Metabolic Syndrome and Metabolic Abnormalities in Bipolar Disorder: A Meta-Analysis of Prevalence Rates and Moderators
Abstract
Objective
Patients with bipolar disorder have high levels of cardiovascular disease risk factors. The presence of metabolic syndrome significantly influences future cardiovascular disease morbidity and mortality. The authors sought to clarify the prevalence and moderators of metabolic syndrome in bipolar patients, accounting for subgroup differences.
Method
The authors searched MEDLINE, PsycINFO, EMBASE, and CINAHL through April 2012 for research reporting metabolic syndrome prevalence rates in bipolar patients. Medical subject headings “metabolic syndrome” and “bipolar” were used in the title, abstract, or index term fields. Manual searches were conducted using the reference lists from identified articles.
Results
The search yielded 81 articles in 37 publications (N=6,983). The overall metabolic syndrome rate was 37.3% (95% confidence interval [CI]=36.1–39.0) using any standardized metabolic syndrome criteria. Compared with general population groups, bipolar patients had higher metabolic syndrome rates (odds ratio=1.98; 95% CI=1.74–2.25). In bipolar patients, older age had a modest effect on the metabolic syndrome rate. The strongest moderator was the region in which the study took place, with the highest rates observed in New Zealand and Australia (64.2% [95% CI=38.3–83.9]) and North America (49.3% [95% CI=29.7–69.3]). Metabolic syndrome was significantly more prevalent in patients currently treated with antipsychotics (45.3% [95% CI=39.6–50.9] than in patients who were antipsychotic free (32.4% [95% CI=27.5–37.4]; odds ratio=1.72 [95% CI=1.24–2.38]).
Conclusions
These findings strongly support the claim that patients with bipolar disorder are at high risk for metabolic syndrome and related cardiovascular morbidity and mortality and require regular monitoring and adequate preventive efforts and treatment for cardio-metabolic risk factors. These findings further suggest that the risk of metabolic syndrome is greater in bipolar patients taking prescribed antipsychotic medication.
Patients with bipolar disorder have nearly five times the age-, race-, and sex-adjusted risk of cardiovascular diseases (1). The observed premature mortality in these patients is largely due to this greater incidence of cardiovascular disease (2). In order to help clinicians focus more on cardiovascular disease risks, the concept of the metabolic syndrome has been introduced. In the general population, the metabolic syndrome is associated with a twofold higher rate of cardiovascular outcomes and a 1.5-fold higher all-cause mortality rate (3). Metabolic syndrome is characterized by a clustering of adverse risk factors for cardiovascular disease and type 2 diabetes, including central obesity, impaired glucose metabolism, dyslipidemia, and hypertension. The criteria for defining metabolic syndrome include those formulated by the National Cholesterol Education Program (the Adult Treatment Panel III [ATP-III] and adapted ATP-III [ATP-III-A] criteria [4, 5]) and the criteria of the International Diabetes Federation (IDF) (6). Current definitions for metabolic syndrome (Table 1) aim for ease of use in clinical settings, and they have similar diagnostic thresholds. However, the role of abdominal obesity is central to the IDF definition, providing ethnicity-specific thresholds for waist circumference (7).
