Efficacy of Functional Remediation in Bipolar Disorder: A Multicenter Randomized Controlled Study
Abstract
Objective
The authors sought to assess the efficacy of functional remediation, a novel intervention program, on functional improvement in a sample of euthymic patients with bipolar disorder.
Method
In a multicenter, randomized, rater-blind clinical trial involving 239 outpatients with DSM-IV bipolar disorder, functional remediation (N=77) was compared with psychoeducation (N=82) and treatment as usual (N=80) over 21 weeks. Pharmacological treatment was kept stable in all three groups. The primary outcome measure was improvement in global psychosocial functioning, measured blindly as the mean change in score on the Functioning Assessment Short Test from baseline to endpoint.
Results
At the end of the study, 183 patients completed the treatment phase. Repeated-measures analysis revealed significant functional improvement from baseline to endpoint over the 21 weeks of treatment (last observation carried forward), suggesting an interaction between treatment assignment and time. Tukey’s post hoc tests revealed that functional remediation differed significantly from treatment as usual, but not from psychoeducation.
Conclusions
Functional remediation, a novel group intervention, showed efficacy in improving the functional outcome of a sample of euthymic bipolar patients as compared with treatment as usual.
It is well established that 40% to 60% of patients with bipolar disorder experience neurocognitive impairment not only during acute mood episodes but also during euthymic periods (1, 2). These rates are quite similar to those reported for functional impairment. In fact, it is estimated that only one-third of patients achieve full social and occupational recovery and return to their premorbid functional levels. Moreover, neurocognitive deficits, together with other clinical and sociodemographic factors, are thought to contribute to functional impairment (3, 4).
In recent years, functional outcome has become an issue of major concern not only in clinical settings but also because it represents a social and economic burden for society (5). Given the impact of neurocognitive impairment on daily functioning (6–8), there is a need to develop adjunctive therapies that target neurocognitive skills in order to enhance everyday functioning (9, 10).
Until recently, cognitive remediation strategies were regarded primarily as an intervention for schizophrenia (11), but little is known about neurocognitive remediation in affective disorders (12–17). To our knowledge, only one study (12), using an open-label design, has actually focused on bipolar patients. It found that 18 bipolar patients with residual depressive symptoms improved both symptomatically and in their psychosocial functioning.
In the present study, we sought to assess the efficacy of functional remediation, a neurocognitive intervention designed specifically for bipolar patients. This therapy involves neurocognitive techniques, training, psychoeducation on cognition-related issues, and problem-solving within an ecological framework (9), in an approach that attempts to avoid the problems with generalizability of learning that occurred in several cognitive remediation studies in schizophrenia.
To the best of our knowledge, this is the first randomized controlled trial to compare functional remediation with a psychoeducational intervention and treatment as usual. The primary outcome measure was improvement in global psychosocial functioning. We hypothesized that the patients in the functional remediation group would experience greater improvement in global psychosocial functioning compared with the other two groups.
Method
Study Design and Sample
This was a multicenter, randomized, rater-blind outpatient trial conducted between 2009 and 2011. It included three parallel arms (1:1:1) in order to evaluate the efficacy of functional remediation as an add-on therapy compared with psychoeducation and treatment as usual in bipolar disorder. A total of 268 outpatients were enrolled across 10 centers in Spain. These expert centers are integrated in the well-recognized Spanish network for research on mental disorders, the Center for Biomedical Research Network on Mental Health (Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM), which has broad experience in research and clinical management, backed up by several peer-reviewed publications on bipolar disorder. To ensure treatment fidelity, the coordinating center organized three meetings before the start of the study to train participating therapists in the two active interventions.
