Perinatal Choline Effects on Neonatal Pathophysiology Related to Later Schizophrenia Risk
Abstract
Objective
Deficient cerebral inhibition is a pathophysiological brain deficit related to poor sensory gating and attention in schizophrenia and other disorders. Cerebral inhibition develops perinatally, influenced by genetic and in utero factors. Amniotic choline activates fetal α7-nicotinic acetylcholine receptors and facilitates development of cerebral inhibition. Increasing this activation may protect infants from future illness by promoting normal brain development. The authors investigated the effects of perinatal choline supplementation on the development of cerebral inhibition in human infants.
Method
A randomized placebo-controlled clinical trial of dietary phosphatidylcholine supplementation was conducted with 100 healthy pregnant women, starting in the second trimester. Supplementation to twice normal dietary levels for mother or newborn continued through the third postnatal month. All women received dietary advice regardless of treatment. Infants’ electrophysiological recordings of inhibition of the P50 component of the cerebral evoked response to paired sounds were analyzed. The criterion for inhibition was suppression of the amplitude of the second P50 response by at least half, compared with the first response.
Results
No adverse effects of choline were observed in maternal health and delivery, birth, or infant development. At the fifth postnatal week, the P50 response was suppressed in more choline-treated infants (76%) compared with placebo-treated infants (43%) (effect size=0.7). There was no difference at the 13th week. A CHRNA7 genotype associated with schizophrenia was correlated with diminished P50 inhibition in the placebo-treated infants, but not in the choline-treated infants.
Conclusions
Neonatal developmental delay in inhibition is associated with attentional problems as the child matures. Perinatal choline activates timely development of cerebral inhibition, even in the presence of gene mutations that otherwise delay it.
Infants who later develop schizophrenia already have signs of developmental delay (1–9). Genes associated with schizophrenia, such as NRG1, ErbB4, and DISC1, are expressed at high levels in the fetus, where they normally promote synaptogenesis (10). Environmental risk factors associated with later expression of psychotic symptoms in offspring include maternal malnutrition (from causes ranging from famine to micronutrient deficiency), depression, anxiety, infection, and cigarette smoking (11–15). Abnormalities in the development of cerebral inhibitory neurons are a key pathophysiological defect in schizophrenia (16). Cerebral inhibition develops during fetal and early postnatal life (17, 18). Newborns whose parents are psychotic or whose mothers had depression or anxiety disorders or smoked cigarettes during pregnancy have diminished inhibition (19). Schizophrenia and related mental illnesses are common disorders, and the genetic and perinatal environmental risk factors associated with them occur with high frequency in the general population. Thus, as for other illnesses with early developmental origins, primary prevention must take the form of a safe, population-wide intervention that promotes normal development even in healthy women and infants.
GABA, the inhibitory neurotransmitter of the mature brain, is excitatory in early fetal brain development. Expression of the membrane chloride transporter KCC2 that switches GABA from excitatory to inhibitory is stimulated by activation of postsynaptic α7-nicotinic acetylcholine receptors (17). α7-Nicotinic receptors are expressed at 10-fold higher levels in fetal hippocampus than in adult hippocampus, perhaps reflecting this critical developmental function, but these receptors are diminished in the hippocampal tissue of persons with schizophrenia (20, 21). Innervation of postsynaptic α7-nicotinic receptors by cholinergic axons does not occur until the end of the third trimester (22). Instead, they are activated by the millimolar concentrations of choline in the amniotic fluid (23, 24).
Several maternal risk factors for schizophrenia are associated with decreased choline availability for the fetus (24). Because of the demands for choline as a lipid component of cell membranes, the pregnant woman must increase her dietary intake of foods rich in choline, such as meats and eggs. Malnutrition may prevent that increase. Choline also may be sequestered in the mother’s liver as a result of stress from trauma, anxiety, or depression, thus depriving the fetus. Choline levels are regulated in part by phenylethanolamine methyl transferase (PEMT). Polymorphisms in the gene PEMT associated with diminished choline levels are also associated with schizophrenia (25).
