A Nationwide Study on the Risk of Autoimmune Diseases in Individuals With a Personal or a Family History of Schizophrenia and Related Psychosis
Abstract
Objective
Previous research has found an increased risk of schizophrenia in individuals with autoimmune diseases and smaller but significant associations with a family history of autoimmune diseases. This study investigates, for the first time, the association between schizophrenia and subsequent autoimmune diseases (the reverse temporality) and also considers the effect of infections, a possible risk factor for both schizophrenia and autoimmune diseases.
Method
Danish nationwide registers were linked to establish a cohort of 3.83 million people, identifying 39,364 individuals with schizophrenia-like psychosis and 142,328 individuals with autoimmune disease. Data were analyzed using survival analysis and adjusted for calendar year, age, and sex.
Results
Individuals with schizophrenia had an elevated risk of subsequent autoimmune diseases, with an incidence rate ratio of 1.53 (95% CI=1.46–1.62). Among persons without hospital contacts for infections, the effect of having schizophrenia was smaller, with an increased incidence rate ratio of 1.32 (95% CI=1.22–1.43) for autoimmune diseases. For individuals with schizophrenia as well as hospital contacts for infections, the combined risk of autoimmune diseases was 2.70 (95% CI=2.51–2.89). A family history of schizophrenia slightly increased the overall risk of developing autoimmune diseases (incidence rate ratio=1.06, 95% CI=1.02–1.09). Autoimmune diseases developed subsequently in 3.6% of people with schizophrenia, and 3.1% of people with autoimmune diseases had a family history of schizophrenia.
Conclusions
The increased risk of subsequent autoimmune diseases in individuals with schizophrenia may involve neuropsychiatric manifestations from the undiagnosed autoimmune disease, medical treatment or lifestyle associated with schizophrenia, or common etiological mechanisms, such as infections and shared genetic factors.
Several studies have found positive associations between autoimmune diseases and psychotic disorders (1–3). Some autoimmune diseases have a high prevalence of neuropsychiatric symptoms and have been suggested to be causally linked with schizophrenia (1, 4–7). An increased risk of schizophrenia is observed in both longitudinal and cross-sectional studies of people with autoimmune diseases (1, 3, 8), linking type 1 diabetes (3), celiac disease (9), autoimmune thyroid diseases (2, 3), and systemic lupus erythematosis (4), among other illnesses, with a raised risk of schizophrenia. Furthermore, epidemiological studies have found positive associations between a family history of autoimmune diseases and an increased risk of psychotic disorders (2, 10). Additionally, studies of patients with schizophrenia without other known somatic comorbidity have found diverse immune alterations, elevated autoantibody levels, and increased autoantibody reactivity (11–13).
The risk of nonaffective psychosis has been shown to increase with the temporal proximity of autoimmune disease diagnosis, suggesting shared etiological factors or common pathogenic mechanisms (2). These processes may include aberrant immunological (10, 14) or infectious processes (3). Some autoimmune diseases involve brain-reactive antibodies or can affect the brain through inflammation, possibly causing neural dysfunction (15). Brain-reactive antibodies have been found in patients with both psychotic disorders (10, 16) and specific autoimmune diseases, such as systemic lupus erythematosus (4, 7), and are believed to cause some of the psychotic symptoms associated with these diseases (15). Infections are prime candidates for initiating autoimmune diseases and may cause autoantibody-mediated brain disorders (15, 17). Infections have also been identified as a possible risk factor for schizophrenia, especially in individuals with autoimmune diseases (3, 18). The associations between autoimmune diseases and schizophrenia may also be a result of shared risk genes (2, 19, 20), and findings of suspected susceptibility genes for both autoimmune diseases and schizophrenia within the major histocompatibility complex support this hypothesis (21, 22). Psychological stress, medical treatment, or pathophysiology related to schizophrenia may also be a trigger for autoimmune disease activity.
Previous studies have investigated the risk of psychotic disorders in individuals with an autoimmune disease or a family history of autoimmune diseases. In the present study, we investigated the reverse temporal direction—that is, the risk of autoimmune diseases in individuals with a previous diagnosis of schizophrenia and related psychosis. We also explored the influence of infections. In order to examine possible genetic associations, we additionally investigated whether the risk of developing autoimmune diseases is increased in individuals with a family history of schizophrenia and related psychosis.
