Abnormalities of Dorsolateral Prefrontal Function in Women With Premenstrual Dysphoric Disorder: A Multimodal Neuroimaging Study
Abstract
Objective
To investigate the neural substrate of premenstrual dysphoric disorder (PMDD), the authors used [15O]H2O positron emission tomography (PET) regional cerebral blood flow (rCBF) and blood-oxygen-level-dependent (BOLD) functional MRI (fMRI) signal measurements during working memory in conjunction with a 6-month hormone manipulation protocol.
Method
PET and fMRI scans were obtained from women with prospectively confirmed PMDD and asymptomatic comparison subjects while they completed the n-back task during three hormone conditions: ovarian suppression induced by the gonadotropin-releasing hormone agonist leuprolide acetate, leuprolide plus estradiol, and leuprolide plus progesterone. Fifteen patients and 15 matched comparison subjects underwent PET imaging. Fourteen patients and 14 comparison subjects underwent fMRI. For each hormone condition, rCBF was measured with [15O]H2O PET, and BOLD signal was measured with fMRI, both during an n-back working memory paradigm. Global Assessment of Functioning Scale (GAF) scores and clinical characteristics were obtained for each patient before hormone manipulation, and symptoms were measured before and during the protocol.
Results
In both the PET and fMRI studies, a main effect of diagnosis was observed, with PMDD patients showing greater prefrontal activation than comparison subjects. In the patient group, the degree to which dorsolateral prefrontal cortex activation was abnormally increased correlated with several dimensions of disease: disability as indicated by GAF scores, age at symptom onset, duration of PMDD, and differences in pre- and postmenses PMDD symptoms.
Conclusions
Abnormal working memory activation in PMDD, specifically in the dorsolateral prefrontal cortex, is related to PMDD severity, symptoms, age at onset, and disease burden. These results support the clinical relevance of the findings and the proposal that dorsolateral prefrontal cortex dysfunction represents a substrate of risk for PMDD. The concordance of the fMRI and PET data attests to the neurobiological validity of the results.
Premenstrual dysphoric disorder (PMDD) is a serious condition that causes significant suffering in 2%–8% of women of reproductive age worldwide and their families (1, 2).
While symptoms of PMDD correspond with menstrual cycle phase, no differences have been detected in plasma hormone levels—including estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH)—between women with PMDD and healthy comparison subjects (5, 6). We previously reported (7) that PMDD symptoms remitted during ovarian suppression induced by gonadotropin-releasing hormone (GnRH) agonist in women with PMDD; when physiological levels of ovarian steroids were replaced, typical symptoms recurred in women with PMDD, but no symptoms emerged in women without PMDD under the same conditions. These findings suggest that women with PMDD have an underlying behavioral sensitivity to the normal physiological events of the menstrual cycle. Although the symptoms undoubtedly involve brain mechanisms, the neural substrate of this differential response has yet to be fully characterized.
There is substantial evidence that hormones affect brain function in healthy women. Neuroimaging studies have demonstrated that ovarian steroids modulate activity in brain regions and circuits involved in processes relevant to the symptoms of PMDD, including the functions of the prefrontal cortex, reward systems, and stress neurocircuitry (8–11). In contrast, few imaging studies have investigated brain function in PMDD, but some differences from comparison subjects have been observed in both the luteal (symptomatic) and follicular (nonsymptomatic) phases of the menstrual cycle (12–14). Similarly, abnormalities of verbal recall have been observed in women with PMDD during both luteal and follicular phases of the menstrual cycle (15). These findings are of particular interest because some of the neurofunctional abnormalities were observed after menstruation, when women with PMDD are not typically symptomatic (16). This observation may be consistent with an underlying trait-like neural vulnerability. However, it remains unclear whether trait or state characteristics of brain function are related to PMDD.
In order to clarify the relationship between brain function and disability in PMDD, we took a multimodal neuroimaging approach, using both fMRI and PET together with a cognitive task that accesses dorsolateral prefrontal cortex circuitry, important in both the cognitive and affective components of PMDD, to validate these associations. If the behavioral impairments of PMDD were linked to altered brain function at the trait level, then not only would abnormal task-related neural activation be seen in women with this disorder, but also the magnitude of those changes would be related to the degree of disability and disease burden, as measured independently of hormone manipulation and brain imaging. To test this hypothesis and the proposal that PMDD involves abnormal reactions to normal hormone levels, we used PET and fMRI to compare neural responses of patients with PMDD and healthy comparison subjects undergoing a 6-month pharmacological protocol that allowed us to carefully control the hormonal milieu to which each woman’s brain was exposed. Additionally, we investigated the relationship between activation in affected brain regions during this protocol and illness severity measured before initiation of the protocol.