| Variable | ATP-III | ATP-III-A | IDF |
|---|---|---|---|
| Waist circumference (cm) | Men >102, women >88 | Men >102, women >88 | Men ≥94, women ≥80 |
| Blood pressure (mmHg) | ≥130/85b | ≥130/85b | ≥130/85b |
| HDL (mg/dL) | Men <40, women <50 | Men <40, women <50 | Men <40, women <50 |
| Triglycerides (mg/dL) | ≥150 | ≥150 | ≥150 |
| Glucose (mg/dL) | ≥110c | ≥100c | ≥100c |
The causes of increased metabolic syndrome are multifactorial (8) and include an unhealthy lifestyle exacerbated by psychiatric symptoms (9), adverse effects of pharmacological treatments (10, 11), and poorer access to and quality of physical health care (11, 12). As in the general population, genetic and geographical environmental differences may be partially responsible for the observation that estimated rates of metabolic syndrome vary across countries of origin. For example, adopting the ATP-III definition (4), age-adjusted metabolic syndrome population rates are 18.4% for men and 14.4% for women in Europe, 28.8% for men and 31.8% for women in South Asia, 15.5% for men and 23.4% for women of Afro-Caribbean descent (13), and 15.7% for men and 23.7% for women in the United States (14, 15). Although an earlier review article (16) reported differences in metabolic syndrome rates across different countries, to the best of our knowledge, meta-analytic research comparing metabolic syndrome rates across geographical regions in patients with bipolar disorder is lacking. It is also clinically relevant to understand whether the risk profile is the same depending on gender, age, illness duration, and diagnostic subgroup (13–15) in order to detect high-risk groups that should especially be screened and treated. Similarly, it remains to be explored whether metabolic syndrome rates in patients with bipolar disorder differ between treatment settings and between those who are taking antipsychotics and those who are not. If risk stratification were observed, it would help guide clinicians in monitoring and treatment decisions.
Given the uncertainties outlined above, we conducted a systematic review and meta-analysis aiming to clarify the prevalence rate of metabolic syndrome in patients with bipolar disorder, taking into account variations in geographical region, gender, age, illness duration, setting, antipsychotic medication use, and diagnostic subgroups. Our secondary aim was to evaluate studies comparing the prevalence of metabolic syndrome in patients with bipolar disorder and age- and gender-matched healthy comparison subjects.
Method
Inclusion and Exclusion Criteria
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard (17). We focused on patients with defined bipolar disorder irrespective of age, and we were interested in the prevalence rate of metabolic syndrome in each clinical setting (inpatient, outpatient, or mixed). All studies examining metabolic syndrome using ATP-III, ATP-III-A, or IDF criteria were included. We also included studies looking at components of metabolic syndrome such as smoking, which is a closely affiliated cardiovascular disease risk factor, although not an integral component. We excluded studies using any nonstandardized definitions of metabolic syndrome and those with inadequate data for extraction of metabolic syndrome rates.
Search Criteria and Critical Appraisal
We searched MEDLINE, PsycINFO, EMBASE, and CINAHL for articles published through April 1, 2012. The key words “metabolic syndrome” and “bipolar” were entered in the title, abstract, or index term fields. Manual searches were also conducted using the reference lists from recovered articles. Methodological appraisal of each study was performed according to PRISMA standards, including evaluation of bias (confounding, overlapping data, and publication bias) (17). Publication bias was tested using Egger’s regression method (18) and the Begg-Mazumdar test (19), with p<0.05 suggesting the presence of bias. In addition, we created a funnel graph to display the study-specific effect estimates in relation to the standard error.
Statistical Analysis
A meta-analysis based on the studies described above was performed to obtain an optimal estimation of the prevalence of metabolic syndrome in the population with bipolar disorder. The effect size used for the prevalence rate of metabolic syndrome was the proportion, but all analyses were performed by converting proportions into logits. As indicated by Lipsey and Wilson (20), logits are preferred over proportions because the mean proportion across studies underestimates the size of the confidence interval around the mean proportion (because of the compression of the standard error as p approaches 0 or 1) and overestimates the degree of heterogeneity across effect sizes. Lipsey and Wilson indicate that this is especially the case when the observed proportions are <0.2 or >0.80, as was the case in some of the included studies. The logit method circumvents these problems and is the preferred method, especially given our interest in between-study differences. However, for ease of interpretation, all final results were back-converted into proportions. To examine the homogeneity of the effect size distribution, the Q statistic was used (21). When the Q statistic is rejected, the effect size distribution is not homogeneous, implying that the variability in the prevalence rates of metabolic syndrome between studies is larger than can be expected on the basis of sampling error (the error associated with the fact that the estimates of prevalence in the individual studies are based on different samples of subjects). In this case, we produced a mixed random-effects model implying that the observed variance stems from three sources: 1) variance from subject-level sampling error, 2) variance from study characteristics that we could identify (e.g., geographical region), and 3) variance from other systematic random or unmeasured sources. In these analyses, several study characteristics were incorporated, including geographical area, mean age of the study sample, criteria used to define metabolic syndrome (ATP-III, ATP-III-A, IDF, or modified), diagnosis (bipolar disorder I or bipolar disorder mixed), and type of setting (outpatient or inpatient).
