the effectiveness of this design. For both tasks, the trials were divided evenly into two blocks and within each block, all delays, reinforcement conditions, and working memory and control trials were randomized.
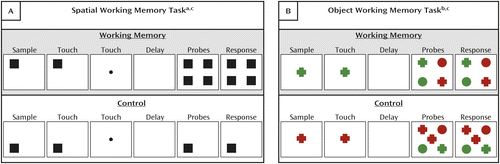
a In the spatial working memory trials (part A, top), a sample stimulus appeared at one of the four corners of the touch screen. The monkey had to touch it. Immediately following this response, a fixation cue stimulus appeared at the center of the screen. The monkey had to touch the fixation cue stimulus and thus could not remember the target location by continuing to touch it. A randomly selected delay (1, 4, 8, or 16 seconds) ensued. At the end of the delay period, choice probes appeared at each corner of the screen. The monkey had to touch the probe at the location occupied by the sample stimulus appearing earlier in the trial. Spatial memory control trials (part A, bottom) were distinguished by the appearance of a single probe at the same location occupied by the sample stimulus appearing earlier in the trial.
b In the object working memory trials (part B, top), a sample stimulus appeared at the center of the touch screen. The monkey had to touch it. A randomly selected delay (1, 4, 8, or 16 seconds) ensued. At the end of the delay period, choice probes appeared at the corners of the screen, distinct in color and shape. The monkey had to touch the probe that matched the sample stimulus appearing earlier in the trial. Object memory control trials (part B, bottom) were distinguished by the reappearance of the sample stimulus at the center of the screen. In an effort to offset a potential order effect, the object memory task had two reinforcement conditions, distinguished by color. The animals were paired to counterbalance assignments to THC or vehicle; therefore, blue or yellow objects indicated double reinforcement for three pairs of monkeys while red or green objects indicated double reinforcement for the other four pairs of monkeys.
c For all trials and both tasks, the monkeys were allowed 20 seconds to respond to the sample stimulus and 20 seconds to respond to the choice probes. If a monkey failed to respond during the allotted times, the trial was recorded as an omission.
THC and Vehicle Administration
At least 2 months before THC or vehicle administration, a polyethylene catheter was inserted into a jugular vein, tunneled subcutaneously, and attached to a vascular access port at the center of the animal’s back (24). Monkeys resumed training following a 10-day recovery period.
The National Institute on Drug Abuse’s Drug Supply Program provided THC (in 100% ethanol). Each day (∼15 minutes before administration), the ethanol was evaporated under a stream of purified nitrogen and the THC was suspended in a vehicle containing ∼1% Tween-80 and 0.9% saline (25). The THC and vehicle solutions were stored in light-protected test tubes on ice until administered through the indwelling vascular access ports.
Study Design
Figure 2 shows the number of weeks each monkey pair participated in each of the four study periods (baseline, acute 1, repeated dosing, and acute 2). At a mean age of 27.6 months (SD=1.1), the monkeys began 4–5 weeks of the baseline training period, during which no THC was administered (24). Performance during the last week of the baseline period (week 0 of the persistent effects study) was used to counterbalance group assignments, which ensured that the mean levels of baseline performance accuracy were not significantly different between the THC and vehicle groups on the spatial memory trials (t=0.47, df=12, p=0.64), double-reinforcement object memory trials (t=−0.94, df=12, p=0.36), or single-reinforcement object memory trials (t=−1.04, df=12, p=0.32) (21).
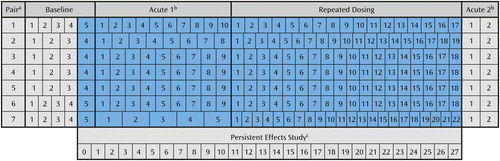
a Within each pair, one monkey received THC and the other received vehicle.
b Acute effects were determined by assessing performance immediately before and 30 minutes after administration of THC or vehicle.
c Persistent effects were determined by assessing performance 23 or 71 hours after administration of THC or vehicle.
