Neuropsychological Impairments in Schizophrenia and Psychotic Bipolar Disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study
Abstract
Objective
Familial neuropsychological deficits are well established in schizophrenia but remain less well characterized in other psychotic disorders. This study from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium 1) compares cognitive impairment in schizophrenia and bipolar disorder with psychosis, 2) tests a continuum model of cognitive dysfunction in psychotic disorders, 3) reports familiality of cognitive impairments across psychotic disorders, and 4) evaluates cognitive impairment among nonpsychotic relatives with and without cluster A personality traits.
Method
Participants included probands with schizophrenia (N=293), psychotic bipolar disorder (N=227), schizoaffective disorder (manic, N=110; depressed, N=55), their first-degree relatives (N=316, N=259, N=133, and N=64, respectively), and healthy comparison subjects (N=295). All participants completed the Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological battery.
Results
Cognitive impairments among psychotic probands, compared to healthy comparison subjects, were progressively greater from bipolar disorder (z=–0.77) to schizoaffective disorder (manic z=–1.08; depressed z=–1.25) to schizophrenia (z=–1.42). Profiles across subtests of the BACS were similar across disorders. Familiality of deficits was significant and comparable in schizophrenia and bipolar disorder. Of particular interest were similar levels of neuropsychological deficits in relatives with elevated cluster A personality traits across proband diagnoses. Nonpsychotic relatives of schizophrenia probands without these personality traits exhibited significant cognitive impairments, while relatives of bipolar probands did not.
Conclusions
Robust cognitive deficits are present and familial in schizophrenia and psychotic bipolar disorder. Severity of cognitive impairments across psychotic disorders was consistent with a continuum model, in which more prominent affective features and less enduring psychosis were associated with less cognitive impairment. Cognitive dysfunction in first-degree relatives is more closely related to psychosis-spectrum personality disorder traits in psychotic bipolar disorder than in schizophrenia.
Kraepelin’s distinction between bipolar disorder and schizophrenia as fundamentally different disorders stands as a pillar of psychiatric nosology. Yet, in addition to overlapping clinical features, similarities in response to antipsychotic medication and in neuroimaging and genetic findings show considerable diagnostic overlap, especially in bipolar patients with a history of psychosis (1–4). These similarities raise fundamental questions about the boundaries and distinctiveness of these disorders.
Cognitive Deficits in Schizophrenia and Bipolar Disorder
Many neuropsychological studies have reported a generalized cognitive deficit in schizophrenia that is present at the first episode of psychosis, relatively stable over time, and largely independent of clinical status or antipsychotic treatment (5–9). These neuropsychological deficits are recognized as an important cause of functional disability (10–12). Studies of neuropsychological deficits in bipolar disorder have shown more modest impairments compared with schizophrenia, but few large studies have directly compared deficits in these disorders using identical testing and recruitment strategies.
Two main findings are emerging from cognitive studies of bipolar patients. First, small studies of acutely ill patients followed through to clinical stabilization and larger studies of clinically stable patients indicate that neuropsychological deficits are enduring trait-like features in bipolar disorder rather than disturbances present only during acute episodes of illness (13, 14). Second, cognitive deficits have been observed more consistently in bipolar patients with psychosis than in nonpsychotic bipolar patients (15, 16).
The lack of a sharp diagnostic boundary between bipolar disorder and schizophrenia is highlighted by the continued use of the schizoaffective disorder diagnosis, which has both schizophrenia and affective symptoms. It remains unclear whether schizoaffective disorder represents a discrete intermediate disorder (17), a variant of schizophrenia or bipolar disorder, or part of a dimensional continuum between schizophrenia and bipolar disorders (18, 19). Available findings are inconsistent, but some data indicate that the cognitive performance of schizoaffective patients lies intermediate between schizophrenia and affective disorder groups (20–23).
Familial Patterns of Cognitive Deficits Across Schizophrenia and Bipolar Disorder
Cognitive disturbances in family members of schizophrenia patients are well established (24–26). Deficits in episodic memory, working memory, and attention have been reported in unaffected offspring, unaffected monozygotic and dizygotic co-twins, and other unaffected relatives (27–30). Among the few studies evaluating cognition in relatives of individuals with bipolar disorder, deficits have been reported in verbal learning and memory, working memory, executive function, face memory, and response inhibition (31–34). Findings in relatives of bipolar patients (35) and direct comparison of relative groups across the two disorders (36) have been inconsistent. Systematic investigation is needed to evaluate potential differences in the severity and familiality of cognitive dysfunction in relatives of individuals with bipolar disorder and schizophrenia.