Method
Registers
Since 1968, the Danish Civil Registration System has recorded information, such as gender, first-degree relatives, and date of birth, on all people living in Denmark (23). A unique Civil Registration System number is assigned to each individual at birth that ensures accurate linkage between the different registers. The Psychiatric Central Register contains data on all admissions to and discharges from psychiatric inpatient facilities since 1969 and visits to psychiatric outpatient care and emergency department contacts since 1995 (24). The National Hospital Register holds information on all admissions to and discharges from hospitals for autoimmune diseases and infections since 1977 (type 1 diabetes since 1987) and registration of outpatients and emergency department contacts since 1995 (25). The Danish version of ICD-8 (26) was the diagnostic system used from 1969 to 1993, and ICD-10 was used from 1994 onward (27). All personal information from the registers is anonymized when used for research purposes, and the present study was approved by the Danish Data Protection Agency.
Study Population
The total study population included all persons born in Denmark between January 1, 1945, and December 31, 2010, and who were alive and a resident of Denmark during our study period. They were followed from January 1, 1987, until onset of the autoimmune disease in question, death, emigration from Denmark, or December 31, 2010, whichever came first. Follow-up was initiated from 1987, since type 1 diabetes could not be distinguished from type 2 diabetes in the register system until then, and all previous diagnoses of diabetes were excluded. In order to classify the affected cases as incident, all persons with a previous diagnosis of schizophrenia and related psychosis or autoimmune diseases during the time period between 1977 and 1986 were excluded, and for schizophrenia and related psychosis we additionally excluded persons with a diagnosis between 1969 and 1986, since the Psychiatric Central Register has a longer available registration period than the National Hospital Register (as described above).
Assessment of Psychiatric Disorders, Autoimmune Diseases, and Infections
Individuals and relatives were classified with schizophrenia if they had a hospital or clinic contact for schizophrenia or schizophrenia-like psychoses (including schizotypal personality disorder) as diagnosed by the treating psychiatrist (ICD-8: 295, 297, 298.39, and 301.83; ICD-10: F20–F29). Individuals with bipolar disorder with psychosis were included as a comparison group (ICD-8: 296.19 and 296.39; ICD-10: F30 and F31). The date at onset was defined as the first day of the first hospital contact for the relevant disease, irrespective of other previous diagnosis.
Each person could have a history of more than one autoimmune disease and/or infection. We omitted all ICD-8 diagnoses that bore the modification code “suspected” or “not found,” and ICD-10 diagnoses with similar codes were also omitted. Persons were classified as having autoimmune disease if they were registered as having one of the 30 autoimmune diseases listed in Table S1 of the online data supplement that accompanies this article and previously included in recent studies (2, 3). All hospital contacts for infections were included (see Table S1 in the data supplement), except for persons with AIDS/HIV infections, who were omitted (ICD-8: 07983; ICD-10: B20–24).
Statistical Analysis
Incidence rate ratios, also referred to as relative risks, of each autoimmune disease were estimated by log-linear Poisson regression using the GENMOD procedure in SAS, version 9.3 (SAS Institute, Cary, N.C.). All estimates were adjusted for calendar year, age, and sex. Age, calendar year, and the occurrence of schizophrenia in cohort members and their first-degree relatives were treated as time-dependent variables, whereas sex was considered time independent. The p values and 95% confidence intervals were based on likelihood ratio tests.
Results
Risk of Autoimmune Diseases in Individuals With Schizophrenia
A cohort of 3.77 million persons born in Denmark between 1945 and 2000 was followed from their 10th birthday or 1987, whichever came last, until 2010 (69 million person-years at risk). During this period, 39,364 persons were diagnosed for the first time with schizophrenia (17,644 female members and 21,720 male members); of these, 1,401 persons (3.6%) had a subsequent autoimmune disease diagnosis (776 female members and 625 male members). From these, 793 persons (56.6%) also had a hospital contact for infection before the autoimmune disease diagnosis. A total of 142,328 individuals were diagnosed with an autoimmune disease during the study period, and 1,401 of these persons (1.0%) had previously been diagnosed with schizophrenia. Of those with an autoimmune disease, 53,709 (37.7%) had a previous hospital contact for infection.