During week 1 of the persistent effects study, at a mean age of 28.6 months (SD=1.2), the monkeys began 5–10 weeks of acute period 1, during which various doses of THC (15–240 μg/kg, generally in an ascending order) or vehicle were administered (for details, see reference 21 and the methodological information in the data supplement accompanying the online version of this article). During acute period 1 (and acute period 2) working memory performance was measured immediately before and 30 minutes after administration of THC or vehicle. Acute period 1 revealed the dose at which each monkey in the THC group became acutely intoxicated, as reflected by a marked decline of performance across tasks. On the basis of these findings, during the 17–22 weeks of the repeated-dosing period the monkeys assigned to the THC group received either 120 (N=3) or 240 (N=4) μg/kg of THC 5 days a week. For weeks 1–27 of the persistent effects study, working memory performance was measured 23 hours (Tuesday–Friday) or 71 hours (Monday) after the most recent administration of THC or vehicle. At week 28, the monkeys began 2 weeks of acute testing (acute period 2), during which two doses of THC (120 and 240 μg/kg), common to acute period 1 and the repeated-dosing period, were administered.
Statistical Analyses
For each task and delay, seven performance measures were obtained. Results for the primary measure of interest, working memory accuracy rate (correct trials as a percentage of those completed), are reported here. The remaining measures (initiation latency, control trial accuracy rate, and completion rate and reaction latency for both working memory and control trials) are presented in the online data supplement for the spatial working memory task.
The primary analysis was done separately for each task and delay by week because no significant effects of day were detected in preliminary analyses. For accuracy rates, the number of correct trials out of completed trials was modeled by a binomial distribution with a logit link function and with monkey treated as a normal random effect. A subsequent summary measure, area under the curve (AUC), for accuracy rates was computed as the weekly average of the daily AUC: 1.5 multiplied by the accuracy rate at the 1-second delay, plus 3.5 multiplied by the accuracy rate at the 4-second delay, plus 6 multiplied by the accuracy rate at the 8-second delay, plus 4 multiplied by the accuracy rate at the 16-second delay. AUC was assumed to follow a normal distribution, and monkey was treated as a normal random effect. Based on the observed weekly accuracy rates (Figure 3 parts A, C, and E), a segmented linear model over weeks was implemented, with response at baseline (week 0) used as a covariate. As illustrated in Supplemental Figure 1 (in the online data supplement), two connected line segments (referred to subsequently as phase 1 and phase 2) were used to model the logit of response and the AUC (online Supplemental Figures 2A and 2B show the goodness of fit for the segmented line in comparison to the smoothed data). A second model that combined delays was implemented to allow comparisons across delays.
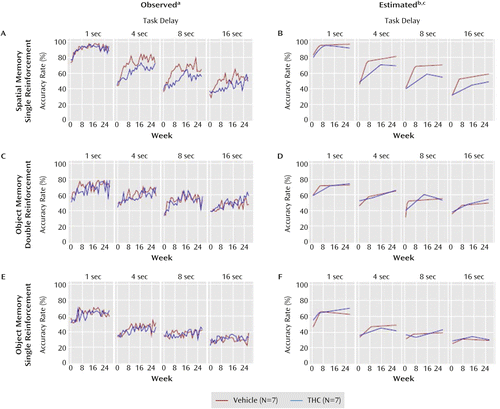
a Left panels show the mean observed working memory accuracy rates by week and delay for the vehicle and THC groups on the spatial task (panel A) and on the double-reinforcement (panel C) and single-reinforcement (panel E) trials of the object task.
b Right panels show the mean estimated working memory accuracy rates derived from the two-phase statistical models of the observed data for the spatial task (panel B) and for the double-reinforcement (panel D) and single-reinforcement (panel F) trials of the object task.
c For both groups on both tasks and both object reinforcement conditions, accuracy rates increased for an initial period of time (phase 1) and then reached a point (change point) before the rate of increase substantially slowed, flattened, or slightly declined (phase 2).