In the inbred mouse strain DBA/2, which has genetically diminished expression of hippocampal α7-nicotinic receptors, dietary supplementation of maternal choline from conception through weaning was found to significantly increase cerebral inhibition in the adult offspring (26). We conducted a similar randomized placebo-controlled trial of effects of perinatal choline on the development of cerebral inhibition in human infants. Perinatal choline supplementation is already advocated in the media because animal models show that it facilitates cognitive function in offspring (27). This is the first randomized human trial conducted under a U.S. Food and Drug Administration (FDA) Investigational New Drug exemption for the purpose of ameliorating a pathophysiological deficit associated with risk for illness. Because the infants in this study were too young to show the effects of the intervention on a wider behavioral repertoire, a group of children who had been recorded electrophysiologically as infants 4 years earlier were studied to assess the behavioral sequelae of diminished infant P50 inhibition (28).
Method
Healthy pregnant women were recruited from Denver obstetrical practices at the beginning of their second trimester. They had no self-reported illicit substance or medical marijuana use in the past 6 months, alcohol dependence, or current nicotine use (confirmed by urinary cotinine levels). Exclusion criteria included maternal history of trimethylaminuria, renal disease, liver disease, or Parkinson’s disease; maternal history of fetal death, fetal congenital malformation, fetal genetic abnormality; and current multiparous pregnancy. Women were also excluded if any fetal abnormality was revealed on initial ultrasound examination. The study was approved by the University of Colorado Institutional Review Board. Mothers were compensated for bringing their infants to the laboratory for recording.
After providing written informed consent, including the assent of the father when available, women completed a 7-day placebo lead-in. Participants whose compliance exceeded 70% by medication vial count were randomly assigned to identical-appearing placebo or drug early in the second trimester. Mothers received standard prenatal care, including standard folate and multivitamin supplements and high-resolution fetal ultrasound examinations during pregnancy. Parents’ psychiatric histories were elicited with the Structured Clinical Interview for DSM-IV Axis I Disorders. The principal diagnoses among parents were major depressive disorder, panic disorder, agoraphobia, generalized anxiety disorder, obsessive-compulsive disorder, and posttraumatic stress disorder. Presence of current psychiatric symptoms in the mother was defined as having sufficient symptoms to meet criteria for an anxiety or depression diagnosis during the pregnancy or an illness with onset prior to pregnancy with continued symptoms causing impairment during the pregnancy.
Mothers were visited at least three times by the study nurse during pregnancy to inquire about compliance with the study medication and prenatal care. Compliance was not otherwise verified. Ten of the mothers were discovered at these visits to have smoked during pregnancy, despite abstinence at enrollment. Because smoking was a prespecified exclusion criterion, their infants’ electrophysiological data were analyzed separately but were included in the safety assessments. Clinical data were acquired by the study nurse or gathered from the hospital records. The infants’ general development was checked at 6 months with the Mullen Scales of Early Learning (29), which assess five developmental domains: 1) gross motor skills, 2) visual perception and discrimination, 3) fine motor planning and control, 4) receptive language, and 5) expressive language. An early learning composite score, derived from four of the scales (all but the gross motor subscale), estimates cognitive ability.
To assess longer-term sequelae of abnormal neonatal cerebral inhibition, we studied a cohort of 93 children who had been recruited 4 years earlier from the same population as the infants in the clinical trial (with the same inclusion and exclusion criteria) and had undergone P50 recordings as newborns (28). We administered the Child Behavioral Checklist, a parental report of symptoms, to the mothers of 50 of these children (54%) at a mean age of 41 months.
Choline Administration as Phosphatidylcholine
Choline is catabolized by intestinal flora to trimethylurea, which has an obnoxious odor. Phosphatidylcholine cannot be catabolized, is well absorbed, and is interchangeably metabolized with choline; oral intake of phosphatidylcholine increases serum choline levels (24). Doses of 3,600 mg each morning and 2,700 mg each evening were administered from a mean of 17.2 weeks (SD=2.1) after the mother’s last menstrual period until delivery. Phosphatidylcholine is 13%–15% choline; thus, this daily dose should provide approximately 900 mg of choline per day, about the same amount as in three large eggs. All mothers, regardless of treatment assignment, were fully advised of dietary practices that would result in similar levels of choline intake. After birth, infants received 100 mg of phosphatidylcholine in an oral suspension once daily or placebo, equivalent to the same treatment as in utero. Choline supplementation for the infant continued to 52 weeks after the mother’s last menstrual period before the pregnancy (until approximately 3 months of age) as estimated by first-trimester ultrasound. Postmortem studies have shown continually increasing KCC2 expression over this same period (30). The Institute of Medicine recommendation for adequate choline intake for infants is 125 mg/day (31).