Individuals with schizophrenia had a 53% increase in the risk of subsequent diagnosis of one or more autoimmune diseases (incidence rate ratio=1.53, 95% confidence interval [CI]=1.46–1.62). Autoimmune diseases were more frequent among women, but there was no significant gender difference in the association between autoimmune diseases and schizophrenia. Persons with schizophrenia had a significantly increased risk of subsequent diagnoses of a range of autoimmune diseases, and the only significant negative association was found with seropositive rheumatoid arthritis (Table 1). The risk was elevated most for those autoimmune diseases with suspected presence of brain-reactive antibodies (incidence rate ratio=1.91, 95% CI=1.78–2.04), compared with the risk associated with the remaining group of autoimmune diseases (incidence rate ratio=1.21, 95% CI=1.11–1.30).
| Autoimmune Diseases | Number of Cases | Number of Exposed Cases | Persons With Schizophrenia Compared With Persons Without Schizophrenia | First 5 Years After Onset of Schizophrenia (Concurrent Period)b | Five or More Years After Onset of Schizophrenia (Delayed Period) | |||
|---|---|---|---|---|---|---|---|---|
| Incidence Rate Ratio | 95% CI | Incidence Rate Ratio | 95% CI | Incidence Rate Ratio | 95% CI | |||
| Persons without schizophrenia (reference) | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Any autoimmune disease | 142,328 | 1,401 | 1.53 | 1.46–1.62 | 1.55 | 1.42–1.69 | 1.52 | 1.43–1.63 |
| Autoimmune disease with suspected presence of brain-reactive antibodies | 75,087 | 849 | 1.91 | 1.78–2.04 | 1.78 | 1.58–1.99 | 1.99 | 1.82–2.16 |
| Autoimmune hepatitis | 1,878 | 40 | 3.51 | 2.51–4.73 | 3.04 | 1.67–5.02 | 3.79 | 2.52–5.43 |
| Autoimmune thyroiditis | 3,386 | 23 | 0.90 | 0.58–1.33 | 0.88 | 0.38–1.71 | 0.91 | 0.54–1.44 |
| Celiac disease | 2,350 | 20 | 1.33 | 0.82–2.03 | 1.08 | 0.43–2.19 | 1.49 | 0.82–2.46 |
| Guillain-Barré syndrome | 1,648 | 24 | 2.73 | 1.77–3.99 | 4.00 | 2.29–6.39 | 1.78 | 0.85–3.23 |
| Multiple sclerosis | 9,759 | 83 | 1.57 | 1.29–1.90 | 1.64 | 1.19–2.19 | 1.53 | 1.18–1.95 |
| Sjögren’s syndrome | 1,994 | 19 | 1.31 | 0.80–2.00 | 1.53 | 0.66–2.96 | 1.21 | 0.65–2.03 |
| Systemic lupus erythematosus | 2,101 | 19 | 1.57 | 0.96–2.39 | 1.41 | 0.61–2.74 | 1.67 | 0.89–2.82 |
| Thyrotoxicosis | 17,308 | 136 | 1.10 | 0.93–1.30 | 1.05 | 0.78–1.39 | 1.13 | 0.91–1.38 |
| Type 1 diabetes | 28,272 | 478 | 2.83 | 2.58–3.10 | 2.60 | 2.21–3.04 | 2.96 | 2.64–3.30 |
| Other autoimmune diseases | 80,979 | 642 | 1.21 | 1.11–1.30 | 1.32 | 1.16–1.48 | 1.14 | 1.02–1.26 |
| Alopecia areata | 1,377 | 9 | 1.05 | 0.50–1.90 | – | – | 0.99 | 0.35–2.13 |
| Ankylosing spondylitis | 3,661 | 21 | 0.78 | 0.49–1.16 | 0.93 | 0.47–1.64 | 0.67 | 0.35–1.15 |
| Crohn’s disease | 12,117 | 98 | 1.33 | 1.08–1.61 | 1.05 | 0.74–1.44 | 1.55 | 1.20–1.97 |
| Idiopathic thrombocytopenic purpura | 1,660 | 14 | 1.36 | 0.76–2.21 | 1.78 | 0.76–3.44 | 1.10 | 0.47–2.14 |
| Iridocyclitis | 9,220 | 80 | 1.18 | 0.94–1.46 | 1.31 | 0.91–1.81 | 1.10 | 0.82–1.45 |
| Pernicious anemia | 1,082 | 19 | 2.59 | 1.58–3.95 | 3.81 | 1.90–6.70 | 1.91 | 0.91–3.45 |
| Polymyalgia rheumatica | 2,396 | 11 | 0.61 | 0.31–1.04 | 1.14 | 0.41–2.46 | 0.43 | 0.17–0.88 |
| Primary adrenocortical insufficiency | 785 | 20 | 3.81 | 2.36–5.79 | 3.77 | 1.62–7.33 | 3.84 | 2.10–6.37 |
| Primary biliary cirrhosis | 478 | 9 | 2.65 | 1.26–4.81 | – | – | 3.37 | 1.53–6.33 |