Method
Participants
The study participants (Table 1) were women who had regular cycles, were medication free, were not medically ill (as assessed by history, physical examination, neurological examination, MRI, gynecological examination, pap smear within the last year, laboratory tests, and electrocardiogram), and were not pregnant. They were paid for participation according to NIH volunteer guidelines. The study protocol was approved by the NIH Combined Neurosciences Institutional Review Board and Radiation Safety Committee, and all women provided written consent.
| Characteristic | PMDD Patients | Comparison Subjects | ||
|---|---|---|---|---|
| Age (years) | Mean | SD | Mean | SD |
| PET | 37.3 | 7.8 | 36.5 | 8.5 |
| fMRI | 38.1 | 8.2 | 36.0 | 8.0 |
| Body mass index (kg/m2) | ||||
| PET | 27.4 | 5.3 | 25.7 | 4.9 |
| fMRI | 27.4 | 5.4 | 24.6 | 4.8 |
| Education (years) | ||||
| PET | 15.8 | 1.6 | 15.5 | 1.5 |
| fMRI | 15.7 | 2.3 | 16.0 | 1.8 |
| Global Assessment of Functioning Scale scores | ||||
| PET | 62.9 | 6.5 | 91.0 | 1.7 |
| fMRI | 62.9 | 6.3 | 92.0 | 2.9 |
| Age at onset (years) | ||||
| PET | 27.0 | 11.3 | ||
| fMRI | 25.4 | 11.4 | ||
| Duration (years) | ||||
| PET | 10.3 | 8.3 | ||
| fMRI | 12.7 | 9.3 | ||
| Menses-related change in negative affectb | ||||
| PET | 43.0 | 10.0 | ||
| fMRI | 42.7 | 11.3 | ||
| N | N | |||
| Race | ||||
| PET | 6 black, 9 white | 6 black, 9 white | ||
| fMRI | 7 black, 1 Asian, 6 white | 4 black, 1 Asian, 9 white | ||
| Handedness | ||||
| PET | 15 right | 15 right | ||
| fMRI | 14 right | 12 right, 2 left | ||
Comparison subjects were recruited through advertisements. They had no history of menstrual-related mood or behavior disturbances, as confirmed during the 2 months before the study with the same daily self-ratings used by the patients, and they had no current or past axis I diagnoses, including alcohol and substance abuse, as confirmed by the SCID.
Hormone Manipulation Protocol
Throughout the 6-month protocol (Figure 1), participants received monthly injections of the GnRH agonist leuprolide acetate (3.75 mg i.m.). Leuprolide suppresses ovarian function and the secretion of endogenous estradiol and progesterone. For the first 3 months, women received leuprolide only. Following this initial phase, the women entered a 3-month hormone add-back phase while continuing to receive monthly leuprolide injections. Women were randomly assigned to receive 5 weeks of transdermal 17β-estradiol, 0.1 mg/day, or progesterone vaginal suppositories, 200 mg b.i.d., in a double-blind crossover design with a 2-week washout between hormone administration periods. In addition, during the fifth week of estradiol add-back, all women received both estradiol and progesterone to induce menses. Plasma estradiol and progesterone levels were measured at each study visit and before each imaging session (Table 2).