The five-site Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium (Maryland Psychiatric Research Center, University of Chicago/University of Illinois at Chicago, University of Texas–Southwestern, Wayne State University/Harvard University, and the Institute of Living/Yale University) was organized to address questions about diagnostic boundaries and familiality of intermediate phenotypes. Large samples of probands with schizophrenia, schizoaffective disorder, or bipolar disorder with a history of psychosis were recruited from the community, along with their available first-degree relatives, using identical inclusion criteria and testing procedures. The broad aim was to compare the severity and familiality of a wide range of potential intermediate phenotypes across schizophrenia and psychotic bipolar disorder.
In this article, we present the neuropsychology data from the B-SNIP study. The Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological battery was administered to all participants. The primary aims were 1) to contrast cognitive impairments in schizophrenia and bipolar disorder with psychosis, 2) to examine cognitive impairment in schizoaffective disorder relative to the two primary disorders of interest, 3) to examine the familiality of cognitive impairments across schizophrenia and psychotic bipolar disorder, and 4) to determine the extent of cognitive impairment among nonpsychotic relatives with and without elevated cluster A personality traits.
Method
Participants
Probands.
Patients were referred by mental health providers or recruited through advertisements and talks at community organizations and support groups. Patients with a history of psychotic symptoms were recruited if they had at least one available first-degree relative 15–65 years of age willing to participate in the study. Probands were required to have a DSM-IV diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder with a history of psychosis, determined at consensus diagnostic meetings after review of data gathered using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (37), information about the proband’s medical and psychiatric history obtained from relatives, and available medical charts. Clinical symptom ratings were assessed using the Positive and Negative Syndrome Scale (PANSS) (38), the Montgomery-Åsberg Depression Rating Scale (MADRS) (39), and the Young Mania Rating Scale (40), and functional status was assessed with the Social Functioning Scale (41). The Schizo-Bipolar Scale (18), which assesses a dimension of illness from prototypical bipolar disorder to prototypical schizophrenia, was also completed. To maintain reliability in ratings, diagnosticians from all sites underwent initial training and reviewed cases during monthly cross-site diagnostic meetings.
Family members.
First-degree relatives were assessed with the SCID and the Structured Interview for DSM-IV Personality (SIDP-IV) (42). As done in previous work (43), our a priori plan was to cast a broad net for ascertaining the presence of relevant elevated personality features by identifying individuals within one criterion of a cluster A (odd or eccentric) or cluster B (dramatic, emotional, or erratic) diagnosis. Given this project’s primary focus on identifying psychosis-related features, the psychotic-like features that characterize cluster A personality disorders were the primary traits of interest. Secondarily, cluster B traits were evaluated because of their potential overlap with bipolar symptoms.
Healthy comparison subjects.
Healthy volunteers were recruited through print and electronic media and research registries. Healthy comparison subjects were required to have no personal history of a psychotic disorder or recurrent depression (based on the SCID and consensus review) and no known immediate family history of these disorders.
All participants had no history of seizures or head injury with loss of consciousness >10 minutes; had a negative urine drug screen for common drugs of abuse on the day of testing; had no diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months; had no change in medication (and were generally clinically stable over the past month); had no history of systemic medical or neurological disorder likely to affect cognitive abilities; had an age-corrected Wide-Range Achievement Test, 4th edition, reading test standard score >65; and were sufficiently fluent in English to complete neuropsychological testing.
The demographic and clinical characteristics of probands and first-degree relatives are summarized in Tables 1 and 2. To address statistically significant group differences for sex distribution and age in proband and relative groups, age- and sex-stratified normative data (44) were used to compute subtest and composite scores for each participant on the BACS. Subtest and composite scores were also computed based on age- and sex-stratified data from the present comparison group. Composite scores anchored to B-SNIP controls and Keefe norms (44) were highly correlated (r values, >0.98). To be consistent with the existing literature and to facilitate direct comparison with previous studies, performance was referenced to published normative data (44). To address the uneven distribution of race among groups, race was used as a covariate in all analyses. All major findings were unchanged after including race as a covariate. Estimated marginal means are presented in the figures.