Lastly, we pooled data from individual studies to calculate the odds ratio and used Wald tests to compare the statistical prevalence of metabolic syndrome between 1) patients treated with antipsychotic drugs and antipsychotic-free patients, 2) bipolar I patients and mixed/unspecified diagnostic groups, and 3) patients with bipolar disorder and age- and gender-matched general population comparison subjects.
Results
Participants
The initial electronic database search resulted in 989 hits (Figure 1). From 84 candidate articles after the exclusion criteria were applied, our search generated 37 articles containing 81 analyses. The list of included studies is presented in Appendix 1 of the data supplement that accompanies the online edition of this article. The data set comprised 6,983 unique patients, and published studies involved sample sizes that ranged from seven to 1,093 participants. Details on the included studies are presented in Appendix 2 in the online data supplement. The funnel plot was rather asymmetrical, as can be seen in Figure 2. A subset of five analyses examined only patients with bipolar I disorder; no separate data for other diagnostic subgroups were available. Of 37 main individual study analyses, five were conducted among inpatients (N=568), seven were conducted in outpatient settings (N=1,708), and 25 were conducted in mixed samples (N=4,707). The mean age was 42.8 years (SD=5.8) (N=6,286 participants; 33 studies). The mean illness duration was 13.5 years (SD=5.7) (N=1,170 participants; nine studies), and 53.7% of the included participants (N=5,303; 30 studies) were women. The available data were too limited to include analyses on ethnicity differences or separate medications. A list of 36 studies that were excluded because of overlapping samples or lack of separate data for bipolar disorder patients is presented in Appendix 3 in the online data supplement.
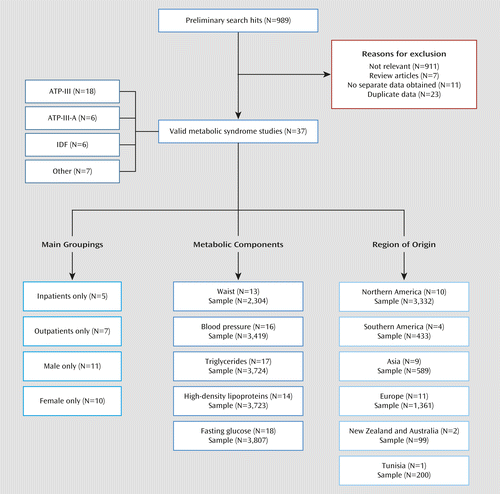
a ATP-III=Adult Treatment Panel III criteria for metabolic syndrome; ATP-III-A=Adult Treatment Panel–Adapted criteria; IDF=International Diabetes Federation criteria.
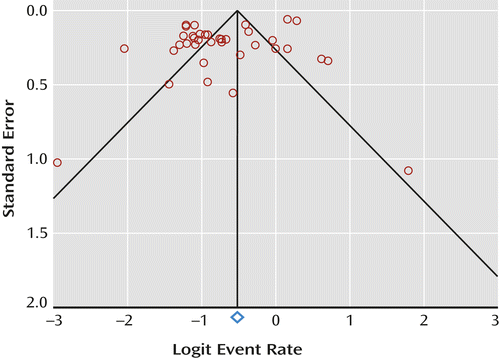
a Begg-Mazumdar test: Kendall’s tau b=0.19, p=0.094. Egger’s bias=–2.81 (95% CI=–5.26 to –0.38), p=0.02.