Statistical Analysis
The ratio of P50 amplitudes discriminates patients with schizophrenia from healthy subjects with larger effect size than the amplitude of either the first or second response alone or their difference (32). However, ratios have more variance than the underlying amplitude measurements. Therefore, nonparametric analyses less affected by this variance have been used to detect genetic and treatment effects. The statistical plan established before the trial specified values ≥0.5 of the P50 ratio as indicating diminished inhibition. Values ≥0.5 for the P50 ratio have previously been associated with variants in the CHRNA7 gene complex, including transmission of alleles that segregate with schizophrenia (33). Analysis of the P50 ratios recorded in this study showed a significant admixture of two components, a lower one with a mean value of 0.24 (SD=0.12) and a higher one with mean value of 0.57 (SD=0.19; χ2=8.50, df=3, p=0.037). P50 ratios ≥0.5 in this study are above the 0.98 probability distribution of the lower component, the same criterion that was used to specify an affected phenotype in previous genetic studies.
Analyses of infants’ other outcomes, including height, weight, and head circumference, were adjusted for gestation, with estimated due dates based on an early-pregnancy ultrasound.
Chi-square tests, Fisher’s exact tests, and t tests were used to compare the treatment groups. For all analyses, an alpha of 0.05 (two-tailed) was the criterion for significance. A post hoc power analysis showed that the groups included in the 1-month analysis had 85% power for determining the observed difference in primary outcome. The power to observe rare side effects was too small to be meaningful.
Results
A total of 193 women were screened, and 100 women were randomly assigned to receive choline or placebo. Seven women withdrew from the study or were lost to follow-up before the infant electrophysiological recording was made (see Figure S1 in the data supplement that accompanies the online edition of this article). Ten women were excluded from the primary outcome analysis because of smoking detected after randomization.
Safety and Tolerability of Choline Treatment
There were no significant differences in maternal outcome during pregnancy and delivery between the 46 women who received choline and the 47 who received placebo (Table 1). The frequency of maternal side effects did not differ significantly between treatment groups. All pregnancies resulted in live births. Seven placebo-treated and four choline-treated women delivered prior to 37 weeks after their last menstrual period. There were no overall differences between groups in Apgar scores, weight, length, head circumference, and perinatal complications (Table 2). At 1 month of age, there were still no significant differences between the two infant groups in measurements or possible side effects from the choline treatment. At 6 months, there were no differences in performance on the Mullen Scales of Early Learning.
| Measure | Placebo Group (N=47) | Choline Group (N=46) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Weight gain (lb) | 32.6 | 14.8 | 32.7 | 18 |
| N | % | N | % | |
| Pregnancy-related hypertension | 3 | 6.4 | 11 | 23.9 |
| Proteinuria | 8 | 17.0 | 8 | 17.4 |
| Edema | 7 | 14.9 | 15 | 32.6 |
| Blurred vision or scotoma | 4 | 8.5 | 5 | 10.9 |
| Gestational diabetes | 2 | 4.3 | 3 | 6.5 |
| Premature labor | 7 | 14.9 | 9 | 19.6 |
| Bleeding | 7 | 14.9 | 4 | 8.7 |
| Polyhydramnios | 2 | 4.3 | 2 | 4.3 |
| Oligohydramnios | 2 | 4.3 | 4 | 8.7 |
| Infant small for gestational age | ||||
| For women <160 cm in height | 0/14 | 0.0 | 3/9 | 33.3 |
| For women ≥160 cm in height | 1/33 | 3.0 | 4/37 | 6.4 |
| Infant large for gestational age | 1 | 2.1 | 2 | 4.3 |
| Cesarean delivery | 11 | 23.4 | 15 | 32.6 |
| Forceps delivery | 1 | 2.1 | 1 | 2.2 |
| Vacuum delivery | 4 | 8.5 | 4 | 8.7 |
| Breech delivery | 5 | 10.6 | 4 | 8.7 |