| Variablea | Schizophrenia Group (N=293) | Schizoaffective Depressed Group (N=55) | Schizoaffective Manic Group (N=110) | Psychotic Bipolar Group (N=227) | Healthy Comparison Group (N=295) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 35.87 | 12.84 | 38.22 | 11.93 | 35.76 | 11.72 | 36.19 | 12.79 | 37.64 | 12.63 | n.s. |
| Education (years)b | 12.74 | 2.25 | 12.63 | 2.17 | 13.28 | 2.22 | 14.18 | 2.37 | 15.10 | 2.57 | ≤0.001 |
| WRAT-4, reading test, standard scorec | 93.96 | 15.52 | 93.49 | 15.29 | 98.28 | 14.29 | 101.35 | 13.76 | 103.09 | 13.80 | ≤0.001 |
| N | % | N | % | N | % | N | % | N | % | ||
| Maled | 198 | 67.6 | 29 | 52.7 | 38 | 34.5 | 85 | 37.4 | 123 | 41.7 | ≤0.001 |
| Racee | ≤0.001 | ||||||||||
| Caucasian | 136 | 46.4 | 29 | 52.7 | 60 | 54.5 | 169 | 74.4 | 186 | 63.3 | |
| African American | 139 | 47.4 | 22 | 40.0 | 44 | 40.0 | 47 | 20.7 | 80 | 27.2 | |
| Other | 18 | 6.1 | 4 | 7.3 | 6 | 5.5 | 11 | 4.8 | 28 | 9.5 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| PANSSf | |||||||||||
| Total score | 65.2 | 16.9 | 66.2 | 17.0 | 68.5 | 14.4 | 53.4 | 14.1 | ≤0.001 | ||
| Positive score | 16.6 | 5.6 | 16.6 | 5.2 | 18.2 | 4.6 | 12.8 | 4.5 | ≤0.001 | ||
| Negative score | 16.7 | 5.9 | 16.4 | 5.3 | 15.4 | 4.7 | 12.1 | 4.0 | ≤0.001 | ||
| YMRSg | 5.3 | 5.8 | 5.1 | 5.9 | 7.9 | 6.8 | 5.9 | 6.6 | ≤0.01 | ||
| MADRSh | 8.6 | 7.8 | 15.4 | 9.5 | 14.7 | 10.5 | 10.4 | 9.2 | ≤0.001 | ||
| N | % | N | % | N | % | N | % | ||||
| Medications | |||||||||||
| Antipsychotic | 264 | 90.1 | 51 | 92.7 | 92 | 83.6 | 164 | 72.2 | n.s. | ||
| Mood stabilizer | 44 | 15.0 | 20 | 36.4 | 57 | 51.8 | 119 | 52.4 | ≤0.001 | ||
| Lithium | 16 | 5.5 | 6 | 10.9 | 13 | 11.8 | 64 | 28.2 | ≤0.001 | ||
| Antidepressant | 115 | 39.2 | 34 | 61.8 | 61 | 55.5 | 102 | 44.9 | n.s. | ||
| Sedative/anxiolytic | 56 | 19.1 | 12 | 21.8 | 35 | 31.8 | 66 | 29.1 | n.s. | ||
| Stimulant | 10 | 3.4 | 1 | 1.8 | 6 | 5.5 | 23 | 10.1 | n.s. | ||
| Anticholinergic | 52 | 17.7 | 10 | 18.2 | 15 | 13.6 | 20 | 8.8 | n.s. | ||
| Variable | Relatives of Schizophrenia Probands (N=316) | Relatives of Schizoaffective Depressed Probands (N=64) | Relatives of Schizoaffective Manic Probands (N=133) | Relatives of Psychotic Bipolar Probands (N=259) | Healthy Comparison Subjects (N=295) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years)a | 43.20 | 14.97 | 41.72 | 15.93 | 40.10 | 16.61 | 40.49 | 15.94 | 37.64 | 12.63 | ≤0.001 |
| Education (years)b | 13.99 | 2.41 | 14.41 | 2.82 | 13.77 | 2.93 | 14.61 | 2.73 | 15.10 | 2.57 | ≤0.001 |
| WRAT-4, reading test, standard scorec | 97.40 | 15.06 | 97.49 | 16.48 | 101.27 | 15.17 | 103.21 | 13.93 | 103.09 | 13.80 | ≤0.001 |
| N | % | N | % | N | % | N | % | N | % | ||
| Maled | 95 | 30.1 | 19 | 29.7 | 42 | 31.6 | 85 | 32.8 | 123 | 41.7 | <0.05 |
| Racee | ≤0.001 | ||||||||||
| Caucasian | 170 | 53.8 | 40 | 62.5 | 83 | 62.4 | 208 | 80.3 | 186 | 63.3 | |
| African American | 128 | 40.5 | 23 | 35.9 | 40 | 30.1 | 43 | 16.6 | 80 | 27.2 | |
| Other | 18 | 5.7 | 1 | 1.6 | 10 | 7.5 | 8 | 3.1 | 28 | 9.5 | |
| History of psychosis | 39 | 12.3 | 12 | 18.8 | 15 | 11.3 | 23 | 8.9 | |||
| Relatives with no history of psychosis | |||||||||||
| Elevated cluster A traits | 44 | 13.9 | 10 | 15.6 | 21 | 15.8 | 31 | 12.0 | |||
| Elevated cluster B traits | 18 | 5.7 | 1 | 1.6 | 10 | 7.5 | 18 | 6.9 | |||
| Not elevated | 215 | 68.0 | 41 | 64.1 | 85 | 63.9 | 184 | 71.0 | |||
Antipsychotic dosage in chlorpromazine equivalents (45), benztropine (anticholinergic) dosage, and the presence or absence of current antipsychotics, mood stabilizers, and antidepressants were minimally related to composite scores on the BACS across all proband groups (r values, <0.21; see Table 1 and the data supplement that accompanies the online edition of this article). A previous history of substance abuse or dependence was also minimally related to performance on the BACS (r values, <0.18). Site effects were nonsignificant. Clinical ratings of psychosis and mood symptoms had minimal associations with data from the BACS (r values, <−0.19). As each of these parameters accounted for less than 5% of the variance in data from the BACS, they were not used as covariates in the analyses reported below. (Additional details about the study sample and treatment history are available in the data supplement.)