The primary analyses allowed separate segmented linear models fitted to the THC group and to the vehicle group for both logit of weekly accuracy rates and also for weekly AUCs. Each model had an initial slope representing linear accuracy improvement (phase 1) followed at a particular change point by a possibly different slope of linear accuracy improvement (phase 2) for the remainder of the 27 weeks. The parameters for each model, taking into account the repeated observations within each monkey over the weeks of the study, were estimated by the method of maximum likelihood, which is a commonly used, effective method of estimation in complex models. These estimates provided the basis for Figures 3 and 4, as well as the basis for related inference. The maximum likelihood estimation for this nonlinear repeated-effects model was implemented in SAS with the NLMIXED procedure (SAS Institute, Cary, N.C.). A more detailed justification of this method is given in the online data supplement, which also contains additional study details.
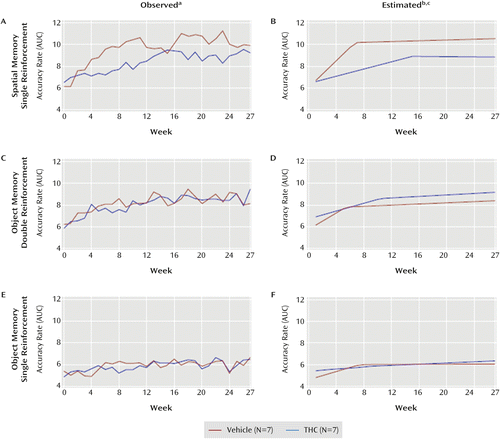
a Left panels show the mean observed area under the delay curves by week for the vehicle and THC groups on the spatial task (panel A) and on the double-reinforcement (panel C) and single-reinforcement (panel E) trials of the object task.
b Right panels show the mean estimated area under the delay curves derived from the two-phase statistical models of the observed data for the spatial task (panel B) and the double-reinforcement (panel D) and single-reinforcement (panel F) trials of the object task.
c For both groups on both tasks and both object reinforcement conditions, accuracy rates increased for an initial period of time (phase 1) and then reached a point (change point) before the rate of increase substantially slowed, flattened, or slightly declined (phase 2).
Analysis of the second acute period was similar to that used for the first acute period (21). The percentage change of accuracy rates, from before to after THC or vehicle administration, was computed for the THC doses administered repeatedly and was assumed to follow a normal distribution, while period, group, delay, and their interactions were treated as fixed effects.
Results
Persistent Effects
Figure 3 parts A and B display observed and estimated accuracy rates over time for the spatial working memory trials. Accuracy rates increased significantly for both groups on all delays during phase 1 (t≥4.83, df=13, p≤0.001 in all cases). However, the improvement during phase 1 was significantly slower for the THC group on the 4-, 8-, and 16-second delays (t≤–4.16, df=13, p≤0.001 in all cases). Additionally, the length of phase 1 was significantly longer for the THC group by 3.2, 9.5, and 8.4 weeks for the 1-second (t=2.60, df=13, p=0.03), 4-second (t=5.10, df=13, p<0.001), and 8-second (t=4.34, df=13, p=0.001) delays, respectively. For the 16-second delay, the difference was 9.0 weeks (t=1.59, df=13, p=0.14). During phase 2 the only significant slope effects were on the 1-second delay for the THC group, which was negative (t=–2.37, df=13, p=0.04), and on the 4-second delay for the vehicle group, which was positive (t=2.67, df=13, p=0.02). The estimated difference between the THC and vehicle groups at the change point (Figure 3 part B) was negative for all delays but not significant (p>0.14 in all cases), and the phase 2 slopes (i.e., the rate of change in accuracy between the change point and week 27) did not significantly differ for the 4-, 8-, or 16-second delay (p>0.19 in each case). However, for the 1-second delay the difference was significant (t=−2.63, df=13, p=0.03).