Participants
Patients included in the study were between the ages of 18 and 55 years and had diagnoses of bipolar I or II disorder according to DSM-IV-TR criteria. Patients were required to have had 3 months of clinical remission before entering the randomization phase. Euthymia was defined as Young Mania Rating Scale score ≤6 (18, 19) and a Hamilton Depression Rating Scale (HAM-D) score ≤8 (20, 21). The screening period was 3 months to facilitate the follow-up of all potential participants prospectively and to ensure clinical remission for 12 consecutive weeks. All patients had to have a moderate to severe degree of functional impairment, as indicated by a score ≥18 on the Functioning Assessment Short Test (22); to ensure that patients were impaired in several domains, they also had to have a score ≥4 in the cognitive domain and a score ≥2 in another domain of the scale.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by an independent ethics committee or an institutional review board at all study sites. All patients received extensive information about the study and provided written informed consent before they were enrolled in the study.
Exclusion criteria were an IQ <85, any medical condition that could affect neuropsychological performance (such as neurological diseases), any comorbid psychiatric condition (including substance abuse or dependence within the past 3 months), or ECT within the past year. Patients were also excluded if they had participated in any structured psychological intervention, such as psychoeducation or cognitive remediation, within the past 2 years.
Interventions
Patients were assigned in a 1:1:1 ratio to receive 21 weeks of functional remediation, psychoeducation, or treatment as usual, stratified by age, sex, and education level. Randomization was accomplished with the use of a computer-generated sequence. In all three groups, pharmacological treatment was prescribed according to local guidelines for the management of bipolar patients.
Functional remediation.
The functional remediation program consisted of 21 weekly sessions, each lasting 90 minutes (Table 1). This intervention addresses neurocognitive issues such as attention, memory, and executive functions, but it focuses even more on enhancing functioning in daily routine. The content of the intervention is based on ecological tasks to be performed in two settings, in the clinic as well as at home. Patients were trained with exercises for memory, attention, problem solving and reasoning, multitasking, and organization in order to improve their functional outcome. Most of the techniques were based on paper-and-pencil tasks and group activities. (For detailed information on the rationale of this intervention, see reference 9. A manual on the intervention is currently in press.)
| Week | Session Content |
|---|---|
| 1 | Introduction to functional remediation: the role of the family. Enhancing practice and reinforcement |
| 2 | What are the most common cognitive dysfunctions in bipolar disorder? Myths and realities |
| 3 | Factors influencing cognitive impairment |
| 4 | What is attention? Strategies for improving it |
| 5 | Strategies for improving attention and its application in daily life |
| 6 | What is memory? Strategies for improving it |
| 7 | Memory: Agenda and other external help |
| 8 | Internal strategies for improving memory |
| 9 | Other strategies for improving memory and the application in daily life |
| 10 | Reading and remembering |
| 11 | Puzzle: retrieving information from the past |
| 12 | Executive functions: self-instructions and self-monitoring |
| 13 | Executive functions: programming and organizing activities |
| 14 | Executive functions: programming activities, establishing priorities, and time management |
| 15 | Executive functions: problem-solving technique |
| 16 | Executive functions: solving problems |
| 17 | Managing stress situations |
| 18 | Training in communication abilities |
| 19 | Improving communication |
| 20 | Improving autonomy and interpersonal relationships |
| 21 | Final session |
Psychoeducation.
The psychoeducation also consisted of 21 weekly sessions of 90 minutes each, aimed at preventing recurrences of bipolar illness by improving four main issues: illness awareness, treatment adherence, early detection of prodromal symptoms of relapse, and lifestyle regularity (23, 24).
Treatment as usual.
In the treatment-as-usual group, patients received prescribed pharmacological treatment without any adjunctive psychosocial therapy.
Assessments
Functional assessment.
The primary efficacy outcome was the change in total score on the Functioning Assessment Short Test from baseline to the end of the intervention. The Functioning Assessment Short Test is a 24-item scale assessing disability in patients with bipolar disorder. It has shown validity, reliability, and sensitivity to change (22). It assesses six functional domains: autonomy (the capacity to make decisions and do things by oneself), occupational functioning (the capacity to maintain a paid job, efficiency of performing tasks at work, working in the field in which the patient was educated, and earning according to the level of the employment position), cognitive functioning (the ability to concentrate, perform simple mental calculations, solve problems, and learn and recall new information), financial issues (the capacity to manage one’s finances), interpersonal relationships (relations with friends and family, involvement in social activities, sexual relationships, and the ability to defend one’s own interests), and leisure time (the capacity to engage in sports or physical activities and to enjoy hobbies). Higher scores indicate higher degrees of functional impairment. As this was the primary outcome measure in this study, a single-blind evaluation was conducted in order to reduce bias. All sites ensured that raters would be blind to treatment allocation throughout the study; ratings were made at each site by an investigator who was specifically trained and assigned to rate functioning and was not involved in treatment delivery.