Procedures
All participants were assessed with the BACS battery, which provides a brief (30 minutes), reliable, and valid test of global neuropsychological function (44, 46) and is widely used in schizophrenia research (47, 48). All tests were scored by two independent examiners, and 15% of cases were randomly selected for review of scoring accuracy by staff at NeuroCog Trials. The BACS consists of six subtests covering four cognitive domains (verbal memory, processing speed, reasoning/problem solving, and working memory). Extreme subtest scores were truncated to z-score=−4.0 before a composite score was computed.
Statistical analyses were conducted in sequence to address the four major study aims.
Probands
Schizophrenia and bipolar disorder.
An analysis of variance (ANOVA) was used to compare cognitive performance in schizophrenia and bipolar probands, the two primary and larger proband groups, with performance in healthy comparison subjects. Simple contrasts were used to clarify significant findings in omnibus testing using a Hochberg correction for multiple comparisons (49). Profile analyses were conducted using a repeated-measures multivariate analysis of variance (MANOVA), with the group-by-subtest interaction being the key statistical test.
Schizoaffective disorder.
Schizoaffective manic and schizoaffective depressed probands were considered together with schizophrenia and bipolar probands in an ANOVA. Next, the association between performance on the BACS and Schizo-Bipolar Scale score was examined in the combined proband sample.
First-Degree Relatives
Familiality.
A heritability analysis to estimate familiality of cognitive function was performed using the SOLAR (Sequential Oligogenic Linkage Analysis Routine) software package (50). In a design such as ours, an estimate of familiality (h2) represents the portion of phenotypic variance accounted for by family membership. To test for the significance of familiality, a maximum likelihood ratio test compared a model in which phenotypic variation is explained by family membership to a model assuming that no variation is explained by familial factors. A correction was applied to account for ascertainment bias, since families were recruited through the identification of a psychotic proband and not a representative community sample (51). Because of the larger sample sizes and the primary focus on capturing both the traditional diagnostic dichotomy of primary interest and the prototypical domains anchoring a mood-psychosis dimension (52), familiality estimates and group comparisons among relatives were restricted to schizophrenia and bipolar disorder.
Relatives of schizophrenia and bipolar disorder probands.
An ANOVA was used to compare the cognitive performance of relatives of probands in the two primary groups with that of healthy comparison subjects before taking into consideration elevated personality traits among relatives. Simple contrasts were used to clarify significant findings in omnibus testing, using a Hochberg correction for multiple comparisons (49).
Personality traits.
Among relatives of schizophrenia and bipolar probands with no history of psychosis, a two-way ANOVA was used to assess the relationship of proband diagnosis and cluster A personality traits with cognitive dysfunction. Because psychosis-related traits were of primary interest, relatives who met criteria for cluster A traits were included in the cluster A group regardless of their cluster B traits. In secondary analyses, composite scores on the BACS from individuals with cluster A traits were compared with scores from individuals with cluster B traits within and across diagnostic category. This analysis was repeated after excluding those who met criteria for both cluster A and cluster B traits (nine relatives of schizophrenia probands and six relatives of bipolar probands), with no change in the findings. Finally, nonpsychotic relatives with neither cluster A nor cluster B traits were compared with healthy comparison subjects and relatives with elevated personality traits of interest.
Results
Schizophrenia and Bipolar Disorder Proband Comparisons
Global neuropsychological performance differed significantly across the schizophrenia, bipolar disorder, and healthy comparison groups (F=129.11, df=2, 811, p<0.001) (Figure 1). Both patient groups were impaired compared with healthy individuals, and schizophrenia probands (z=−1.42) were more impaired than bipolar probands (z=−0.77) (F=32.12, df=1, 518, p<0.001). The composite score on the BACS was significantly correlated with social function (53) in both schizophrenia probands (r=0.27, p<0.001) and psychotic bipolar probands (r=0.31, p<0.001). This level of association was consistent with estimates in the literature (41, 54) and indicates that cognitive deficits have functional significance across psychotic disorders.