| Meconium fluid staining | 4 | 8.5 | 4 | 8.7 |
| Abnormal fetal heart rate | 4 | 8.5 | 4 | 8.7 |
| Nuchal cord | 8 | 17.0 | 11 | 23.9 |
| Rh incompatible | 5 | 10.6 | 11 | 23.9 |
| Gestational age <259 days | 7 | 14.9 | 4 | 8.7 |
| Meconium aspiration | 7 | 14.9 | 6 | 13.0 |
| Maternal symptoms | ||||
| Nausea | 15 | 31.9 | 14 | 30.4 |
| Back pain | 15 | 31.9 | 11 | 23.9 |
| Headache | 10 | 21.3 | 15 | 32.6 |
| Diarrhea | 14 | 29.8 | 10 | 21.7 |
| Vomiting | 10 | 21.3 | 8 | 17.4 |
| Shortness of breath | 8 | 17.0 | 7 | 15.2 |
| Abdominal pain | 6 | 12.8 | 8 | 17.4 |
| Dizziness | 8 | 17.0 | 6 | 13.0 |
| Fatigue | 5 | 10.6 | 7 | 15.2 |
| Flatulence | 3 | 6.4 | 6 | 13.0 |
| Anxiety | 6 | 12.8 | 1 | 2.2 |
| Limb pain | 5 | 10.6 | 2 | 4.3 |
| Urinary urgency | 3 | 6.4 | 3 | 6.5 |
| Palpitations | 3 | 6.4 | 3 | 6.5 |
| Paresthesia | 4 | 8.5 | 1 | 2.2 |
| Anorexia | 2 | 4.3 | 2 | 4.3 |
| Chest pain | 3 | 6.4 | 1 | 2.2 |
| Rash | 4 | 8.5 | 0 | 0.0 |
| Body odor | 0 | 0.0 | 0 | 0.0 |
| Measure | Placebo Group (N=47) | Choline Group (N=46) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Status at delivery | ||||
| Male baby | 22 | 46.8 | 26 | 56.5 |
| Jaundice | 25 | 53.2 | 15 | 32.6 |
| Respiratory distress | 1 | 2.1 | 2 | 4.3 |
| Mean | SD | Mean | SD | |
| Duration of gestation (days) | 271 | 14 | 273 | 17 |
| Birth weight (g) | 3,193 | 540 | 3,114 | 636 |
| Birth length (cm) | 50.0 | 2.7 | 49.0 | 2.2 |
| Head circumference | 34.2 | 1.9 | 34.0 | 5.5 |
| Apgar score at 1 minute | 7.7 | 1.3 | 7.5 | 2.0 |
| Apgar score at 5 minutes | 8.7 | 0.7 | 8.7 | 1.0 |
| Status at adjusted age of 4 weeks | ||||
| Weight (g) | 4,580 | 575 | 4,342 | 868 |
| Head circumference (cm) | 37.5 | 1.4 | 36.7 | 5.7 |
| Length (cm) | 55.0 | 2.3 | 53.3 | 8.4 |
| Status at adjusted age of 12 weeks | ||||
| Weight (g) | 6,211 | 744 | 5,764 | 1,125 |
| Head circumference (cm) | 41.0 | 3.3 | 39.6 | 6.3 |
| Length (cm) | 60.4 | 4.2 | 58.7 | 9.4 |
| N | % | N | % | |
| Adverse events reported for at least four infants | ||||
| Diarrhea | 14 | 29.8 | 12 | 26.1 |
| Flatulence | 8 | 17.0 | 6 | 13.0 |
| Constipation | 5 | 10.6 | 7 | 15.2 |
| Cough/runny nose | 6 | 12.8 | 5 | 10.9 |
| Reflux | 4 | 8.5 | 4 | 8.7 |
| Fever | 3 | 6.4 | 4 | 8.7 |
| Vomiting | 3 | 6.4 | 3 | 6.5 |
| Thrush/yeast infection | 2 | 4.3 | 3 | 6.5 |
| Fishy body odor | 0 | 0.0 | 2 | 4.3 |
| Mean | SD | Mean | SD | |
| Mullen Scales of Early Learning at 6 months (T scores)b | ||||
| Gross motor scale | 47.9 | 6.7 | 47.1 | 7.5 |
| Fine motor scale | 51.2 | 6.2 | 51.8 | 6.8 |
| Visual reception scale | 50.2 | 9.1 | 48.0 | 6.6 |
| Receptive language scale | 48.9 | 7.4 | 46.6 | 5.9 |
| Expressive language scale | 41.7 | 4.4 | 41.7 | 7.6 |
| Early learning composite score | 96.7 | 6.8 | 93.5 | 9.6 |
Electrophysiological Assessment of Infants’ Inhibitory Brain Function
Electrophysiological recordings were obtained from 93 infants. Seven did not sleep or had poor quality recordings. Ten women were discovered to have smoked during the first trimester, and some were subsequently observed smoking during nursing visits during the study; their infants’ recordings were not considered in the primary analysis. Two recordings were performed, one at a mean gestation-adjusted age of 33 days, and the other at a mean adjusted age of 89 days at the termination of choline administration. Fifty-two infants received both recordings; data from all infants, whether recorded on one or both days, were analyzed. There were no differences between the choline and placebo groups (or between these two groups and participants who were excluded from the primary analysis) in maternal age, parental socioeconomic status, parental psychiatric illnesses, gestational age at birth, or infant’s sex (see Table S1 in the online data supplement). Week of treatment initiation was not significantly associated with inhibition of the auditory P50 cerebral evoked response.