| Psoriasis vulgaris | 13,269 | 190 | 2.13 | 1.84–2.45 | 3.04 | 2.48–3.67 | 1.59 | 1.29–1.95 |
| Seropositive rheumatoid arthritis | 15,768 | 82 | 0.75 | 0.60–0.93 | 0.60 | 0.38–0.88 | 0.83 | 0.64–1.06 |
| Ulcerative colitis | 22,289 | 146 | 0.99 | 0.84–1.16 | 1.02 | 0.79–1.30 | 0.97 | 0.77–1.19 |
| Autoimmune diseases with too few cases to calculate the individual riskc | 6,701 | 25 | 0.72 | 0.47–1.04 | 0.76 | 0.38–1.33 | 0.69 | 0.40–1.11 |
Risk of Autoimmune Diseases According to Time Since First Registration of Schizophrenia and the Association With Infections
The risk of autoimmune diseases did not significantly differ by time after the first hospital contact for schizophrenia, divided into a concurrent period (0–4 years after onset of schizophrenia) and a delayed period (5 or more years after onset of schizophrenia). The only specific disease for which there was a significant difference in risk between the concurrent and delayed period was psoriasis (p<0.0001). When the time periods were more detailed, there was a significant effect of time since the first schizophrenia diagnosis (p=0.003). The incidence rate ratio of any autoimmune disorder decreased from 2.10 (95% CI=1.78–2.45) in the first year after the onset of schizophrenia to 1.45 (95% CI=1.24–1.69) 15 or more years after the onset of schizophrenia (p=0.001 for no difference [see Table 2]). Among persons with schizophrenia but no hospital contacts for infections, there was no significant increase in the risk of autoimmune diseases, compared with persons without schizophrenia, in the time period 15 or more years after onset of schizophrenia (incidence rate ratio=1.07, 95% CI=0.81–1.38) (Figure 1). Among persons with a hospital contact for infection, we found a significant effect of schizophrenia in all six time intervals (from an incidence rate ratio of 2.21 [95% CI=1.77–2.70] the first year after the schizophrenia diagnosis to an incidence rate ratio of 1.58 [95% CI=1.29–1.91] 15 years after), when compared with persons with a hospital contact for infection but without schizophrenia.
| History of Schizophrenia | Autoimmune Diseases in Persons With Schizophrenia | Autoimmune Diseases in Persons With Schizophrenia and No Hospital Contacts for Infections | Autoimmune Diseases in Persons With Schizophrenia and Hospital Contacts for Infections | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence Rate Ratio | 95% CI | Cases With Autoimmune Diseases | Incidence Rate Ratio | 95% CI | Cases With Autoimmune Diseases | Incidence Rate Ratio | 95% CI | Cases With Autoimmune Diseases | |
| Completed years since first diagnosis of schizophreniab | |||||||||
| <1 | 2.10 | 1.78–2.45 | 154 | 1.73 | 1.35–2.18 | 67 | 3.86 | 3.10–4.73 | 87 |
| 1–2 | 1.37 | 1.18–1.57 | 191 | 1.21 | 0.97–1.48 | 88 | 2.36 | 1.94–2.85 | 103 |
| 3–4 | 1.44 | 1.25–1.66 | 190 | 1.36 | 1.10–1.65 | 92 | 2.36 | 1.92–2.86 | 98 |
| 5–9 | 1.53 | 1.39–1.68 | 425 | 1.36 | 1.18–1.57 | 192 | 2.61 | 2.29–2.96 | 233 |
| 10–14 | 1.56 | 1.39–1.75 | 284 | 1.28 | 1.06–1.53 | 114 | 2.82 | 2.42–3.27 | 170 |
| ≥15 | 1.45 | 1.24–1.69 | 157 | 1.07 | 0.81–1.38 | 55 | 2.77 | 2.26–3.34 | 102 |
| Total with schizophrenia | 1.53 | 1.46–1.62 | 1,401 | 1.32 | 1.22–1.43 | 608 | 2.70 | 2.51–2.89 | 793 |
| No history of schizophrenia (reference) | 1.00 | (Reference) | 140,927 | 1.00 | (Reference) | 88,011 | 1.75 | 1.73–1.77 | 52,916 |
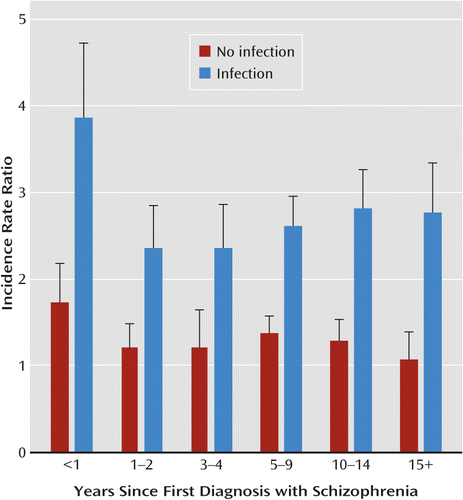
a The reference group is people without hospital contacts for schizophrenia and infections.