Prevalence of Metabolic Syndrome in Patients With Bipolar Disorder
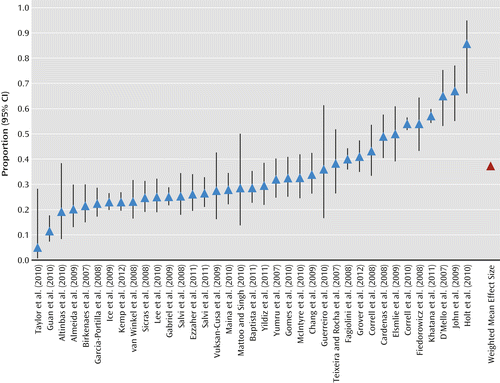
Based on a meta-analysis involving 37 studies with 6,983 unique patients with bipolar disorders, the estimated weighted mean prevalence rate of metabolic syndrome defined with standardized criteria was 37.3% (95% confidence interval [CI]=36.1–39.0). The rates were 29.9% using the definition from ATP-III (N=2,204 from 18 studies; 95% CI=28.0–31.9), 41.5% using ATP-III-A (N=2,799 from 10 studies; 95% CI=39.6–43.4), and 35.6% using IDF (N=1,321 from 11 studies; 95% CI=33.0–38.2). The rate using ATP-III-A or IDF criteria was 43.9% (N=1,160 from six studies; 95% CI=41.4–46.5). In Figure 3, the distribution of the estimated prevalence rates of metabolic syndrome of each individual study, ordered from small to large, is presented with the weighted mean prevalence rates. The Q statistic indicates that the distribution of metabolic syndrome prevalence rates of individual studies was not homogeneous (χ2=585.8, df=36, p<0.0001), implying that the variability in the prevalence rates between studies is larger than can be expected on the basis of sampling error. Consequently, in a next step, we examined the potential role of several study characteristics to explain systematic differences in prevalence rates of metabolic syndrome between studies.
Mixed-Model Analyses
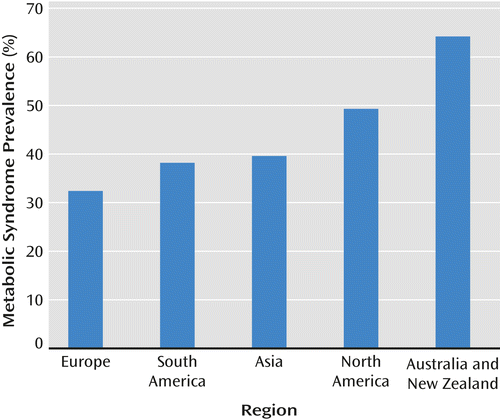
Under the assumption that the variance beyond subject-level error was derived partly from systematic factors that can be identified and partly from unidentified random sources, a mixed model was estimated with the following variables: 1) region where the study took place, 2) mean age of the study participants, 3) criteria used to define metabolic syndrome, 4) diagnostic subgroups, and 5) type of setting. Results indicated that only study region (F=3.21, df=5, 13.2, p=0.041) and mean age of the samples (F=5.23, df=1, 15, p=0.037) significantly explained part of the heterogeneity of the prevalence estimates between the included studies. The prevalence of metabolic syndrome was highest in New Zealand and Australia. In this region, the prevalence rate was 64.2% (95% CI=38.3–83.9), which was significantly higher than in South America at 38.2% (95% CI=20.2–60.0) and in Europe at 32.4% (95% CI=17.4–52.1). Furthermore, the prevalence rate was significantly higher in North America at 49.3% (95% CI=29.7–69.3) than in Europe. No significant differences between other regions were observed in prevalence rates. The mean prevalence in Asian countries was 39.6% (95% CI=24.0–57.6). Tunisia was the only African country with a mean prevalence of 30.0% (95% CI=10.1–62.1). The metabolic syndrome rates across different geographical regions are summarized in Figure 4. With respect to age of study participants, the results revealed that the prevalence of metabolic syndrome was higher in studies with a higher mean age of participants. Furthermore, differences in prevalence rates of different studies could not be explained by illness duration, the criteria used to define metabolic syndrome, diagnostic subgroups, type of treatment setting (inpatient or outpatient), or year of publication (data not presented).
Analyses by Pooling Data of Individual Studies
Six of the included studies also reported metabolic syndrome prevalence in bipolar patients taking antipsychotic medication (N=298) compared with antipsychotic-free bipolar patients (N=339). Bipolar patients who were taking antipsychotic medication were at a significantly greater risk of metabolic syndrome relative to antipsychotic-free patients (odds ratio=1.72, 95% CI=1.24–2.38; χ2=10.99, df=1, p<0.0001): 45.3% (95% CI=39.6–50.9) and 32.4%, respectively (95% CI=27.5–37.4).