The primary outcome measure was the P50 inhibition ratio—that is, the amplitude of the P50 response to the second of paired auditory stimuli divided by the amplitude of the response to the first stimulus (Figure 1A). A smaller ratio indicates an inhibition of the cerebral response to the repeated stimulus. The mean P50 ratio was 0.45 (SD=0.24) at age 33 days and 0.39 (SD=0.24) at age 89 days, similar to the values obtained in a previous study of 83 infants of parents with no mental illness and no tobacco exposure (19). The a priori data analysis plan dichotomized P50 ratios into those <0.5, considered intact cerebral inhibition, and those ≥0.5, considered diminished inhibition (Figure 1B).
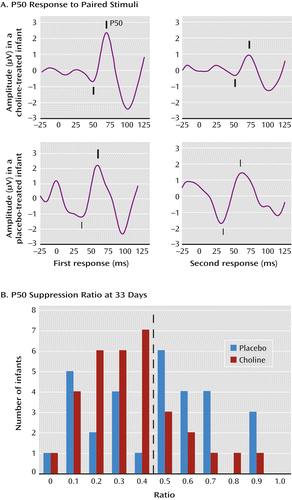
a Panel A shows recordings of P50 averaged evoked potentials in two infants. The gestation-adjusted age is 30 days for the infant treated with choline and 29 days for the infant treated with placebo. For each infant, the two auditory stimuli were delivered 0.5 seconds apart. The diminished amplitude of the second response relative to the first demonstrates cerebral inhibition, quantified as the P50 ratio, which was 0.38 in the choline-treated infant and 0.92 in the placebo-treated infant. Positive potential is upward; amplitudes were measured from the preceding negative potential, both indicated by tick marks. Panel B is a histogram of the P50 ratio at a mean adjusted age of 33 days. The dashed line demarcates the normal level of P50 inhibition, with a ratio <0.5. More choline than placebo-treated infants were in this normal range (χ2=6.90, df 1, p=0.009).
CHRNA7 rs3087454 infant genotype was correlated with P50 inhibition at 33 days in the placebo group (rs=0.38, df=30, p=0.032), whereas the choline-treated group showed no effect (rs=–0.05, df=28, n.s.) (Figure 2; see also Figure S2 in the online data supplement).
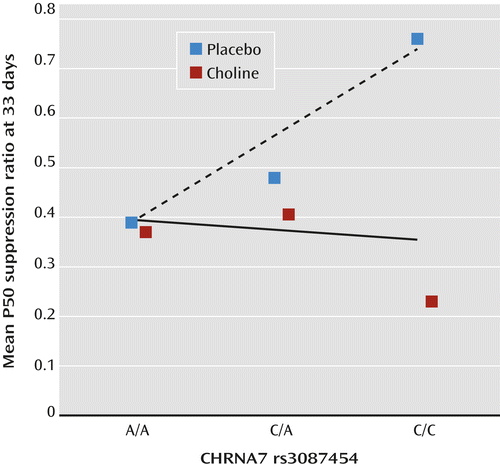
a In the placebo-treated infants, a significant correlation of P50 ratio with rs3087454 was observed (rs=0.38, df=30, p=0.032, dashed line). There was no significant correlation for the choline-treated infants (solid line). Mean values are shown here; for individual values, see Figure S2 in the online data supplement.