There was a significant multiplicative interaction of having a diagnosis of schizophrenia and infections (p=0.004) on the risk of developing autoimmune diseases (Figure 2). The risk of subsequent autoimmune diseases in those with schizophrenia and no hospital contacts for infections was elevated, with an incidence rate ratio of 1.32 (95% CI=1.22–1.43).
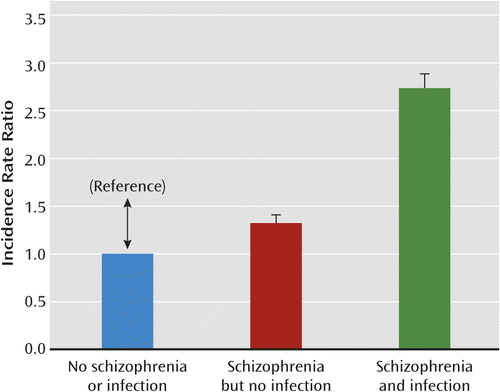
a Data indicate a significant multiplicative interaction between schizophrenia and infection on the risk of autoimmune diseases (p=0.004).
Risk of Autoimmune Diseases in Individuals With a Family History of Schizophrenia
We followed a cohort of 3.83 million persons born in Denmark between 1945 and 2010, with known identity of the mother, to study the effect of a family history of schizophrenia on the risk of developing autoimmune diseases. The cohort was followed from birth or 1987 until 2010. During this period, 3,382 individuals out of the 110,761 individuals with autoimmune diseases (3.1%) had a parent or sibling with a diagnosis of schizophrenia.
There was a small but significantly increased risk of one or more autoimmune diseases among individuals with a family history of schizophrenia (incidence rate ratio=1.06, 95% CI=1.02–1.09) (Table 3), and the risk was still significantly elevated after additional adjustments for a personal history of schizophrenia (incidence rate ratio=1.04, 95% CI=1.01–1.08). The presence of schizophrenia in a relative was associated with significantly increased risk of seven autoimmune diseases (as summarized in Table 3), and after adjustments for a personal history of schizophrenia, the presence of schizophrenia in a relative was associated with significantly increased risk of autoimmune hepatitis (incidence rate ratio=1.48, 95% CI=1.12–1.90), systemic lupus erythematosus (incidence rate ratio=1.46, 95% CI=1.12–1.86), Sjögren’s syndrome (incidence rate ratio=1.46, 95% CI=1.09–1.92), and iridocyclitis (incidence rate ratio=1.14, 95% CI=1.01–1.29), out of the 30 autoimmune diseases examined.
| Autoimmune Diseases | Relative Risk | |||
|---|---|---|---|---|
| Number of Cases | Number of Exposed Cases | Incidence Rate Ratio | 95% CI | |
| Persons without a family history of schizophrenia (reference) | 1.00 | (reference) | ||
| Any autoimmune disorder | 110,761 | 3,382 | 1.06b | 1.02–1.09 |
| Pernicious anemia | 749 | 34 | 1.46 | 1.01–2.02 |
| Autoimmune hemolytic anemia | 311 | 12 | 1.44 | 0.76–2.45 |
| Idiopathic thrombocytopenic purpura | 2,204 | 52 | 1.13 | 0.85–1.48 |
| Thyrotoxicosis | 12,009 | 429 | 1.10 | 1.00–1.21 |
| Autoimmune thyroiditis | 2,617 | 87 | 1.03 | 0.82–1.26 |
| Type 1 diabetes | 21,133 | 646 | 1.12 | 1.04–1.21 |
| Primary adrenocortical insufficiency | 607 | 20 | 1.11 | 0.69–1.69 |
| Multiple sclerosis | 7,175 | 217 | 0.94 | 0.82–1.07 |
| Guillain-Barré syndrome | 1,294 | 43 | 1.27 | 0.92–1.70 |
| Iridocyclitis | 7,443 | 266 | 1.15b | 1.01–1.29 |
| Crohn’s disease | 10,520 | 291 | 0.99 | 0.88–1.11 |
| Ulcerative colitis | 18,340 | 530 | 0.97 | 0.89–1.06 |
| Autoimmune hepatitis | 1,299 | 60 | 1.58b | 1.20–2.03 |
| Primary biliary cirrhosis | 255 | 15 | 1.80 | 1.02–2.92 |
| Celiac disease | 2,736 | 65 | 1.02 | 0.79–1.29 |
| Pemphigus | 185 | 7 | 1.60 | 0.68–3.17 |
| Pemphigoid | 124 | 5 | 1.41 | 0.50–3.10 |
| Psoriasis vulgaris | 9,932 | 299 | 0.99 | 0.88–1.11 |
| Alopecia areata | 1,405 | 39 | 1.08 | 0.77–1.46 |
| Vitiligo | 1,012 | 36 | 1.29 | 0.91–1.78 |
| Seropositive rheumatoid arthritis | 9,697 | 294 | 0.94 | 0.84–1.06 |
| Juvenile arthritis | 4,145 | 55 | 0.91 | 0.69–1.18 |
| Wegener’s granulomatosis | 380 | 11 | 0.90 | 0.46–1.56 |
| Dermatopolymyositis | 469 | 15 | 1.14 | 0.65–1.83 |