Clinical and sociodemographic assessment.
The diagnosis of bipolar disorder was confirmed through a clinical interview based on the Structured Clinical Interview for DSM-IV (25). Clinical and sociodemographic data included age, sex, education level, occupational status, body mass index, diagnosis, number and type of episodes, illness duration, age at first hospitalization, age at illness onset, number of hospitalizations, number of suicide attempts, history of psychosis, pharmacological treatment and adherence, family psychiatric history, comorbidities, and several course specifiers, such as rapid cycling, atypical symptoms, melancholia, and psychotic depression.
Neuropsychological assessment.
Patients were tested with a comprehensive neuropsychological battery. It consisted of different tasks divided into six cognitive domains: 1) estimated IQ, which was evaluated with the WAIS-III vocabulary subtest; 2) the processing speed index, which consists of two subtests of the WAIS-III, the digit-symbol coding and symbol search; 3) executive function, which was tested by set shifting, verbal fluency, planning, and response inhibition using the Computerized Wisconsin Card Sorting Test (26), the Stroop Color-Word Interference Test (27), the phonemic (F-A-S) and categorical (animal naming) components of the Controlled Oral Word Association Test (28), the Trail Making Test, part B (29), and the Rey-Osterrieth Complex Figure (30, 31); 4) visual memory and verbal learning/memory, which were assessed with the Rey Osterrieth Complex Figure for visual memory and the California Verbal Learning Test (32) and the Logical Memory Scale (WMS-III) (33) for learning/memory; 5) the working memory index, which was tested with three subtests of the WAIS-III: arithmetic, digits forward and backward, and letter-number sequencing; and 6) attention, which was tested with the Trail Making Test, part A (29), administered together with the Continuous Performance Test–II, version 5 (34), to measure sustained attention.
The neuropsychological evaluation lasted from 100 to 150 minutes, depending on the subject. Participants were offered a 10-minute break in the middle of the assessment, if needed.
Reassessment.
The patients underwent clinical, neuropsychological, and functional reassessment after completion of the intervention, 21 weeks after randomization. Criteria for discontinuation during the study were one or more of the following: missing more than five sessions in any of the intervention groups; hospitalization for any type of episode or clinically meaningful affective relapse; or withdrawal of consent.
Statistical Analysis
Data were analyzed using SPSS, version 18. As a first step, descriptive analyses were conducted. Next, repeated-measures analyses of variance were conducted to assess the impact of the three different treatment arms on participants’ scores on the Functioning Assessment Short Test from the baseline assessment to the postintervention assessment. Effect sizes were also calculated to quantify the effect of the intervention within and between groups. The same procedure was applied to test changes in neurocognition and clinical variables.
In a secondary analysis, the primary efficacy measure (change from baseline in the Functioning Assessment Short Test total score) was evaluated at 6 months with a mixed-model repeated-measures analysis. The model included factors for pooled center, time, baseline Functioning Assessment Short Test score, treatment, and treatment-by-time interaction.
For the main statistical analyses, the last observation carried forward was used to minimize the effect of attrition rates at 6-month follow-up.
Results
A chart of the patient flow through the study is available in the data supplement that accompanies the online edition of this article. A total of 268 patients were enrolled in the study, 29 of whom (10.8%) did not enter the randomization process, for various reasons (withdrew consent, no longer met study criteria, lost to follow-up). The remaining 239 patients were randomly assigned to a treatment arm and entered the 21-week intervention phase. During the intervention, 28.6%, 24.4%, and 17.5% of the patients discontinued in the functional remediation, psychoeducation, and treatment-as-usual groups, respectively (not significantly different between groups).