Five of the included studies investigated metabolic syndrome prevalence only in patients with bipolar I disorder (N=1,347). These rates were compared with those in mixed or unspecified diagnostic groups (N=5,496). Compared with mixed or unspecified diagnostic groups (39.1%; 95% CI=37.8–40.3), bipolar I patients had a significantly lower risk of metabolic syndrome (24.0%; 95% CI=21.8–26.3) (odds ratio=2.03, 95% CI=1.77–2.32, χ2=103.92, df=1, p<0.001). However, this odds ratio is not adjusted for age, and patients with mixed or unspecified diagnostic groups were older (mean age, 43.1 years [SD=6.2]) than bipolar I patients (mean age, 36.6 years [SD=1.8]) (t=4.21, df=8.1, p=0.003). As a result, results might only reflect age differences.
Individual Metabolic Abnormalities in Patients With Bipolar Disorder
Eleven studies reported the rate of obesity, defined as waist circumference more than 102 cm in men and 88 cm in women (ATP-III or ATP-III-A criteria), while only one study reported the rate of obesity defined as waist circumference more than 94 cm in men and 80 cm in women (IDF criteria).
Odds of Metabolic Syndrome in Bipolar Patients Relative to Comparison Subjects
Six of the included studies reported metabolic syndrome prevalence rates of bipolar patients (N=1,252; 48% men; mean age, 42.2 years [SD=5.2]) relative to age- and gender-matched healthy comparison subjects from the general population (N=87,861; 45.2% men; mean age, 41.1 years [SD=6.3]).
Discussion
General Findings
To our knowledge, this is the first meta-analysis of metabolic syndrome and its components in patients with bipolar disorder. We found that 37.3% of unselected bipolar patients have metabolic syndrome. Our meta-analysis also supports a greater prevalence of metabolic syndrome in bipolar patients relative to the general population. McIntyre et al. (16) documented a greater hazard for metabolic syndrome among bipolar individuals in 12 countries in Europe, Australia, Asia, North America, and South America (N=2,250 in 20 studies). Our meta-analysis adds to the literature that the odds ratio for metabolic syndrome is almost twice as high for bipolar patients relative to age- and gender-matched healthy comparison subjects. Moreover, metabolic syndrome rates were consistently high, regardless of syndrome definition and treatment setting. However, the presence of antipsychotics—consistently associated with cardio-metabolic risk and metabolic syndrome (23, 24)—was significantly associated with metabolic syndrome in bipolar patients. Regarding individual metabolic syndrome criteria, approximately one-half of the patients with bipolar disorder had abdominal obesity, one-half were hypertensive, one in six had significant fasting hyperglycemia (according to the 100 mg/dL threshold), and about 40% had abnormal levels of either HDL or triglycerides. We found 81 valid analyses in 37 studies published in the period from 2005 to April 2012. This indicates that cardio-metabolic risk in patients with bipolar disorder has been a research focus for only the past 8 years, but it is clearly becoming recognized as a key consideration in the long-term health of bipolar patients.
When considering the metabolic syndrome, identifying patients who currently have or who are at high risk for metabolic disorders is a clinical imperative. Knowledge about factors that are associated with the highest metabolic syndrome rates can help identify patients at greater risk. Consistent with population studies (13, 14, 22), no significant differences were found between men and women, indicating that both sexes need the same attention. In contrast, the prevalence of metabolic syndrome was higher in older patients. The fact that longer illness duration was not related to higher metabolic syndrome rates may be due to the limited variation in available illness duration data. However, it is also possible that biological age and not illness duration influences metabolic syndrome rates most strongly, as seen in the general population where advancing age is among the strongest cardio-metabolic risk factors (25). Considering our meta-analytic data, it might be hypothesized that a cumulative long-term effect of poor health behaviors and medication use places an older patient at greater risk of cardio-metabolic disorders. Because we had limited metabolic syndrome data on individual medications and no data in treatment-naive patients, we were not able to draw any conclusions on the precise extent to which use of specific medications accounts for metabolic syndrome hazard in this population. For example, lithium and valproic acid are also associated with significant weight gain (26–28). A recent meta-analysis (28) demonstrated that patients receiving lithium gained more weight than those receiving placebo (odds ratio=1.89, 95% CI=1.27–2.82; p=0.002). However, we did find that patients taking antipsychotics were at greater risk for metabolic syndrome relative to those who were not. Future studies involving drug-naive bipolar patients are recommended.