| Polymyalgia rheumatica | 796 | 34 | 1.19 | 0.83–1.65 |
| Myasthenia gravis | 422 | 17 | 1.42 | 0.84–2.23 |
| Scleroderma | 629 | 21 | 1.10 | 0.69–1.66 |
| Systemic lupus erythematosus | 1,493 | 63 | 1.47b | 1.13–1.88 |
| Sjögren’s syndrome | 1,045 | 52 | 1.48b | 1.11–1.94 |
| Ankylosing spondylitis | 2,967 | 98 | 1.04 | 0.84–1.26 |
Sensitivity Analysis and Similar Associations With Bipolar Disorder
When the cohort was restricted to persons born after 1977, for whom complete lifetime follow-up data on hospital contacts in the registers were available, the results were not appreciably different with regard to schizophrenia.
We included persons with bipolar disorder as a group with another type of psychosis and demonstrated that they also had increased risk of subsequent autoimmune diseases compared with persons without bipolar disorder (incidence rate ratio=1.71, 95% CI=1.56–1.88; N=461). Furthermore, the risk of autoimmune diseases was elevated the most in individuals with both bipolar disorder and hospital contacts for infections (incidence rate ratio=2.91, 95% CI=2.58–3.28). However, a family history of bipolar disorder did not significantly increase the risk of autoimmune diseases (incidence rate ratio=1.03, 95% CI=0.97–1.09; N=1,204).
Discussion
In this study, individuals diagnosed with schizophrenia and related psychosis were at increased risk of subsequent diagnosis of autoimmune diseases, in particular the group of diseases with suspected presence of brain-reactive antibodies. There was a significant multiplicative interaction between having both schizophrenia and hospital contacts for infections that increased the risk of subsequent autoimmune diseases. In persons with schizophrenia but no hospital contacts for infections, the risk of autoimmune diseases was less elevated and decreased with time since onset of schizophrenia to a nonsignificant level in the time period 15 or more years after the diagnosis. A family history of schizophrenia slightly increased the risk of later development of autoimmune diseases.
Until now, most research has indicated an increased risk of psychotic disorders in individuals with autoimmune diseases and an elevated co-occurrence of schizophrenia or bipolar disorder (2, 8). Our study found that the relationship is bidirectional because in individuals with schizophrenia, the risk of a subsequent diagnosis of an autoimmune disease was increased by 53%, and in individuals with bipolar disorder, the risk was increased by 71%. Patients with schizophrenia had a significantly increased risk of subsequent diagnosis of autoimmune hepatitis, type 1 diabetes, psoriasis vulgaris, Guillain-Barré syndrome, multiple sclerosis, primary adrenocortical insufficiency, Crohn’s disease, pernicious anemia, and primary biliary cirrhosis. In the study by Eaton et al. (2), which examined the risk of schizophrenia associated with previous autoimmune diseases (the reverse temporality), most of these autoimmune diseases were also significantly associated with an elevated risk of a subsequent schizophrenia spectrum diagnosis, except for pernicious anemia and primary biliary cirrhosis, of which there were not enough cases to calculate the risk. The association of a range of autoimmune diseases with schizophrenia, including the negative association with rheumatoid arthritis, has also been found in a recent prevalence study of a national sample from Taiwan (8).
Infections are among the prime candidates for initiating autoimmune diseases (17) and are also possible risk factors for schizophrenia (3, 18). In our study, among persons with schizophrenia and no hospital contacts for infections, the increase in the risk of autoimmune diseases was reduced to a 32% increase.