The participants’ baseline clinical and demographic characteristics at baseline are summarized in Table 2. There were no significant differences across the three groups in most of the variables, suggesting that the randomization process was effective. Illness duration differed significantly between the psychoeducation and treatment-as-usual groups (F=3.27, df=2, p=0.04).
| Functional Remediation (N=77) | Psychoeducation (N=82) | Treatment as Usual (N=80) | ||||
|---|---|---|---|---|---|---|
| Demographic and Clinical Variables | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 40.59 | 9.10 | 39.25 | 8.85 | 40.47 | 8.69 |
| Education (years) | 12.64 | 4.06 | 13.27 | 3.66 | 13.22 | 3.54 |
| Body mass index | 27.10 | 4.91 | 26.60 | 5.47 | 27.53 | 5.53 |
| Estimated premorbid IQ | 105.92 | 12.51 | 103.20 | 11.63 | 107.66 | 14.31 |
| Age at illness onset (years) | 25.76 | 8.46 | 26.83 | 8.61 | 24.29 | 7.69 |
| Illness duration (years) | 14.83 | 9.69 | 12.69 | 8.63 | 16.38 | 8.79 |
| Total number of episodes | 11.86 | 12.54 | 9.93 | 12.13 | 13.03 | 12.27 |
| Number of manic episodes | 3.08 | 3.85 | 3.01 | 3.68 | 2.88 | 3.28 |
| Number of hypomanic episodes | 3.08 | 5.58 | 1.70 | 4.44 | 2.92 | 5.24 |
| Number of depressive episodes | 4.81 | 6.28 | 3.89 | 5.28 | 6.08 | 6.25 |
| Number of mixed episodes | 1.29 | 4.25 | 1.56 | 6.01 | 1.19 | 2.61 |
| Number of hospitalizations | 2.79 | 3.64 | 2.56 | 2.51 | 2.51 | 2.26 |
| Functioning Assessment Short Test score | 30.62 | 9.87 | 29.63 | 10.64 | 29.63 | 9.38 |
| Hamilton Depression Rating Scale score | 4.09 | 2.51 | 4.14 | 2.68 | 4.32 | 2.59 |
| Young Mania Rating Scale score | 1.43 | 1.83 | 1.68 | 2.12 | 1.32 | 1.77 |
Functional Improvement
Longitudinal repeated-measures analyses addressing the treatment effect of the primary outcome measure showed significant differences between groups (Pillai’s Trace=0.065; F=6.51, df=2, p=0.002), suggesting an interaction between treatment allocation and time (pretreatment to posttreatment assessment) (Figure 1).
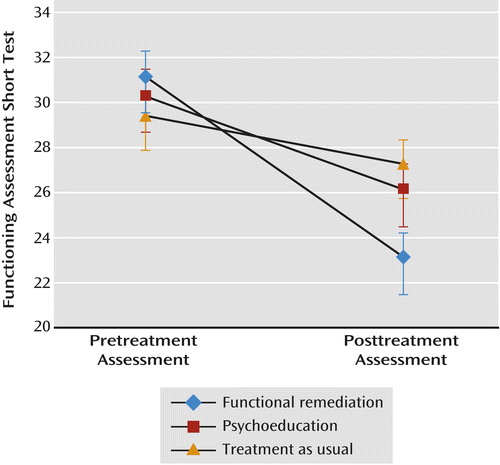
a Functional impairment is measured with the Functioning Assessment Short Test; higher scores indicate greater impairment. Change for the functional remediation group was significantly different from change for the treatment-as-usual group (Pillai’s Trace=0.065; F=6.51, p=0.002). Error bars indicate standard error of the mean.
When Tukey post hoc tests were performed, functional remediation differed significantly from treatment as usual (p=0.001), but fell short of significance when compared with psychoeducation (p=0.056).