Other than the ratio between the first and second P50 responses, the latencies and amplitudes of the individual P50 responses did not differ between the treatment groups. P50 ratios were correlated with the amplitudes of the P50 responses to the second stimulus (first assessment, r=0.69, p<0.001; second assessment, r=0.78, p<0.001) but not to the first stimulus (first assessment, r=0.11, p=0.375; second assessment, r=–0.09, p=0.5).
Of 10 infants whose mothers smoked during pregnancy, eight received placebo and two received choline. Postnatal smoking was not regulated. P50 recording was completed in nine of the 10 tobacco-exposed infants at the first assessment and in seven of the 10 at the second assessment. Five of the seven placebo-treated infants and both the choline-treated infants had P50 ratios <0.5 at the first recording. At the second recording, however, four of the five placebo-treated infants and one of the two choline-treated infants had P50 ratios ≥0.5. (Other subgroup analyses are summarized in Table S3 in the online data supplement).
Sequelae of Diminished Infant P50 Inhibition in Later Childhood
Among the children whose recordings from 4 years earlier indicated deficient P50 suppression in infancy, 24 had greater attentional symptoms at 3.5 years compared with 26 children with intact P50 suppression in infancy, as indicated by parent report on the Child Behavior Checklist (2.75 items [SD=1.51] compared with 1.46 items [SD=1.39]; Wilcoxon rank-sum p=0.004).
Discussion
Choline treatment of mothers during the last two trimesters of pregnancy and of their infants during the first 12 weeks of postnatal life was safely tolerated by both mother and infant. By approximately 5 postnatal weeks adjusted for duration of gestation, infants treated with choline were significantly more likely to have normal cerebral inhibition. The selection of inhibition of the auditory P50 cerebral evoked response as the primary outcome measure reflects issues in the assessment of developmental risk for schizophrenia. The primary measurement must meet four requirements: it must reflect a developmental process whose outcome is measurable in newborns, that occurs in the time window of observation and treatment, that is likely to be directly affected by the treatment, and that has a longer-term relationship to later mental illnesses. All four requirements were addressed by the inhibition of the auditory P50 cerebral evoked response. The measurement of P50 inhibition was previously shown to be feasible in human newborns (28), the switch of GABA from an excitatory to an inhibitory neurotransmitter is a well-established fetal developmental milestone (17, 30), the mouse model experiment showed that a similar mouse inhibition is affected by perinatal choline treatment (26), and family and genetic studies have shown that P50 inhibition in adults and newborns is related to transmission of risk for schizophrenia (19, 33).
Transient Developmental Delay and Risk for Later Schizophrenia
Other evidence of early developmental delay associated with later schizophrenia has been reported. A landmark behavioral observation showed dystonia and poor coordination in home films of infants who would later develop schizophrenia (2). Recent structural MR imaging has shown differences in brain volume in male infants who have parents with schizophrenia (34). Children who later develop schizophrenia have delayed development of attentional control as well as delayed motor, verbal, social, and general cognitive development (1–9). The apparent normalization of cerebral inhibition by 13 weeks in the placebo group is similar to the transient nature of other developmental delays previously observed in infants, children, and adolescents vulnerable to later psychosis (1–9). The apparent normalization may reflect compensatory mechanisms. Mouse Chrna7 null mutants eventually develop GABA-mediated inhibition (17). Maternal oxytocin released during labor, which also promotes the change in polarity of GABA receptor activation, may be one contributor to this compensatory, if delayed, developmental process (35). Although developmental delays may be transient, the risk for later psychosis persists into adult life. In regard to cerebral inhibition, postmortem brain tissue from persons with schizophrenia continues to show only partial conversion of chloride transporters to the adult KCC2 type (30).