A family history of schizophrenia significantly increased the risk of autoimmune diseases by 6% in the present study, and a family history of autoimmune diseases has previously been found to increase the risk of schizophrenia by 10%, hence the much stronger positive association between the two diseases when occurring in the same individual seems less likely to be primarily a result of shared risk genes. Furthermore, a family history of bipolar disorder was not significantly associated with autoimmune diseases, even in the reverse association as studied by Eaton et al. (2) However, the association with family history should be interpreted with caution because it is a crude indicator of variation in individual genes and a larger effect might be found if specific genes are identified (28). A family history of schizophrenia increased the risk of autoimmune hepatitis, type 1 diabetes, Sjögren’s syndrome, iridocyclitis, primary biliary cirrhosis, and pernicious anemia, and in the Eaton et al. study, a family history of most of these autoimmune diseases also increased the risk of schizophrenia spectrum disorders. The following autoimmune diseases had an increased risk both in individuals with schizophrenia and when a family history of schizophrenia was present: autoimmune hepatitis, type 1 diabetes, primary biliary cirrhosis, and pernicious anemia. Genetic components may influence the association in regard to these distinct autoimmune diseases, and genetic markers within the major histocompatibility complex have been associated with both occurrence of autoimmune diseases and schizophrenia (4, 21). The negative association between schizophrenia and rheumatoid arthritis has been found in more than a dozen studies and may in fact be a result of the interplay of genetic influences (29–31). However, a family history of rheumatoid arthritis was not protective in our study, and ascertainment bias may also be involved (32). Some patients with schizophrenia and related psychosis may have a genetic vulnerability toward dysregulation of the immune system, which could make them more susceptible toward acquiring infections, thereby increasing the risk of autoimmune diseases and possibly psychosis. Studies have indicated increased incidence of infections in people with some autoimmune diseases, which may also be the case in schizophrenia, particularly around symptom exacerbation, but the literature is not consistent (33–35).
Factors other than shared etiological components could be responsible for the associations between schizophrenia and autoimmune diseases. Antipsychotic medications and related side effects may have an effect on alterations of the immune system. However, there is no evidence from previous research that antipsychotic medications induce autoimmune diseases, and we previously found that the incidence of autoimmune diseases are also elevated before the diagnosis of schizophrenia and initiation of antipsychotic medication (3). Additionally, patients with schizophrenia are at increased risk of smoking and alcohol and drug abuse, which may increase the risk of developing autoimmune diseases. Psychological stress associated with schizophrenia may also be a trigger for autoimmune disease activity and infections leading to hospital contacts. However, if the increased risk is a result of lifestyle, psychological stress, or medication, the risk could be expected to increase with time after the diagnosis of schizophrenia because of longer exposure periods; however, the risk actually decreased over time in people with schizophrenia but no hospital contacts for infections.
Both schizophrenia and autoimmune diseases could be present in an individual long before entry into treatment and diagnosis, which suggests that the timing of onsets as well as the temporal order of onsets is uncertain. Autoimmune diseases, such as celiac disease, often have a long duration of untreated illness and may not require a hospital contact, since treatment can often be conducted by a general practitioner (9). A hospital contact for schizophrenia could increase the subsequent risk of autoimmune diseases as a result of detection bias, but the risk in our study was elevated in approximately the same degree in the concurrent period as in the delayed period after the first hospital contact for schizophrenia. Furthermore, celiac disease, for instance, was not significantly elevated in our cohort based on hospital contacts, which may be a result of underdiagnosis, since screening studies of patients with schizophrenia have indicated an increased prevalence and our previous study found increased incidence before the diagnosis of schizophrenia (3, 39). Lastly, previous studies indicate that patients with schizophrenia are in fact suffering from undertreatment of somatic comorbidity, possibly explaining the increased mortality among those with schizophrenia (40), hence the prevalence of autoimmune diseases may actually be underestimated.
Conclusions
We found robust associations between hospital contacts for both schizophrenia-like psychosis and bipolar disorder and subsequent first-time diagnosis of autoimmune diseases requiring a hospital contact, especially in persons with a contact for an infection as well as psychosis, hence the general pattern seemed similar across schizophrenia and bipolar disorders. Infections may act as a common pathological mechanism for both psychosis and autoimmune diseases, or persons with psychosis may simply be more susceptible to acquiring infections than the general population, increasing the risk of autoimmune diseases. Individuals with psychosis may also be genetically predisposed to acquiring infections and developing autoimmune diseases because of an altered immune system. However, a family history of schizophrenia only slightly increased the risk of autoimmune diseases, and a family history of bipolar disorder did not significantly increase the risk, making it less likely to be the sole explanation of our findings. Neuropsychiatric manifestations from the not yet diagnosed autoimmune disease could also be involved, or it may be that the treatment or lifestyle associated with psychosis raises the risk for autoimmune diseases.