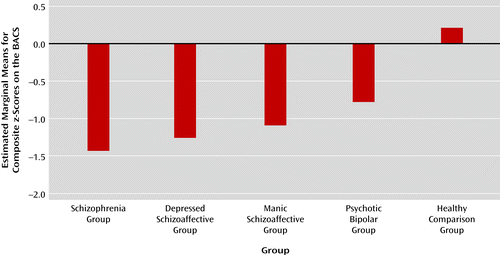
a Cognitive function compared with test norms in four proband groups and the healthy comparison group. Schizophrenia probands demonstrated significantly greater global neuropsychological deficits than bipolar probands; schizoaffective probands were intermediate and differed from the two primary diagnostic groups.
Profile comparisons.
Repeated-measures MANOVA testing for profile differences indicated a significant group-by-subtest interaction (F=9.69, df=10, 1580, p<0.001). However, when the comparison group was excluded, the group-by-subtest interaction for proband groups was not significant, indicating that their pattern of performance did not differ across cognitive domains (Figure 2).

a The patterns of subtest performance on the Brief Assessment of Cognition in Schizophrenia indicate a similar profile of cognitive dysfunction across psychotic disorders.
Schizoaffective disorder.
Schizoaffective probands were significantly less impaired than schizophrenia probands (F=4.51, df=1, 456, p=0.03) and were more impaired than bipolar probands (F=12.97, df=1, 390, p=0.008). Differences between the schizoaffective subtypes (depressed or manic) were not significant.
Bipolar-schizophrenia dimension.
To characterize neurocognitive function along a bipolar-schizophrenia dimension, composite scores from the BACS were examined in relation to scores on the Schizo-Bipolar Scale (18). As illustrated in Figure 3, overall cognitive performance declined as affective features became less prominent and the persistence of psychosis became more prominent (r=−0.25, p<0.001). The progressive reduction in cognitive scores from prototypical bipolar disorder to prototypical schizophrenia and the intermediate scores of the schizoaffective case subjects support a continuum model of cognitive deficits from schizophrenia to bipolar disorder.

a Consistent with a dimensional model of psychosis, cognitive performance declines progressively as affective symptoms become less prominent and psychotic features more pronounced and pervasive.
Familiality
Familiality estimates of cognitive function were significant in all groups and did not differ by proband diagnosis (Table 3).
| Schizophrenia Pedigrees | Bipolar Pedigrees | |||
|---|---|---|---|---|
| Instrument | h2 | 90% CI | h2 | 90% CI |
| WRAT-4, reading test | 0.75 | 0.60–0.90 | 0.70 | 0.54–0.86 |
| BACS composite | 0.50 | 0.37–0.63 | 0.61 | 0.42–0.79 |
| BACS subtests | ||||
| Verbal memory | 0.51 | 0.35–0.67 | 0.42 | 0.26–0.58 |
| Digit sequencing | 0.49 | 0.34–0.64 | 0.51 | 0.35–0.67 |
| Token motor | 0.32 | 0.16–0.48 | 0.39 | 0.21–0.57 |
| Verbal fluency | 0.33 | 0.17–0.49 | 0.52 | 0.34–0.70 |
| Symbol coding | 0.40 | 0.22–0.58 | 0.47 | 0.29–0.65 |
| Tower | 0.29 | 0.16–0.42 | 0.45 | 0.27–0.63 |
Relatives of schizophrenia and bipolar disorder probands.
There were no significant differences in composite scores on the BACS for first-degree relatives with a history of psychosis compared with their respective probands. Only relatives with no history of psychotic symptoms were included in the following analyses. There was a significant group difference in composite scores on the BACS among the relative groups and the healthy comparison group (F=7.02, df=2, 800, p=0.001). Simple contrasts indicated that relatives of schizophrenia probands had significant impairments compared with healthy comparison subjects, but there was no significant difference between relatives of psychotic bipolar probands and healthy comparison subjects (Figure 3). As with probands, the relative groups did not differ in their pattern of neuropsychological strengths and weaknesses (see the online data supplement).
Personality traits in relatives.
When compared with healthy comparison subjects, relatives exhibited significant impairments on the BACS composite scores when either cluster A or cluster B features were present. Among those who had elevated axis II traits (either cluster A or cluster B), there was no difference on the composite score between relatives of schizophrenia probands and relatives of bipolar probands. As illustrated in Figure 4, the interaction between axis II status (those with elevated cluster A or B traits versus those with neither traits) and proband diagnosis (schizophrenia versus bipolar) was significant (F=4.05, df=1, 505, p=0.05). This interaction was characterized by significant cognitive deficits in relatives of schizophrenia probands with no personal history of psychosis, even when cluster A and cluster B traits were present (F=8.68, df=1, 507, p=0.003). In contrast, in relatives of bipolar probands, cognitive deficits were observed when either elevated cluster A or cluster B traits were present, but not when axis II traits were nominal. This pattern could not be attributed to differential rates of axis I disorders because rates of disorders such as depression and anxiety disorders were similar in these relative groups. (Correlational analyses were also used to examine personality traits as continuous variables [number of criteria for any cluster A or B disorder]; these findings are presented in the online data supplement.)