Research has indicated that patients with bipolar disorder taking olanzapine either alone or as adjunctive treatment to mood stabilizers gained significantly more weight than control subjects taking placebo (26). Similarly, in a pooled analysis of placebo-controlled trials in patients with acute mania associated with bipolar I disorder (29), olanzapine, quetiapine, and risperidone, but not aripiprazole and ziprasidone, as well as valproic acid and oxcarbazepine were all associated with significantly greater weight gain than placebo. Of note, the pooled metabolic syndrome rate of 37.3% (95% CI=36.1–39.0) in bipolar patients appeared to be significantly higher than the recently reported pooled rate of 32.5% (95% CI=30.1–35.0) across 77 studies and 25,692 patients with schizophrenia (30). However, we advise caution in this interpretation, as the latter are more likely to receive long-term antipsychotic treatment, and these pooled rates are subject to strong regional differences as well as the effects of the criteria used to define metabolic syndrome. Therefore, analyses stratified by region and criteria are needed to directly compare metabolic syndrome risk across different psychiatric disorders. In one study that compared metabolic syndrome rates among patients with schizophrenia and bipolar disorder (10), rates were similar (45.9% in schizophrenia patients and 43.2% in bipolar patients), but patients were selected for being treated with at least one antipsychotic agent at the time of assessment.
We also found that in studies including only bipolar I patients, metabolic syndrome rates were lower than in studies with mixed or unspecified diagnostic groups. The older age of the patients in mixed or unspecified diagnostic groups might be a confounding variable. Another possible reason could be that individuals with bipolar II disorder experience a higher burden of depressive symptoms than those with bipolar I disorder (31). It might therefore be hypothesized that levels of depressive symptoms were higher in the mixed or unspecified diagnostic groups than in studies limited to bipolar I patients. A recent meta-analysis demonstrated that depression and depressive symptoms are associated with a risk of higher metabolic syndrome (32).
Despite the large sample size (N=6,983), data for ethnic minority populations were limited. Consequently, no meta-analytic conclusions can be drawn on the differences in metabolic syndrome between different ethnic populations. In contrast, significant geographical differences were found. Although this finding may be somewhat affected by different syndrome criteria, with IDF criteria being associated with the highest rates, these geographic differences also indicate that additional factors, including genetic vulnerability and environmental (lifestyle) effects, may play a role in modifying metabolic syndrome rates in patients with bipolar disorder. Since we did not have any metabolic syndrome data for early-stage or drug-naive bipolar patients relative to general population comparison subjects, it is not clear whether patients with bipolar disorder have a higher intrinsic vulnerability to metabolic abnormalities in the absence of medication. It is known, however, that compared with healthy subjects, bipolar patients have poorer eating behaviors, are less likely to be physically active, and have lower ability to care for themselves (9). In addition, bipolar disorder is associated with higher rates of tobacco and alcohol abuse, which may negatively affect the risk of metabolic syndrome and cardiovascular disease (33). However, in this meta-analysis, data on smoking habits were too limited to draw any conclusions.
Clinical Implications
Our findings demonstrate that both inpatients and outpatients with bipolar disorder, particularly those taking long-term antipsychotics, are a high-risk group for metabolic syndrome. Our data support the recently developed recommendations from the Canadian Network for Mood and Anxiety Treatments that bipolar patients should be proactively screened for metabolic syndrome risk factors (34). The International Society for Bipolar Disorders guidelines (35) suggest that this can be achieved by establishing a risk profile based on personal and family history of cardiovascular disease and diabetes, body mass index, waist circumference, blood pressure, fasting glucose, lipid profile, smoking status, and alcohol use. Patients treated with medications that have the potential for weight gain and metabolic side effects should have weight and metabolic parameters evaluated even more frequently. Patients treated with antipsychotic medications are a particularly high-risk group.