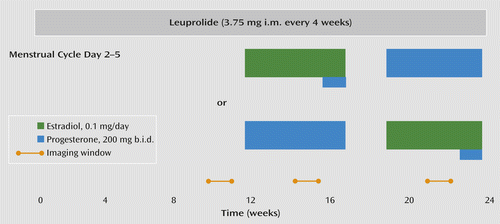
a All participants received injections of the gonadotropin-releasing hormone agonist leuprolide at a dosage of 3.75 mg i.m. every 4 weeks. Plasma follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone levels were measured at each study visit to confirm adequate gonadal suppression. After 3 months of unopposed leuprolide, all participants entered the hormonal add-back phase while continuing to receive monthly leuprolide injections. The women were randomly assigned to receive 5 weeks each of transdermal 17β-estradiol at a dosage of 0.1 mg per day or progesterone vaginal suppositories at a dosage of 200 mg b.i.d. in a double-blind, placebo-controlled crossover fashion with a 2-week washout period between the periods of hormone administration. In addition, during the fifth week of estradiol, all women received both estradiol and progesterone to induce menses. During the hormone add-back phases, women received both patches and suppositories each day (active or placebo, according to add-back phase) in order to blind the treatment team and participants to the hormone being replaced. Scanning windows are indicated by the yellow bars.
| Leuprolide | Progesterone | Estradiol | Repeated-Measures ANOVA | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMDD Patients | Comparison Subjects | PMDD Patients | Comparison Subjects | PMDD Patients | Comparison Subjects | Main Effect of Hormone Condition | Main Effect of Diagnosis | Interaction of Hormone by Diagnosis | ||||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | F | p | F | p |
| Premenstrual Tension Syndrome Scale score | ||||||||||||||||||
| PET | 2.4 | 3.5 | 1.1 | 1.4 | 8.1b | 8.1 | 1.7 | 2.6 | 7.7b | 5.2 | 1.3 | 2.1 | 5.7 | 0.006 | 18.1 | <0.001 | 4.4 | 0.02 |
| fMRI | 2.0 | 3.3 | 1.5 | 2.5 | 8.7b | 6.5 | 0.6 | 1.1 | 6.9b | 6.5 | 0.3 | 0.6 | 3.9 | 0.03 | 27.1 | <0.001 | 7.2 | 0.002 |
| Plasma estradiol (pg/mL) | ||||||||||||||||||
| PET | 25.5 | 14.5 | 26.0 | 12.3 | 35.3 | 23.8 | 32.4 | 18.4 | 127.8 | 72.1 | 112.3 | 44.8 | 59.2c | <0.001 | 0.5 | 0.5 | 0.4 | 0.7 |
| fMRI | 27.3 | 14.7 | 34.5 | 13.3 | 32.0 | 14.1 | 36.4 | 12.9 | 121.0 | 60.4 | 135.0 | 89.4 | 39.4c | <0.001 | 0.8 | 0.4 | 0.08 | 0.9 |
| Plasma progesterone (ng/mL) | ||||||||||||||||||
| PET | 2.2 | 5.2 | 0.5 | 0.2 | 10.5 | 6.4 | 14.0 | 6.4 | 0.6 | 1.2 | 0.5 | 0.2 | 59.2d | <0.001 | 0.6 | 0.5 | 2.6 | 0.09 |
| fMRI | 0.3 | 0.1 | 0.4 | 0.2 | 11.1 | 6.4 | 13.5 | 6.1 | 0.3 | 0.2 | 0.4 | 0.2 | 104.3d | <0.001 | 1.2 | 0.3 | 1.0 | 0.4 |
| 0-back task | ||||||||||||||||||
| PET | 98.4 | 2.8 | 99.0 | 1.4 | 99.2 | 1.2 | 98.5 | 4.0 | 98.8 | 3 | 99.6 | 0.3 | 0.3 | 0.7 | 0.07 | 0.8 | 0.9 | 0.4 |
| fMRI | 97.1 | 3.1 | 98.5 | 3.9 | 97.5 | 3.0 | 99.5 | 0.8 | 98.2 | 3.2 | 99.2 | 1.8 | 0.2 | 0.8 | 4.2 | 0.05 | 0.1 | 0.9 |
| 2-back task | ||||||||||||||||||
| PET | 66.5 | 19.9 | 73.9 | 20.1 | 75.1 | 19.1 | 77.7 | 18.0 | 76.5 | 18.2 | 80.5 | 14.4 | 8.8e | 0.001 | 0.6 | 0.5 | 0.6 | 0.6 |
| fMRI | 66.7 | 18.9 | 88.7 | 12.1 | 75.7 | 22.0 | 94.1 | 6.3 | 77.9 | 17.4 | 91.9 | 9.8 | 7.2e | 0.002 | 12.0 | 0.002 | 1.7 | 0.2 |
Rating Scales
Symptom rating forms were completed daily by all women before the study began and during the 6-month leuprolide protocol. The forms included a 12-item visual analogue scale (an extended version of the scale used during the 3-month baseline screening phase) and a modification of the daily rating form (20, 21), both completed each evening. The daily rating form and the visual analogue scale were used to assess the severity of common symptoms of PMDD, to confirm that each woman with PMDD met DSM-IV criteria for PMDD, and to measure symptom severity in all participants during the hormone manipulation study. Finally, all women completed the Rating Scale for Premenstrual Tension Syndrome (24) during each scanning session.