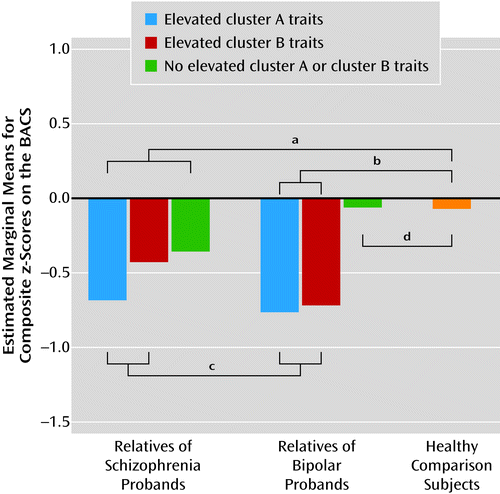
a All relatives of schizophrenia probands (with cluster A or cluster B traits and without; the former was defined as being one criterion from meeting the diagnostic threshold for a disorder in the cluster) exhibited significant levels of cognitive impairment compared to healthy comparison subjects (p<0.001).
b Cognitive performance differed significantly between bipolar proband relatives with cluster A or cluster B traits and healthy comparison subjects (p<0.001).
c Cognitive performance in relatives with cluster A or cluster B traits did not differ significantly within or across disorders.
d Cognitive performance did not differ significantly between relatives of bipolar probands without elevated cluster A or cluster B traits and healthy comparison subjects.
Discussion
To our knowledge, this is the first large-scale study to compare neuropsychological deficits across a range of psychotic proband groups and their first-degree relatives. The findings suggest that there is a continuum of cognitive deficits in psychotic disorders in which schizophrenia anchors one end, with the most severe deficits (z=−1.42), and bipolar disorder anchors the other, with significant but more modest deficits (z=−0.77). These findings parallel those of previous reports of more severe cognitive impairments in schizophrenia than in psychotic affective disorders (14, 23, 55–58) and support dimensional rather than robust categorical models of psychotic disorders (52, 59, 60). The scope and design of this large-sample investigation enabled direct comparison along a schizophrenia-bipolar dimension in a single study. The intermediate cognitive deficits in schizoaffective patients are consistent with this continuum model (Figures 1 and 3). Two factors vary along this continuum—the prominence of affective features and the persistence of psychosis. The relevance and relationships of these factors for determining level of cognitive deficit remains an important issue to address in future research.
Familiality
An additional advantage of the study design was a detailed evaluation of familial patterns of cognitive deficit. The findings indicated a different pattern of cognitive dysfunction in unaffected relatives of schizophrenia compared with relatives of bipolar probands with respect to their association with personality traits. Familiality was significant for the BACS composite and individual subtest scores and was comparable across schizophrenia and bipolar pedigrees.
Personality features.
Among relatives of both schizophrenia and bipolar probands, those with cluster A traits exhibited cognitive impairments similar to those seen in relatives with cluster B traits. This pattern parallels previous findings of medium to large effect sizes for cognitive deficits across a range of cluster A and B disorders, including schizotypal (61), antisocial (62), and borderline (63, 64) personality disorders. The main factor leading to greater overall cognitive deficits in relatives of schizophrenia probands than in relatives of bipolar probands was that relatives of schizophrenia probands as a group demonstrated cognitive deficits regardless of whether cluster A or B personality traits were present, while relatives of bipolar probands did not. This pattern suggests that cognitive deficits in families with a schizophrenia proband are transmitted at least partially independently from factors associated with schizotypal and other personality disorder traits, while in relatives of probands with bipolar disorder, cognitive deficits are more closely linked with elevated cluster A or cluster B personality traits. This may point to broader qualitative differences in the selectivity or penetrance of familial risk mechanisms affecting cognition across psychotic disorders.
General and specific measures.