As a second step regarding the prevention and treatment of metabolic syndrome, psychiatrists, physicians, and other members of the multidisciplinary treatment team should educate and help motivate patients with bipolar disorder to improve their lifestyle through the use of effective behavioral interventions, including smoking cessation, dietary measures, and exercise. If lifestyle interventions do not succeed, the treating physician should consider preferential use of or switching to a lower-risk medication or adding a medication for weight reduction to prevent or treat metabolic abnormalities (36).
Future Research
Variables such as concomitant or previous use of lithium, valproic acid, and antipsychotic medication were not controlled in many available studies. Therefore, future studies should investigate the extent to which the risk for metabolic syndrome in drug-naive and untreated patients is lower than in those with specific pharmacological regimes. Second, given that most bipolar patients receive two or more psychotropic drugs, with some receiving two or more atypical antipsychotics during long-term as well as maintenance treatment (37–39), future studies should examine whether patients being treated with mood stabilizers or antipsychotic polytherapy are at higher risk for developing metabolic abnormalities compared with patients receiving antipsychotic monotherapy. Third, future studies should examine if there is an underlying genetic risk for the development of metabolic abnormalities after pharmacotherapy initiation. Examining whether cardio-metabolic outcomes are moderated by genetic factors, but also by clinical characteristics, should become a clinical research priority. Fourth, interventions that target the individual metabolic syndrome components should be evaluated. Fifth, future research should undertake a comprehensive assessment of metabolic syndrome risk factors following, at the very least, recommended monitoring guidelines and should evaluate the optimal monitoring regimen and interventions in patients treated with antipsychotics, those treated with mood stabilizers, and those treated with both medication classes. To date, audits of metabolic monitoring conducted in patients with bipolar disorder and schizophrenia who are taking antipsychotics show that most patients are not receiving adequate surveillance (40). Long-term follow-up will be required in order to accurately document the emergence of some more distal outcomes, such as diabetes and ischemic heart disease.
Limitations
We wish to acknowledge several limitations in the primary data and our meta-analysis. First, considerable methodological heterogeneity was found across studies, which can only be partly controlled by stratification for metabolic syndrome definition, year of publication, gender, and treatment setting. Second, because our study findings were based on cross-sectional rather than on longitudinal or randomized data, directionality of the association between antipsychotic medication use and observed metabolic parameters cannot be deduced with certainty; it is possible that bipolar patients with higher metabolic risk factors were more likely taking atypical antipsychotics or that patients taking atypical antipsychotics were more likely to subsequently develop signs of metabolic syndrome. Third, a threat to the validity of any meta-analysis is publication bias. We did find indications that studies with a smaller sample size reported either lower or higher prevalence rates of metabolic syndrome than studies in a larger sample. Fourth, there were often missing data on duration of illness. It is important to note that illness duration is often a proxy for duration of medication exposure and is related to the patient’s age. Fifth, there were inadequate data on ethnic distribution and specific medications. Sixth, only a few studies compared metabolic syndrome rates to general population samples of the same region or to other comparison groups. Seventh, lifestyle behaviors were not recorded sufficiently, precluding the meta-analytic assessment of these factors as moderating or mediating variables. Finally, we found a marked variation in the quality of studies with limited sample sizes, a reliance largely on cross-sectional retrospective studies, and insufficient pretreatment information on metabolic syndrome in enrolled participants. Nevertheless, to our knowledge, this is the largest study of metabolic syndrome rates in bipolar disorder and the first formal meta-analysis of this important topic.
Conclusions
Our meta-analysis demonstrated that metabolic syndrome risk factors are highly prevalent in patients with bipolar disorder. Bipolar patients should be designated as a vulnerable population comparable to patients with schizophrenia. Treating psychiatrists should implement the necessary screening assessments and, where necessary, referral for treatment. Multidisciplinary assessment of medical and behavioral conditions is needed, and psychiatric treatment facilities should offer and promote healthy lifestyle interventions. Future research should focus on evaluating interventions that target metabolic syndrome risk factors. It should also examine whether cardio-metabolic outcomes are moderated by clinical and treatment characteristics or by genetic factors and study interventions that may avert or delay adverse cardiovascular outcomes.