Figure 4 parts A and B display observed and estimated AUCs for the spatial task. Compared with vehicle, THC 1) significantly slowed the rate of improvement during phase 1 (t=–2.76, df=12, p=0.02), 2) significantly extended (by 8.4 weeks) the length of phase 1 (t=2.59, df=12, p=0.03), 3) significantly lowered accuracy at the change point between phases (t=–2.60, df=12, p=0.03), but 4) did not significantly affect phase 2 slopes (p=0.71).
In order to compare performance across delays, we fit a model that included all four delays. The difference between change points was significantly smaller for the 1-second delay than for the 4-second (t=2.53, df=55, p=0.02) and 8-second (t=2.43, df=55, p=0.02) delays. For technical reasons, the 16-second delays could not be compared (see the supplemental results in the online data supplement).
Figure 3 parts C and D display observed and estimated accuracy rates for the double-reinforcement object working memory trials. Accuracy rates increased significantly (t≥2.27, df=13, p≤0.04 in all cases) for both groups during phase 1 on all delays except the 1-second delay for the vehicle group (t=2.14, df=13, p=0.06) and the 4-second delay for the THC group (p=0.24). The only significant difference between groups was on the 8-second delay. Compared with vehicle, THC 1) significantly slowed the rate of improvement during phase 1 (t=–3.09, df=13, p=0.009), 2) significantly extended the length of phase 1 (t=6.50, df=13, p<0.001), but 3) did not significantly affect accuracy at the change point between phases (p=0.18), and 4) did not significantly affect phase 2 slopes (p=0.13).
Figure 4 parts C and D display observed and estimated areas under the curve for the double-reinforcement object memory trials. These data revealed no significant differences between groups. The model with all four delays showed that the difference in change points between groups did not depend on delay. These results indicate that THC had no consistent effects on performance of the double-reinforcement object working memory trials.
The observed and estimated accuracy rates (Figure 3 parts E and F) and AUC (Figure 4 parts E and F) for the single-reinforcement object working memory trials also indicate that THC had no consistent effects on performance in these trials (see supplemental results in the online data supplement).
Performance on the other six performance measures did not differ between the THC and vehicle groups, and there were no apparent trends in p values (see Supplemental Tables 1–6 in the online data supplement).
Acute Effects
THC impaired spatial, but not object, working memory accuracy in a delay-dependent manner during the first acute period (21). This same pattern was observed during the second acute period (Figure 5 parts A, B, and C), and the spatial working memory impairments did not differ significantly between the two acute periods (group-by-period interaction: F=0.22, df=1, 70.9, p=0.64; group-by-period-by-delay interaction: F=0.07, df=3, 69.8, p=0.97). Consistent with a delay-dependent effect of THC on spatial working memory, the combined results from the two acute periods revealed an overall significant group-by-delay interaction (F=2.73, df=3, 70.1, p=0.05).
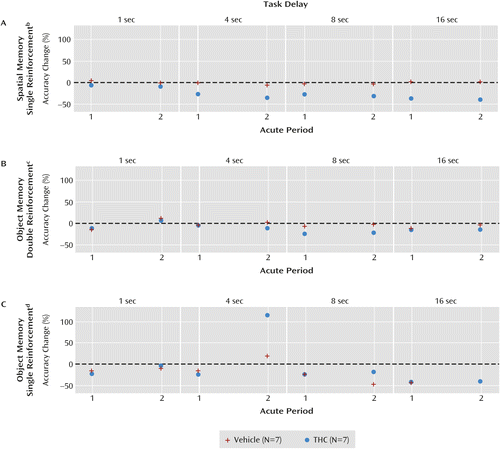
a During the first acute period, increasing doses of THC (or vehicle) were administered in order to determine the dose at which each monkey in the THC group became acutely intoxicated, as reflected by a marked decline of performance across tasks. Acute period 1 preceded the 6-month repeated-dosing study, which used stable doses, and acute period 2 came after the repeated-dosing study. During the second acute period, performance (measured as the percentage change in accuracy from pre- to post-drug administration) of monkeys in the THC group was assessed following administration of the same THC doses used throughout the repeated-dosing study, either 120 μg/kg of THC (three monkeys) or 240 μg/kg of THC (four monkeys).