The observation of significant familiality of cognitive function in schizophrenia is consistent with several previous studies (24, 28, 29, 33, 34), and our findings provide new evidence for a similar pattern in bipolar disorder with a history of psychosis. First, it is noteworthy that familiality estimates were somewhat larger for single word reading than for scores on the BACS. This may reflect a more substantial shared family environment component in which some parents provide a more enriching educational environment. Second, familiality estimates for individual neuropsychological subtests were variable and were generally lower than those observed for the BACS composite score. Individual tests focus more narrowly on specific cognitive processes, while composite scores integrate several cognitive skills regardless of their specific nature. As reflected in the history of intelligence test development, composite measures are often more closely related to functional ability in day-to-day life. Some researchers have suggested that genes with a broad impact on brain development and function may have a general impact on cognition (65, 66). It remains unclear whether examining aggregate effects of multiple genetic factors that affect scores (67) or individual genetic effects on specific cognitive functions will best advance gene discovery in psychotic disorders. Both approaches have potential, as particular genes may be tightly linked to a specific cognitive deficit while global deficits may represent a final common pathway of multiple factors that can be understood using systems biology approaches. The comparative advantages of these approaches remain to be clarified, but our findings in this study support the view that measures of generalized cognitive deficits can be useful for understanding familial factors contributing to bipolar disorder with psychosis (13, 57) as well as schizophrenia (6, 68). Based on the interaction between axis II status and cognitive performance, in which only bipolar relatives with elevated cluster A or cluster B traits exhibited cognitive deficits, a potentially promising phenotyping strategy for tracking risk mechanisms in bipolar disorder might lie in evaluating the co-occurrence of personality traits and cognitive dysfunction.
Limitations
Certain aspects of this study may limit the generalizability of our findings. First, probands who qualified for the study (i.e., those who were clinically stable, had limited current and past substance use, were willing and able to complete the demanding B-SNIP protocol, and had at least one first-degree relative willing and able to participate) may not be fully representative of their respective disorders. This recruitment strategy may have served to exclude some seriously ill individuals or recovered patients in the community. Second, because the study did not examine all types of bipolar disorder, cognition in nonpsychotic bipolar disorder remains to be explored (as well as in certain other disorders, such as psychotic unipolar depression). Third, the possibility of medication effects on cognition are a potential concern, although the minimal correlations between pharmacological treatment and performance on the BACS, the familiality of cognitive function in healthy relatives, and the failure of many pharmacological trials to enhance cognition (69) all suggest that medication effects did not have a major impact on the study findings. Fourth, the relationship between personality traits and cognitive deficits in relatives was based on a relatively small sample of relatives with axis II traits of interest. Finally, while the BACS battery was useful in characterizing general cognitive deficits, the relative utility of this battery as an informative cognitive endophenotyping measure needs to be considered in comparison with other cognitive endophenotyping approaches, especially those targeting specific neurocognitive processes.
1 : Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry 2000; 48:531–538Crossref, Medline, Google Scholar
2 : Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7:405–411Crossref, Medline, Google Scholar
3 : Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet 2006; 141B:76–83Crossref, Medline, Google Scholar
4 : Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses 2012; 6:145–151Crossref, Medline, Google Scholar
5 : Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res 2004; 68:49–63Crossref, Medline, Google Scholar
6 : Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 2007; 64:532–542Crossref, Medline, Google Scholar
7 : Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000; 157:549–559Link, Google Scholar
8 : Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull 2009; 35:403–414Crossref, Medline, Google Scholar
9 : Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry 2009; 21:336–356Crossref, Medline, Google Scholar
10 : Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry 2008; 63:505–511Crossref, Medline, Google Scholar
11 : Functional co-primary measures for clinical trials in schizophrenia: results from the MATRICS Psychometric and Standardization Study. Am J Psychiatry 2008; 165:221–228Link, Google Scholar
12 : The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. J Clin Exp Neuropsychol 2006; 28:260–269Crossref, Medline, Google Scholar
13 : Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis 2006; 194:255–260Crossref, Medline, Google Scholar
14 : A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr Res 2009; 113:167–175Crossref, Medline, Google Scholar
15 : The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry 2007; 62:910–916Crossref, Medline, Google Scholar
16 : Cognitive impairment in affective psychoses: a meta-analysis. Schizophr Bull 2010; 36:112–125Crossref, Medline, Google Scholar
17 : Psychotic bipolar disorders: dimensionally similar to or categorically different from schizophrenia? J Psychiatr Res 2004; 38:47–61Crossref, Medline, Google Scholar
18 : A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res 2011; 133:250–254Crossref, Medline, Google Scholar
19 : Schizoaffective disorders are psychotic mood disorders: there are no schizoaffective disorders. Psychiatry Res 2006; 143:255–287Crossref, Medline, Google Scholar