Similarly, based on the mixed-model repeated-measures analysis, the change from baseline to 6 months on the functioning scale was significantly greater for the functional remediation group compared with the treatment-as-usual group (p=0.001), but not compared with the psychoeducation group.
Effect sizes within the groups confirmed these findings, showing a large effect for functional remediation (d′=0.93), followed by a small effect for psychoeducation (d′=0.41) and no effect for treatment as usual (d′=0.22). The effect sizes between the groups were also calculated: functional remediation compared with treatment as usual (d′=0.3) and psychoeducation compared with treatment as usual (d′=0.09).
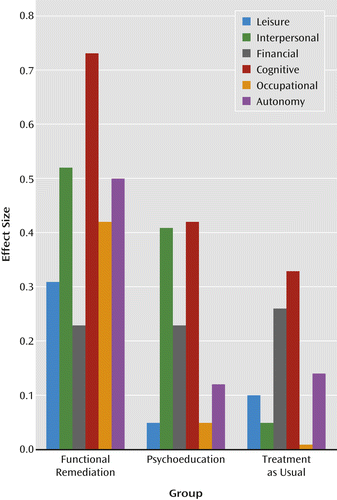
Finally, when changes from the baseline to the posttreatment assessment in the domains of the Functioning Assessment Short Test were analyzed in detail, only two of the six domains were found to be significantly different between groups. Functional remediation showed superiority when compared with treatment as usual in two domains: the interpersonal domain (F=3.95, df=2, p=0.02) and the occupational domain (F=3.57, df=2, p=0.03). Moreover, 5.4% of the patients in the functional remediation group were able to get a job, compared with none in the treatment-as-usual group. Figure 2 presents further details on the within-group effect sizes.
Clinical and Neurocognitive Changes Before and After Intervention
Repeated measures revealed no significant effect of treatment group on the clinical or neurocognitive variables. However, a substantial main effect for time (baseline to posttreatment) was observed when analyzing neurocognitive performance (Pillai’s Trace=0.43; F=7.58, df=15, p<0.001). Tests that were found to show a substantial effect over time, probably as a result of learning and other effects, included perseverative errors on the Wisconsin Card Sorting Test (F=6.86, df=1, p=0.01), Trail Making Test, part A (F=17.47, df=1, p<0.001), and all measures of the California Verbal Learning Test (F=14.23, df=1, p<0.001). (The remaining data are not shown but are available upon request.)
Discussion
The main objective of this multicenter, randomized, rater-blind clinical trial was to evaluate the efficacy of functional remediation, a novel rehabilitation program designed for use in functionally impaired euthymic bipolar patients, compared with pharmacological treatment alone and an established psychoeducational program for bipolar disorder. The results show significantly greater improvements for the functional remediation group on functioning, demonstrating the intervention’s efficacy. Functional remediation showed superiority to treatment as usual in improving psychosocial functioning at study endpoint, with an effect size within the range of most pharmacotherapies and psychosocial therapies. Functional improvement was greater with functional remediation compared with psychoeducation, but the difference fell just short of statistical significance.
To our knowledge, only one previous study, a small, uncontrolled, open trial (12), suggested efficacy of a cognitive remediation program in mildly depressed bipolar patients with functional impairment. That intervention aimed to mitigate residual depressive symptoms and to reduce disability, and it showed some effects. In our sample, patients were required to have relatively low scores on the HAM-D and the Young Mania Rating Scale at baseline in order to diminish the interference of mild depressive or manic symptoms on neurocognitive performance and general functioning.
Interestingly, in the same study (12), the authors observed some improvement in occupational functioning as well, which is in line with our results, as a significantly positive effect was observed in this area (see Figure 2). Occupational functioning is a critical element of functional outcome, and patients with bipolar disorder generally exhibit impairments in this area (35, 36). The functional remediation program thus seemed to help on occupational aspects, which in turn could augment economic autonomy and decrease financial dependence on others. In fact, several patients who received functional remediation were able to get a job or improve their occupational performance after the intervention (5.4% in the functional remediation group and none in treatment-as-usual group).