Use of Surrogate Markers in the Prevention of Illness
Diminished neonatal P50 inhibition as a target for a treatment intended to prevent schizophrenia is an example of increasing efforts toward early treatment of surrogate markers for later illness. The FDA is now authorized to recognize such efforts, because early treatments directed to a surrogate marker may be the only possible strategy to prevent an illness like schizophrenia that will not appear until decades after the critical period of brain development when preventive treatment is possible (36). The strategy has had notable successes and failures for other illnesses. Treatment of the surrogate marker high serum cholesterol prevents later heart disease. In contrast, while pioglitazone treatment of the surrogate marker glucose instability in type 2 diabetes effectively decreases the risk of later heart disease, rosiglitazone, another drug in the same class that also treats glucose instability, unexpectedly increases the risk of heart disease (37). Both drugs bind to peroxisome proliferator-activated receptor gamma (PPARG), but their mixture of PPARG activation and inhibition was found to differ subtly. Relevant to the present study, both nicotine and choline activate α7-nicotinic receptors, but nicotine also profoundly desensitizes nicotinic receptors and choline does not (23). Maternal smoking is associated with poorer neonatal cerebral inhibition and later childhood behavioral abnormalities, whereas choline appears to have benefits (14, 19).
Mechanism of the Treatment
Sensory gating deficiency assessed by P50 auditory evoked potential response to paired stimuli was initially developed as an endophenotype to assess transmission of genetic risk, which is most often through unaffected parents. P50 ratios ≥0.50 were found in 91% of unrelated patients with schizophrenia, compared with 6% of healthy comparison subjects. Generally one of two unaffected parents and about half of unaffected siblings of schizophrenia probands also had decreased P50 inhibition, and transmission of the phenotype through clinically unaffected family members to an ill proband was genetically linked with the CHRNA7 genotype (33). A second group of investigators replicated the genetic association between P50 gating and CHRNA7 genotype (38). Associations with other known schizophrenia risk genes, COMT and NRG1, have also been reported (39, 40).
Other Actions of Choline
Choline also participates in membrane biosynthesis and one-carbon metabolic pathways, including those involved in DNA methylation (42). However, stimulation of α7-nicotinic receptors requires higher levels of choline (KM=300 µM) than activation by choline kinase, required to synthesize membrane sphingomyelin (KM=17 µM), or by choline dehydrogenase, required for its role as a methyl donor (KM=5.7 µM) (23, 43, 44). The implication is that agonism at α7-nicotinic receptors is much more affected by supplemental dietary intake, particularly if the infant has a genetically determined receptor expression deficit.
Longer-Term Clinical Effect
A surrogate marker has never been used as a target for the prevention of a brain illness. Alzheimer’s disease, with its development over a decade, has been considered, but the development of schizophrenia over several decades, spanning development from fetal life to early adulthood, is more daunting. Therefore, the commitment to look for clinical effects is critical. P50 abnormalities appear to predict early childhood inattentiveness, as shown in the cohort initiated 4 years ago, but more such efforts, including the longer-term follow-up of children who have received supplemental choline, will be needed.
Limitations
This is the first randomized controlled trial of choline supplementation in pregnancy for any reason. There are obvious limitations. Because of the small sample in this initial trial, the frequency of most side effects cannot be determined. Nor does the trial establish whether choline supplementation will actually prevent later mental illness in the offspring. The choline dosage approximated recommended dietary levels, which would increase choline intake to twice normal levels in a mother eating an adequate diet. In the mouse experiment, we supplemented at five times dietary levels. Plasma levels were not measured, however, because they primarily reflect the most recent meal and do not reach a fasting steady state. Thus, we do not know the optimal human dosage.
Conclusions
The beneficial effects of many perinatal interventions, such as folic acid to prevent neural tube deficits, were not definitively established until they were applied population-wide (45). The safety profile of phosphatidylcholine would support such administration. Schizophrenia, with its low rate (10%) of parent-to-child transmission and the high population allele frequencies of most risk genes, including CHRNA7, merits population-wide prevention, but determining the ultimate outcome of the choline intervention represents a formidable problem because the illness itself does not appear until decades later. Changes in biomarkers such as P50 inhibition in the neonatal period in studies such as this one are thus the only available indicator of the putative success of intervention in the most vulnerable developmental period.