1 : Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord 2009; 11:657–662Crossref, Medline, Google Scholar
2 : Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001; 58:844–850Crossref, Medline, Google Scholar
3 : The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56:1113–1132Crossref, Medline, Google Scholar
4 : Executive summary of the third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497Crossref, Medline, Google Scholar
5 ;
6 : The metabolic syndrome: a new worldwide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006; 23:469–480Crossref, Medline, Google Scholar
7 : Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–1645Crossref, Medline, Google Scholar
8 : Associations between bipolar disorder and metabolic syndrome: a review. J Clin Psychiatry 2006; 67:1034–1041Crossref, Medline, Google Scholar
9 : Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord 2007; 9:443–452Crossref, Medline, Google Scholar
10 : Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord 2008; 10:788–797Crossref, Medline, Google Scholar
11 : Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry 2009; 194:491–499Crossref, Medline, Google Scholar
12 : Physical illness in patients with severe mental disorders, I: prevalence, impact of medications and disparities in health care. World Psychiatry 2011; 10:52–77Crossref, Medline, Google Scholar
13 : Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans, and white Europeans: a UK population-based cross-sectional study. Diabetologia 2005; 48:649–656Crossref, Medline, Google Scholar
14 : Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287:356–359Crossref, Medline, Google Scholar
15 : The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2003; 163:427–436Crossref, Medline, Google Scholar
16 : Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord 2010; 126:366–387Crossref, Medline, Google Scholar
17 : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097Crossref, Medline, Google Scholar
18 : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634Crossref, Medline, Google Scholar
19 : Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101Crossref, Medline, Google Scholar
20 : Practical meta-analysis. Thousand Oaks, Calif, Sage, 2001Google Scholar
21 : Statistical models for meta-analysis. New York, Academic Press, 1985Google Scholar
22 : Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc 2006; 105:626–635Crossref, Medline, Google Scholar
23 : Antipsychotic drugs and obesity. Trends Mol Med 2011; 17:97–107Crossref, Medline, Google Scholar
24 : Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012; 8:114–126Crossref, Google Scholar
25 : The intersection between aging and cardiovascular disease. Circ Res 2012; 110:1097–1108Crossref, Medline, Google Scholar
26 : Olanzapine in long-term treatment for bipolar disorder. Cochrane Database Syst Rev 2009; 1:CD004367Medline, Google Scholar
27 : Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev 2011; 12:e32–e43Crossref, Medline, Google Scholar
28 : Lithium toxicity profile: a systematic review and meta-analysis. Lancet 2012; 379:721–728Crossref, Medline, Google Scholar
29 : Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord 2010; 12:116–141Crossref, Medline, Google Scholar
30 : Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders: a systematic review and meta-analysis. Schizophr Bull (Epub ahead of print, Dec 29, 2011)Google Scholar
31 : Long-term symptomatic status of bipolar I vs bipolar II disorders. Int J Neuropsychopharmacol 2003; 6:127–137Crossref, Medline, Google Scholar
32 : Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 2012; 35:1171–1180Crossref, Medline, Google Scholar
33 : Impact of substance use on the physical health of patients with bipolar disorder. Acta Psychiatr Scand 2010; 121:437–445Crossref, Medline, Google Scholar
34 : The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry 2012; 24:69–81Medline, Google Scholar
35 : The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord 2009; 11:559–595Crossref, Medline, Google Scholar
36 : Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009; 24:412–424Crossref, Medline, Google Scholar
37 : Advantages and disadvantages of combination treatment with antipsychotics: ECNP Consensus Meeting, March 2008, Nice. Eur Neuropsychopharmacol 2009; 19:520–532Crossref, Medline, Google Scholar
38 : Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry 2011; 72:240–247Crossref, Medline, Google Scholar
39 : Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf 2012; 11:527–542Crossref, Medline, Google Scholar
40 : Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012; 42:125–147Crossref, Medline, Google Scholar