A significant enhancement in interpersonal relationships was also observed in the functional remediation group. The exercises performed in the program may account for some improvement in this area, especially those related to memory, strategies for encoding information, and other training related to enhancing social skills, improving communication with improved assertiveness, and emotion recognition. Moreover, the patients often had to interact among themselves in order to perform exercises or tasks, which may have increased self-confidence in interpersonal management. Another factor favoring interpersonal relationships might be the group nature of the treatment, in which patients were likely to engage in new relationships with other group members.
Functional remediation was not associated with a significantly greater improvement in the cognitive domain of the Functioning Assessment Short Test. Although the numerical improvement was high, patients allocated to the other interventions also experienced improvement. Given that patients had to have a score ≥4 in the cognitive domain, a possible explanation for the lack of a significant treatment effect in this domain may be an overestimation of cognitive disability at baseline.
Changes in neurocognitive performance did not reach statistical significance at study endpoint. Patients in the functional remediation group showed improvement in verbal memory measures (California Verbal Learning Test), but the differences with the other two groups were not statistically significant. Further research is needed to confirm these results, as a possible learning effect as well as other factors must be taken into account. However, the object of functional remediation is general functional improvement, beyond cognitive improvement (9), and this trial’s inclusion criteria required a certain level of functional disability, but not necessarily neuropsychological impairment. This may explain why improvements in functioning were larger and more significant than neuropsychological changes.
In schizophrenia, there is some evidence that improvement in neurocognition leads to an enhancement of functional outcome, which suggests that cognitive remediation is a useful tool in improving both neurocognition and functioning (13, 17, 37). However, our results suggest that even though some cognitive deficits may persist, patients exhibit greater ability and more strategies to cope with those deficits in daily life after having received a specific training. It may be hypothesized that the enhancement of neurocognitive functioning in our patients may account in part, but not entirely, for changes in functional outcome (8). This was expected, given the nature of the functional remediation program and the emphasis on the use of compensatory skills for coping and everyday functioning as well as improving communication, autonomy, and stress management.
Psychoeducation was not associated with a significant improvement in functional outcome when compared with treatment as usual. Psychoeducation is basically aimed at preventing recurrences, and it may not affect functioning in the short term.
Besides the innovation of this program in targeting and improving functioning, it is worth mentioning some other strengths of this study. First, the study design, which included blinding and randomization, the inclusion of three intervention arms, and a relatively large sample size, allowed us to draw solid conclusions on the effectiveness of functional remediation in euthymic bipolar patients. Second, the multicenter nature of the trial, with 10 Spanish centers, suggests a certain degree of validity for the sample’s representativeness in relation to the Spanish population of patients with bipolar disorder.
The study has also some limitations. First, we focused on the immediate effect of the interventions, and our findings cannot be extrapolated to long-term outcome; follow-up data are needed to clarify this issue. Second, the restrictive inclusion criteria with regard to clinical remission (at least 3 months in remission) and functional impairment (a score ≥18 on the Functioning Assessment Short Test) at baseline may limit the generalizability of our results, but they were established to ensure homogeneity of the sample and internal validity. Finally, the lack of parallel tests in the neuropsychological battery at follow-up does not allow us to isolate learning effects, especially in those tests related to verbal and visual memory.
In summary, the functional remediation program proved to be effective in enhancing functioning in patients with bipolar I and II disorder; significant improvements were seen in occupational and interpersonal functioning. Hence, a combination of medication and functional remediation (for patients with relevant disabilities in daily life) may ultimately improve the outcome of patients suffering from bipolar disorder.