20 : Executive deficits in psychotic and bipolar disorders: implications for our understanding of schizoaffective disorder. Eur Psychiatry 2008; 23:20–25Crossref, Medline, Google Scholar
21 : Cognitive discernible factors between schizophrenia and schizoaffective disorder. Brain Cogn 2005; 59:292–295Crossref, Medline, Google Scholar
22 : Neurocognition in early-onset schizophrenia and schizoaffective disorders. J Am Acad Child Adolesc Psychiatry 2010; 49:52–60Crossref, Medline, Google Scholar
23 : Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disord 2006; 8:117–123Crossref, Medline, Google Scholar
24 : Initial heritability analyses of endophenotypic measures for schizophrenia: the Consortium on the Genetics of Schizophrenia. Arch Gen Psychiatry 2007; 64:1242–1250Crossref, Medline, Google Scholar
25 : Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Res 1994; 53:1–12Crossref, Medline, Google Scholar
26 : Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry 1995; 52:821–828Crossref, Medline, Google Scholar
27 : Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry 2002; 159:1514–1520Link, Google Scholar
28 : Recognition memory for faces in schizophrenia patients and their first-degree relatives. Neuropsychologia 2002; 40:2314–2324Crossref, Medline, Google Scholar
29 : Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry 2007; 164:813–819Link, Google Scholar
30 : Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry 2001; 50:98–107Crossref, Medline, Google Scholar
31 : Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry 1999; 45:639–646Crossref, Medline, Google Scholar
32 : Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med 2001; 31:915–922Crossref, Medline, Google Scholar
33 : Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. Br J Psychiatry 2005; 186:378–385Crossref, Medline, Google Scholar
34 : Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res 2012; 196:38–44Crossref, Medline, Google Scholar
35 : Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med 2008; 38:771–785Crossref, Medline, Google Scholar
36 : Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res 2004; 121:207–217Crossref, Medline, Google Scholar
37 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
38 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
39 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
40 : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
41 : Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull 2012; 38:865–872Crossref, Medline, Google Scholar
42 : Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC, American Psychiatric Press, 1997Google Scholar
43 : Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry 1998; 55:830–836Crossref, Medline, Google Scholar
44 : Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res 2008; 102:108–115Crossref, Medline, Google Scholar
45 Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 2010; 67:255–262Crossref, Medline, Google Scholar
46 : The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68:283–297Crossref, Medline, Google Scholar
47 : Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 2007; 164:1061–1071Link, Google Scholar
48 : Abbreviated neuropsychological assessment in schizophrenia: prediction of different aspects of outcome. J Clin Exp Neuropsychol 2009; 31:462–471Crossref, Medline, Google Scholar
49 : A sharper Bonferroni procedure for multiple tests of signicance. Biometrika 1988; 75:800–802Crossref, Google Scholar
50 : Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 1998; 62:1198–1211Crossref, Medline, Google Scholar
51 : Robust inference for variance components models in families ascertained through probands, I: conditioning on proband’s phenotype. Genet Epidemiol 1987; 4:203–210Crossref, Medline, Google Scholar
52 : The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry 2005; 186:364–366Crossref, Medline, Google Scholar
53 : The Social Functioning Scale: the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 1990; 157:853–859Crossref, Medline, Google Scholar
54 : Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry 2012; 11:73–79Crossref, Medline, Google Scholar
55 : Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry 2004; 161:996–1003Link, Google Scholar
56 : Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull 2009; 35:1022–1029Crossref, Medline, Google Scholar
57 : Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry 2007; 62:179–186Crossref, Medline, Google Scholar
58 : Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord 2010; 12:364–375Crossref, Medline, Google Scholar
59 : The Kraepelinian dichotomy: going, going... but still not gone. Br J Psychiatry 2010; 196:92–95Crossref, Medline, Google Scholar
60 : A controlled family study of chronic psychoses: schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45:328–336Crossref, Medline, Google Scholar
61 : Schizotypal disorder and schizophrenia: a profile analysis of neuropsychological functioning in Japanese patients. J Int Neuropsychol Soc 2007; 13:672–682Crossref, Medline, Google Scholar
62 : A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev 2000; 20:113–136Crossref, Medline, Google Scholar
63 . The neuropsychology of borderline personality disorder: a meta-analysis and review. Psychiatry Res 2005; 137:191–202Crossref, Medline, Google Scholar
64 : The neuropsychology of borderline personality disorder: relationship with clinical dimensions and comparison with other personality disorders. J Pers Disord 2009; 23:555–562Crossref, Medline, Google Scholar
65 : Generalist genes: implications for the cognitive sciences. Trends Cogn Sci 2006; 10:198–203Crossref, Medline, Google Scholar
66 : Variants in doublecortin- and calmodulin kinase like 1, a gene up-regulated by BDNF, are associated with memory and general cognitive abilities. PLoS One 2009; 4:e7534Crossref, Medline, Google Scholar
67 : Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav 2008; 7:435–446Crossref, Medline, Google Scholar
68 : Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J Int Neuropsychol Soc 2008; 14:209–221Crossref, Medline, Google Scholar
69 : Cognitive dysfunction in psychiatric disorders: characteristics, causes, and the quest for improved therapy. Nat Rev Drug Discov 2012; 11;141–168Crossref, Medline, Google Scholar