As part of the SCID, a Global Assessment of Functioning Scale (GAF) score (25), measuring overall psychosocial/somatic disturbance and impairment (100=high function, 0=low function/suicidality), was obtained during random points in each woman’s menstrual cycle before the initiation of pharmacological treatment. These scores, obtained independently of the imaging procedures and the pharmacological protocol, were used to examine the relationship between neural activation abnormalities and PMDD-related disability in the patients.
Imaging
The [15O]H2O PET method was used to measure rCBF, and fMRI was used to measure the BOLD signal during each of three different hormone conditions: ovarian suppression with leuprolide only, leuprolide plus estradiol replacement, and leuprolide plus progesterone replacement (Figure 1). Scanning took place during weeks 8–12 of the leuprolide-alone condition and during the third or fourth week of each hormone replacement condition. During both imaging modalities, the n-back working memory test (26), a cognitive paradigm widely used in neuroimaging, was used to probe prefrontally related activation.
n-Back Task
For the n-back task (see supplemental figure 1 in the data supplement that accompanies the online edition of this article), the numbers 1–4 were displayed on a computer monitor on the points of a diamond shape and were shown in random order (one every 2 seconds). The participants responded to each trial by pressing one of four buttons arrayed on a response box in the same configuration as the stimuli on the monitor. During the 0-back sensorimotor control task, participants pressed the button corresponding to the number shown at the time of the current trial, whereas during the 2-back working memory task, they were instructed to press the button corresponding to the number shown two trials previously.
PET Acquisition and Preprocessing
During each hormone condition, rCBF measurements (10 mCi [15O]H2O/scan) were obtained with a GE Advance three-dimensional scanner (General Electric, Waukesha, Wisc.) during 14 scans of 60-seconds each. Scans from the 2-back and 0-back tasks were alternated and were separated by 6 minutes. The SPM5 software package (London, Wellcome Department of Imaging Neuroscience) was used for image attenuation and correction, reconstruction (32 planes, 6.5 mm full width at half maximum), anatomical normalization to an average template, smoothing (10 mm3 Gaussian kernel), and scaling to remove global blood flow variations. First-level single-subject activation maps (2-back > 0-back) were calculated for each scan session (one statistical map per hormone condition) at a significance threshold of p<0.05.
fMRI Acquisition and Preprocessing
During each hormone condition, participants underwent two runs of the n-back task on a GE 3-T scanner using T2*-weighted gradient echo planar imaging (36 axial slices, 4 mm thickness, 1 mm gap; repetition time=3,000 msec, echo time=35 msec, field of view=24 cm, matrix=64×64). Each run consisted of 14 blocks of 24 seconds each, switching between 2-back and 0-back tasks. Images were preprocessed in SPM5 (slice timing and motion correction, coregistration to a standard template, alignment to the first volume for each subject, and spatial normalization to the Montreal Neurological Institute T1-weighted template). Data were then smoothed with a 10 mm3 Gaussian kernel. First-level single-subject activation maps (2-back > 0-back) were calculated identically to the PET analysis.
Between-Group PET and fMRI Activation Analyses
We used the same approach for the group-level analyses of both the PET and fMRI data. First, for each woman, one first-level activation map per hormone condition was entered as a repeated measure, and diagnosis was entered as a between-group measure in a flexible factorial model in SPM5. Next, for each imaging modality, a binary mask of the main effect of task (2-back > 0-back) across all participants at a significance threshold of p<0.05 (uncorrected) was used to restrict the between-group analyses to regions relevant to task performance. Finally, within these imaging modality-specific masks, between-group differences in working memory activation (2-back > 0-back) were evaluated with a significance threshold of p<0.05 (corrected for false discovery rate). These analyses tested for main-effect between-group differences across all three hormone conditions (i.e., any identified group differences occurring regardless of hormonal state). Additionally, to assess the potential effects of task performance, these analyses were repeated with 2-back accuracy as a covariate of no interest.