1 : Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270Link, Google Scholar
2 : Heterogeneity in cognitive functioning among patients with bipolar disorder. J Affect Disord 2008; 109:149–156Crossref, Medline, Google Scholar
3 : Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom 2009; 78:285–297Crossref, Medline, Google Scholar
4 : Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord 2012; 14:217–226Crossref, Medline, Google Scholar
5 : Prevalence and effects of mood disorders on work performance in a nationally representative sample of US workers. Am J Psychiatry 2006; 163:1561–1568Link, Google Scholar
6 : Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disord 2007; 9:103–113Crossref, Medline, Google Scholar
7 : Clinical and neurocognitive predictors of functional outcome in bipolar euthymic patients: a long-term follow-up study. J Affect Disord 2010; 121:156–160Crossref, Medline, Google Scholar
8 : Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J Affect Disord 2008; 109:286–299Crossref, Medline, Google Scholar
9 : Functional remediation for bipolar disorder. Clin Pract Epidemiol Ment Health 2011; 7:112–116Crossref, Medline, Google Scholar
10 : Neurocognitive training in patients with bipolar disorders: current status and perspectives. Psychother Psychosom 2012; 81:250–252Crossref, Medline, Google Scholar
11 : A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 2011; 168:472–485Link, Google Scholar
12 : RESEARCH: Cognitive rehabilitation for bipolar disorder: an open trial for employed patients with residual depressive symptoms. CNS Neurosci Ther 2010; 16:298–307Crossref, Medline, Google Scholar
13 : Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull 1999; 25:657–676Crossref, Medline, Google Scholar
14 : Cognitive training in affective disorders improves memory: a preliminary study using the NEAR approach. J Affect Disord 2010; 121:258–262Crossref, Medline, Google Scholar
15 : A controlled randomized treatment study: the effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatr Scand 2004; 109:70–74Crossref, Medline, Google Scholar
16 : Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatr Scand 2005; 111:193–201Crossref, Medline, Google Scholar
17 : Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am J Psychiatry 2007; 164:437–441Link, Google Scholar
18 : [Spanish version of a scale for the assessment of mania: validity and reliability of the Young Mania Rating Scale]. Med Clin (Barc) 2002; 119:366–371 (Spanish)Crossref, Medline, Google Scholar
19 : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
20 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
21 : [Validation of the Castillian version of the Hamilton Rating Scale for Depression]. Actas Luso Esp Neurol Psiquiatr Cienc Afines 1986; 14:324–334 (Spanish)Medline, Google Scholar
22 : Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health 2007; 3:5Crossref, Medline, Google Scholar
23 : Psychoeducation Manual for Bipolar Disorder. Cambridge, UK, Cambridge University Press, 2006Crossref, Google Scholar
24 : A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003; 60:402–407Crossref, Medline, Google Scholar
25 : Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
26 : Wisconsin Card Sorting Test Manual. Odessa, Fla, Psychological Assessment Resources, 1981Google Scholar
27 : Stroop Color and Word Test. Chicago, Stoelting, 1978Google Scholar
28 : Multilingual Aphasia Examination. Iowa City, University of Iowa, 1976Google Scholar
29 : The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, Ariz, Neuropsychology Press, 1993Google Scholar
30 : Test de copia de una figura compleja. Madrid, TEA Ediciones, 1997Google Scholar
31 : L’examen clinique en psychologie. Paris, Presses Universitairies de France, 1958Google Scholar
32 : California Verbal Learning Test. New York, Psychological Corp, 1987Google Scholar
33 : Weschler Memory Scale, 3rd ed. San Antonio, Tex, Psychological Corp, 1997Google Scholar
34 : Conners’ Continuous Performance Test (CPT-II) Computer Program for Windows. Tonawanda, NY, Multi-Health Systems Inc, 2000Google Scholar
35 : Six-month functional outcome of a bipolar disorder cohort in the context of a specialized-care program. Bipolar Disord 2011; 13:679–686Crossref, Medline, Google Scholar
36 : One-year psychosocial functioning in patients in the early vs late stage of bipolar disorder. Acta Psychiatr Scand 2012; 125:335–341Crossref, Medline, Google Scholar
37 : Cognitive mechanisms, psychosocial functioning, and neurocognitive rehabilitation in schizophrenia. Schizophr Res 2003; 63:219–227Crossref, Medline, Google Scholar