1 : Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006; 163:521–528Link, Google Scholar
2 : Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord 2010; 12:638–646Crossref, Medline, Google Scholar
3 : Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 2011; 168:1303–1310Link, Google Scholar
4 : Immune dysregulation and self-reactivity in schizophrenia: Do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol 2005; 83:9–17Crossref, Medline, Google Scholar
5 : Autoantibody-mediated disorders of the central nervous system. Autoimmunity 2008; 41:55–65Crossref, Medline, Google Scholar
6 : Neuropsychiatric syndromes in patients with systemic lupus erythematosus and primary Sjögren syndrome: a comparative population-based study. Ann Rheum Dis 2009; 68:1541–1546Crossref, Medline, Google Scholar
7 : Behavioral changes in systemic lupus erythematosus are of an autoimmune nature. Nat Clin Pract Rheumatol 2007; 3:592–593Crossref, Medline, Google Scholar
8 : Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry 2012; 200:374–380Crossref, Medline, Google Scholar
9 : Coeliac disease and risk of schizophrenia and other psychosis: a general population cohort study. Scand J Gastroenterol 2007; 42:179–185Crossref, Medline, Google Scholar
10 : Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun 2006; 27:71–80Crossref, Medline, Google Scholar
11 : Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res 2008; 158:83–86Crossref, Medline, Google Scholar
12 : Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J Neuroimmunol 2003; 141:155–164Crossref, Medline, Google Scholar
13 : Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008; 63:801–808Crossref, Medline, Google Scholar
14 : Immune system and schizophrenia. Curr Immunol Rev 2010; 6:213–220Crossref, Medline, Google Scholar
15 : Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol 2009; 9:449–456Crossref, Medline, Google Scholar
16 : Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 2011; 258:686–688Crossref, Medline, Google Scholar
17 : The role of infection in the pathogenesis of autoimmune disease. Semin Immunol 1998; 10:5–13Crossref, Medline, Google Scholar
18 : Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry 2008; 13:470–479Crossref, Medline, Google Scholar
19 : Family history of autoimmune diseases in psychosis. Schizophr Res 1996; 19:33–40Crossref, Medline, Google Scholar
20 : Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophr Res 1996; 20:261–267Crossref, Medline, Google Scholar
21 ;
22 : Autoimmune diseases: genes, bugs and failed regulation. Nat Immunol 2001; 2:759–761Crossref, Medline, Google Scholar
23 : The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 2006; 53:441–449Medline, Google Scholar
24 : The Danish Psychiatric Central Research Register. Scand J Public Health 2011; 39(suppl):54–57Crossref, Medline, Google Scholar
25 : The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull 1999; 46:263–268Medline, Google Scholar
26
27
28 : Modelling the contribution of family history and variation in single nucleotide polymorphisms to risk of schizophrenia: a Danish national birth cohort-based study. Schizophr Res 2012; 134:246–252Crossref, Medline, Google Scholar
29 : Schizophrenia and rheumatoid arthritis: a review. Schizophr Res 1992; 6:181–192Crossref, Medline, Google Scholar
30 : Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci 2012; 1262:56–66Crossref, Medline, Google Scholar
31 : Negative association between schizophrenia and rheumatoid arthritis. Schizophr Bull 1991; 17:669–678Crossref, Medline, Google Scholar
32 : A population-based register study of the association between schizophrenia and rheumatoid arthritis. Schizophr Res 1999; 40:67–74Crossref, Medline, Google Scholar
33 : Incident somatic comorbidity after psychosis: results from a retrospective cohort study based on Flemish general practice data. BMC Fam Pract 2011; 12:132Crossref, Medline, Google Scholar
34 : A prevalence study of urinary tract infections in acute relapse of schizophrenia. J Clin Psychiatry 2013; 74:271–277Crossref, Medline, Google Scholar
35 : Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol 2013; 20:1153–1160Crossref, Medline, Google Scholar
36 : Autoantibodies to serotonin in serum of patients with psychiatric disorders. Psychiatry Res 2003; 121:51–57Crossref, Medline, Google Scholar
37 : Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res 2010; 44:321–330Crossref, Medline, Google Scholar
38 : High prevalence of autoantibodies against phosphoglycerate mutase 1 in patients with autoimmune central nervous system diseases. J Neuroimmunol 2010; 219:105–108Crossref, Medline, Google Scholar
39 : Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull 2011; 37:94–100Crossref, Medline, Google Scholar
40 : Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS ONE 2011; 6:e24597Crossref, Medline, Google Scholar