Correlations Between GAF Scores and Activation
To test the relationship between activation abnormalities and GAF scores, voxel-wise correlational analyses were performed with data from the PMDD patients. Because comparison subjects had near-ceiling GAF scores with little variation, they were not included in the GAF correlation. For patients, parallel analyses were carried out for the PET and fMRI data. As in the between-group activation analyses, a binary mask of the main effect of task (2-back > 0-back) at p<0.05 (uncorrected) was created for each imaging modality to restrict the correlational analysis to regions relevant to task performance. Next, for each patient, the average activation map (2-back > 0-back) for each hormone condition (three activation maps per patient) was entered into a flexible factorial model with the hormone condition as a repeated measure. Finally, mean-centered GAF scores were entered as contrast weights to produce correlational maps, which were thresholded at p<0.05 (false discovery rate corrected).
Additional Post Hoc Analyses
To determine whether the GAF-activation correlations were consistently observed during each hormone condition, post hoc correlational analyses between the GAF scores and the activation data were carried out separately for each of the three hormone conditions. Using an independently derived dorsolateral prefrontal cortex mask (as cytoarchitechtonically defined in standard stereotaxic space in the postmortem human brain [27]), we extracted average activation values from a 4-mm sphere surrounding the most robust voxel in the across-hormone GAF-PET and GAF-fMRI correlations (one per woman per hormone condition) and correlated these values with each woman’s GAF score within each hormone condition using Predictive Analytics Software (PASW Statistics 18.0, 2009).
We also investigated the correlation of dorsolateral prefrontal cortex activity with additional dimensions of pre-existing PMDD disease severity, including reported age at onset of PMDD, duration of PMDD, and the average change from pre- to postmenses in visual analogue scale scores for negative affective symptoms obtained during baseline screening (before leuprolide treatment). Finally, to further examine the relationship of the dorsolateral prefrontal cortex to the hormone manipulation-induced symptoms of PMDD, dorsolateral prefrontal cortex activity was correlated with symptom ratings obtained during the hormone manipulation protocol (i.e., Premenstrual Tension Syndrome Scale scores at the time of each scan and the 7-day average of irritability ratings from the daily rating form during the week of each scan).
Results
The patients and comparison subjects did not differ significantly in age, racial distribution, body mass index, handedness, or education for either imaging modality (Table 1). Five patients with PMDD had a history of major depression, and one patient met criteria for a history of substance abuse disorder (alcohol). The patients’ GAF scores ranged from 51 to 75 (mean=63, SD=6, including two patients with scores >70 who were less severely affected). We observed no differences between GAF scores acquired during luteal and follicular phases (two-sample t test, α=0.05). GAF scores for the comparison subjects ranged from 90 to 100 (mean=92, SD=3). Reported ages at PMDD onset ranged from 11 to 45 years (mean=26.2 years, SD=11.2). The average duration of PMDD was 11 years (range=1–29 years).
Hormone Levels and Behavioral Findings
For both the PET and fMRI cohorts, blood plasma measurements confirmed ovarian suppression by leuprolide and the replacement of the appropriate ovarian steroid during each add-back condition. The hormone levels of patients and comparison subjects did not differ significantly (Table 2).
Patients experienced a recurrence of typical PMDD symptoms while on both estradiol and progesterone replacement, but they were asymptomatic during the leuprolide-only condition. In contrast, comparison subjects experienced no mood or behavioral symptoms during any of the three hormone conditions.
Task Performance During Imaging
All participants performed well above chance (25%) on all runs across all hormone conditions. There was a main effect of hormone condition on 2-back percent accuracy (number of correct responses divided by the total number of possible responses) during both PET and fMRI scanning. Post hoc comparisons between hormone conditions using the Bonferroni correction revealed that this effect was due to lower scores during the leuprolide-only condition, likely because this testing phase occurred first (although other factors cannot be ruled out). A main effect of diagnosis was observed in the fMRI study (patients performed more poorly than comparison subjects in all three hormone conditions), but not in the PET study; patients’ scores did not differ between PET and fMRI, but comparison subjects scored higher in fMRI compared with PET. We observed no significant diagnosis-by-hormone interaction for either imaging study (Table 2). Covarying for performance did not change the activation results.