1 : The detection of schizophrenia in infancy: a preliminary report. J Nerv Ment Dis 1957; 125:1–24Crossref, Medline, Google Scholar
2 : Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Crossref, Medline, Google Scholar
3 : Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol 1999; 11:487–508Crossref, Medline, Google Scholar
4 : Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59:449–456Crossref, Medline, Google Scholar
5 : Early developmental milestones and risk of schizophrenia: a 45-year follow-up of the Copenhagen Perinatal Cohort. Schizophr Res 2010; 118:41–47Crossref, Medline, Google Scholar
6 : Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398–1402Crossref, Medline, Google Scholar
7 : Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ 1994; 309:699–703Crossref, Medline, Google Scholar
8 : Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry 2011; 168:1295–1302Link, Google Scholar
9 : Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med 2012; 42:743–755Crossref, Medline, Google Scholar
10 : Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci 2009; 32:485–495Crossref, Medline, Google Scholar
11 : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
12 : Schizophrenia in the offspring of antenatally depressed mothers in the northern Finland 1966 birth cohort: relationship to family history of psychosis. Am J Psychiatry 2010; 167:70–77Link, Google Scholar
13 : Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010; 67:889–894Crossref, Medline, Google Scholar
14 : Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry 2010; 67:841–849Crossref, Medline, Google Scholar
15 : Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry 1996; 53:25–31Crossref, Medline, Google Scholar
16 : Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 2011; 168:921–929Link, Google Scholar
17 : Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 2006; 314:1610–1613Crossref, Medline, Google Scholar
18 : Research review: cholinergic mechanisms, early brain development, and risk for schizophrenia. J Child Psychol Psychiatry 2010; 51:535–549Crossref, Medline, Google Scholar
19 : Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull 2011; 37:1200–1208Crossref, Medline, Google Scholar
20 : Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Brain Res Dev Brain Res 1997; 101:93–105Crossref, Medline, Google Scholar
21 : Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 1995; 38:22–33Crossref, Medline, Google Scholar
22 : The acetylcholine innervation of cerebral cortex: new data on its normal development and its fate in the hAPP(SW,IND) mouse model of Alzheimer’s disease. J Neural Transm 2005; 112:149–162Crossref, Medline, Google Scholar
23 : Rhesus monkey α7 nicotinic acetylcholine receptors: comparisons to human α7 receptors expressed in Xenopus oocytes. Eur J Pharmacol 2005; 524:11–18Crossref, Medline, Google Scholar
24 : Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006; 26:229–250Crossref, Medline, Google Scholar
25 : A study of the PEMT gene in schizophrenia. Neurosci Lett 2007; 424:203–206Crossref, Medline, Google Scholar
26 : Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology (Berl) 2008; 198:413–420Crossref, Medline, Google Scholar
27 : Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res 1999; 118:51–59Crossref, Medline, Google Scholar
28 : Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport 2008; 19:79–82Crossref, Medline, Google Scholar
29 : Mullen Scales of Early Learning. Circle Pines, Minn, American Guidance Service, 1995Google Scholar
30 : Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 2011; 31:11088–11095Crossref, Medline, Google Scholar
31 : Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Panthothenic Acid, Biotin, and Choline. Washington, DC, National Academy Press, 1998Google Scholar
32 : Probing the relative contribution of the first and second responses to sensory gating indices: a meta-analysis. Psychophysiology 2011; 48:980–992Crossref, Medline, Google Scholar
33 : Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
34 : Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry 2010; 167:1083–1091Link, Google Scholar
35 : Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 2006; 314:1788–1792Crossref, Medline, Google Scholar
36 : Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004; 1:189–195Crossref, Medline, Google Scholar
37 : Revisiting the rosiglitazone story: lessons learned. N Engl J Med 2010; 363:803–806Crossref, Medline, Google Scholar
38 : The promoter -194 C polymorphism of the nicotinic α 7 receptor gene has a protective effect against the P50 sensory gating deficit. Mol Psychiatry 2004; 9:320–322Crossref, Medline, Google Scholar
39 : Effect of catechol O-methyltransferase val(158)met polymorphism on the p50 gating endophenotype in schizophrenia. Biol Psychiatry 2007; 62:822–825Crossref, Medline, Google Scholar
40 : Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry 2011; 168:930–946Link, Google Scholar
41 : Choline enhances perinatal development of sensory inhibition though alpha7-nicotinic receptors. Society of Biological Psychiatry Abstracts, 2012, pp 1010Google Scholar
42 : Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 2012; 95:1060–1071Crossref, Medline, Google Scholar
43 : Phospholipid biosynthetic enzymes in human brain. Lipids 1997; 32:351–358Crossref, Medline, Google Scholar
44 : Choline dehydrogenase: assay, properties, and inhibitors. Biochem Pharmacol 1981; 30:2993–3000Crossref, Medline, Google Scholar
45 : Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 338:131–137Crossref, Medline, Google Scholar