b Panel A: independent of delay, performance on the spatial task during the first acute period was similar to performance during the second acute period for both the THC and vehicle groups.
c Panel B: performance on the double-reinforcement trials of the object task during the first acute period was similar to performance during the second acute period for both the THC and vehicle groups.
d Panel C: performance on the single-reinforcement trials of the object task during the first acute period was similar to performance during the second acute period for both the THC and vehicle groups.
Discussion
The present study in adolescent male rhesus monkeys was designed to determine 1) if the persistent effects of repeated THC administration during adolescence would differentially influence improvements on spatial versus object working memory tasks and 2) if repeated THC exposure would affect sensitivity to the acute effects of THC on working memory performance. We found that 1) relative to vehicle exposure, repeated THC administration impaired the age- and practice-related improvements in accuracy on the spatial working memory task in a delay-dependent manner but did not impair improvements in accuracy on the object working memory task at any delay or reinforcement level and 2) neither tolerance nor sensitivity developed to the acute effects of THC on working memory performance after 6 months of repeated exposure.
Our finding of a persistent THC effect selective for spatial working memory, under well-controlled experimental conditions in adolescent monkeys, is consistent with previous reports in humans that heavy cannabis use during adolescence has a more profound effect on cognitive functions than similar use during adulthood. For example, relative to cannabis use during adulthood, adolescent cannabis use is associated with greater neuropsychological deficits (6–8, 9, 31), altered neural activation patterns in the prefrontal cortex during working memory task performance (3, 4, 32), and an increased risk of developing schizophrenia (10), a disorder characterized by working memory impairments (11). In concert, these findings support the hypothesis that immature cognitive abilities are particularly vulnerable to the deleterious effects of THC and suggest that adolescent cannabis use is an important public health concern. This concern is heightened given recent reports that the proportion of 12–17-year-olds who are experimenting with cannabis is increasing while the proportion who regard smoking cannabis as carrying a great risk of harm is declining (12).
Tolerance/Sensitivity to THC Following Repeated Exposure
Repeated exposure of monkeys to THC for 6 months did not appear to affect sensitivity to the acute impairing effects of THC on spatial working memory previously observed when the monkeys were drug naive (21). Although rodents demonstrate tolerance to cannabinoids (33), evidence from human studies is inconsistent (23), which is likely due to between-study differences in the extent of prior cannabis use (i.e., amount, frequency, and years of use), the interval between most recent cannabis use and the testing day (i.e., withdrawal effects), and the within-study intervals between cannabis or THC exposure and testing (i.e., time of testing relative to peak THC level). However, when THC concentrations and levels of subjective high were similar in groups of occasional versus heavy users, tolerance to the acute impairing effects of THC was more evident in the heavy users (34). The current study controlled the extent of previous THC exposure, the interval between the most recent exposure and testing, and the interval between THC administration and testing. Thus, our finding that repeated exposure does not produce tolerance to the acute impairing effects of THC on working memory suggests that adolescents might remain susceptible to the acute cognitive deficits caused by cannabis regardless of their history of use.
Limitations
It is possible that completing the spatial task before the object task could confound comparisons of performance across tasks. Nonetheless, the motivational demand (i.e., thirst), reinforcer (i.e., water), motor requirements (i.e., responding to stimuli on a touch screen), and attentional requirements (i.e., attending to the sample stimulus by touching it in each trial) were identical for both tasks. In fact, the selective impairment of performance on the spatial task is particularly striking given that the object task 1) was always completed after the spatial task, when motivation might have been reduced owing to partial satiation of thirst and 2) placed a greater load on mnemonic processes associated with recalling the color and shape of a stimulus, versus recalling the location of the stimulus. That is, because accuracy on the more difficult object working memory task, which required similar nonmnemonic processes but greater motivation, was not impaired by THC at any delay, our findings suggest that THC selectively disrupted the mnemonic processes required to maintain visuospatial information transiently. In fact, many studies concerning the effects of cannabinoids on memory tasks in humans, monkeys, and rodents used a fixed order, did not counterbalance the order of task presentations among subjects, or did not specify an order of presentation (35–41). Moreover, THC impairs spatial working memory at doses that do not affect motor or other cognitive processes in adolescent rodents (42). Thus, it seems unlikely that counterbalancing the tasks or always presenting the object task before the spatial task would have altered the current findings. Our findings also suggest that the selective impairment on spatial working memory was not due to impaired motor, motivational, or attentional processes. Indeed, cannabinoids do not cause acute impairments (1) or persistent impairments (43, 44; but see 40) on purely attentional tasks in human cannabis users. Thus, although adolescence is characterized by protracted refinements in mesolimbic incentive-motivational circuits (45), our findings suggest that THC-induced effects on motivational processes are not likely to account for 1) the persistent spatial working memory deficits observed in adolescent cannabis users (41) or 2) the adverse effects of cannabis on educational performance (46).
It is important to note that intravenous THC administration is not isomorphic to smoking cannabis. Cannabis contains other cannabinoids (e.g., cannabidiol), as well as terpenoids and flavonoids (47), some of which could enhance or offset the effects of THC (48, 49).
Potential Clinical Implications
Our findings provide well-controlled experimental data indicating that repeated THC exposure during adolescence causes persistent impairments in working memory processes that 1) are selective for spatial memoranda, 2) persist during the period of exposure, and 3) do not alter the acute cognitive effects of THC. These findings suggest that cannabis use during adolescence may result in poorer academic performance (50) even in the absence of acute drug use, which heightens concerns associated with the increasing use of cannabis among adolescents (12).
1 : The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 2006; 188:425–444Crossref, Medline, Google Scholar
2 : Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 2010; 49:561–572Medline, Google Scholar
3 : The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J Psychoactive Drugs 2010; 42:401–412Crossref, Medline, Google Scholar
4 : Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res 2008; 163:40–51Crossref, Medline, Google Scholar
5 : Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004; 176:239–247Crossref, Medline, Google Scholar
6 : The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev 2008; 1:99–111Crossref, Medline, Google Scholar
7 : The residual cognitive effects of heavy marijuana use in college students. JAMA 1996; 275:521–527Crossref, Medline, Google Scholar
8 : Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep 2001; 3:507–512Crossref, Medline, Google Scholar
9 : Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012; 109:E2657–E2664Crossref, Medline, Google Scholar
10 : Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry 2013; 4:129Crossref, Medline, Google Scholar
11 : Cognitive function in schizophrenia. Int Clin Psychopharmacol 1997; 12(suppl 4):S29–S36Crossref, Medline, Google Scholar
12
13 : What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 2010; 72:101–113Crossref, Medline, Google Scholar
14 : Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res 1978; 143:233–249Crossref, Medline, Google Scholar
15 : Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004; 101:8174–8179Crossref, Medline, Google Scholar
16 : Postnatal development of neural circuits in the primate prefrontal cortex, in Principles of Frontal Lobe Function, 2nd ed. Edited by Stuss DTKnight RT. New York, Oxford University Press, 2012, pp 99–117Google Scholar
17 : Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci 2005; 9:60–68Crossref, Medline, Google Scholar
18 : Working memory performance in typically developing children and adolescents: behavioral evidence of protracted frontal lobe development. Dev Neuropsychol 2007; 31:103–128Crossref, Medline, Google Scholar
19 : Maturation of prefrontal cortex in the monkey revealed by local reversible cryogenic depression. Nature 1977; 267:613–615Crossref, Medline, Google Scholar
20 : Functional development of frontal association cortex in monkeys: behavioural and electrophysiological studies. Neurosci Res Program Bull 1982; 20:471–479Medline, Google Scholar
21 : Delay- and dose-dependent effects of Δ⁹-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology 2012; 37:1357–1366Crossref, Medline, Google Scholar
22 : Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 2005; 81:285–299Crossref, Medline, Google Scholar
23 : Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011; 214:391–401Crossref, Medline, Google Scholar
24 : Acquisition and baseline performance of working memory tasks by adolescent rhesus monkeys. Brain Res 2011; 1378:91–104Crossref, Medline, Google Scholar
25 : Repeated, intermittent delta(9)-tetrahydrocannabinol administration to rats impairs acquisition and performance of a test of visuospatial divided attention. Neuropsychopharmacology 2004; 29:522–529Crossref, Medline, Google Scholar
26 : Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex 2007; 17:175–191Crossref, Medline, Google Scholar
27 : Development of cannabinoid 1 receptor protein and messenger RNA in monkey dorsolateral prefrontal cortex. Cereb Cortex 2010; 20:1164–1174Crossref, Medline, Google Scholar
28 : Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 1993; 260:1955–1958Crossref, Medline, Google Scholar
29 : Non-spatial memory after selective prefrontal lesions in monkeys. Brain Res 1978; 143:313–323Crossref, Medline, Google Scholar
30 : Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol 1972; 36:362–377Crossref, Medline, Google Scholar
31 : Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 2003; 69:303–310Crossref, Medline, Google Scholar
32 : Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011; 216:131–144Crossref, Medline, Google Scholar
33 : Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 2005; 81:300–318Crossref, Medline, Google Scholar
34 : Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 2009; 23:266–277Crossref, Medline, Google Scholar
35 : Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 1994; 115:340–349Crossref, Medline, Google Scholar
36 : Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002; 164:61–70Crossref, Medline, Google Scholar
37 : Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 2001; 25:757–765Crossref, Medline, Google Scholar
38 : Chronic marijuana smoke exposure in the rhesus monkey, II: effects on progressive ratio and conditioned position responding. J Pharmacol Exp Ther 1992; 260:210–222Medline, Google Scholar
39 : Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther 1988; 245:178–186Medline, Google Scholar
40 ;
41 : The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 2007; 26:309–319Crossref, Medline, Google Scholar
42 : Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001; 157:142–150Crossref, Medline, Google Scholar
43 : The residual neuropsychological effects of cannabis: the current status of research. Drug Alcohol Depend 1995; 38:25–34Crossref, Medline, Google Scholar
44 : Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001; 58:909–915Crossref, Medline, Google Scholar
45 : Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol 2010; 20:236–241Crossref, Medline, Google Scholar
46 : The effects of adolescent cannabis use on educational attainment: a review. Addiction 2000; 95:1621–1630Crossref, Medline, Google Scholar
47 : Cannabis—1988. Acta Psychiatr Scand Suppl 1988; 345:108–118Medline, Google Scholar
48 : Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010; 35:764–774Crossref, Medline, Google Scholar
49 : Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry 2010; 197:285–290; correction, 2010; 197:416Crossref, Medline, Google Scholar
50 : Cannabis use and later life outcomes. Addiction 2008; 103:969–976, discussion 977–978Crossref, Medline, Google Scholar
51 : Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry 2010; 167:160–169Link, Google Scholar
52 : Preserved working memory and altered brain activation in persons at risk for psychosis. Am J Psychiatry 2013; 170:1297–1307Link, Google Scholar