Between-Group Differences in PET and fMRI Activation
The results were remarkably consistent across the two imaging modalities. In both data sets, both groups showed a consistent and robust pattern of activation in regions associated with working memory and cognitive control, including the dorsolateral prefrontal cortex, orbitofrontal cortex, cingulate cortex, and inferior and superior parietal lobules. However, a number of highly significant between-group differences were observed.
In both the PET and fMRI activation data, a main effect of group across all hormone conditions taken together was observed at p<0.05, false discovery rate corrected (Figure 2A; see also supplemental table 1 online). In both imaging modalities, the patients showed abnormal prefrontal recruitment, specifically greater activation than comparison subjects throughout the dorsolateral prefrontal cortex (Brodmann’s area [BA] 9/46) bilaterally, as well as in the medial frontal gyrus (BA 8) and the cerebellum (Figure 2A).
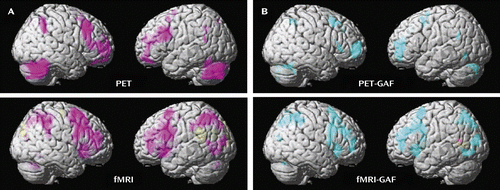
a Panel A shows the between-group differences in activation (2-back > 0-back) using PET and fMRI. Regions in which patients had greater activation than comparison subjects are shown in pink. Panel B shows the correlations between Global Assessment of Functioning Scale (GAF) scores and activation in patients using PET and fMRI. Regions in which these two measures were negatively correlated (the greater the overactivation, the more severe impairment indicated by GAF scores) are shown in blue. For all analyses: p<0.05, false discovery rate corrected; extent threshold=50.
In contrast, regions in which patients showed less activation than comparison subjects were sparse. There were no such findings in the PET data, and only small clusters were found in the cuneus (BA 19), precuneus (BA 7), and lateral temporal cortex (BA 39) in the fMRI data. Covarying for performance demonstrated that the between-group differences were not due to task performance.
Correlations Between GAF Scores and Activation
As in the between-group activation analyses, the correlational findings from the patient PET and fMRI data sets were remarkably congruent (Figure 2B; see also supplemental table 2 online). In both imaging modalities, patients’ 2-back > 0-back activations correlated negatively with GAF scores (the greater the overactivation, the greater the disability as indicated by lower GAF scores) throughout broad swaths of the working memory/executive function pathway bilaterally in regions where this group demonstrated abnormal working memory recruitment: most prominently in the dorsolateral prefrontal cortex (BA 9/46, p<0.005, false discovery rate corrected), as well as in the inferior and superior parietal lobules (BA 40/7). Negative correlations were also observed in the cerebellum in both imaging modalities and were observed in the posterolateral temporal cortex (BA 39) in the fMRI data.
Positive correlations with GAF scores were sparse. There were no such findings in the PET data, and activation correlated positively only in small clusters in the left superior temporal gyrus (BA 22) and the right middle frontal gyrus (BA 10) in the fMRI data.
Anatomical Convergence Between Activation Abnormalities and GAF Activation Correlations
Figure 3 depicts the overlap between maps of the patients’ overactivation and negative correlations between activations and GAF scores. In both the PET and fMRI data, when the correlational and activation findings were viewed together (p<0.05, false discovery rate corrected for both), the patients’ areas of overactivation converged with regions where GAF correlated negatively, indicating that the abnormal neural recruitment in areas involved in working memory is relevant to the degree of clinical disturbance accompanying PMDD.
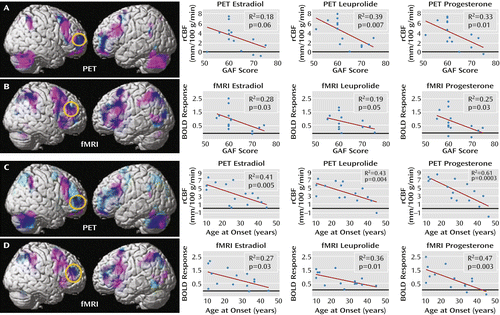
a In the top panels, PET and fMRI scan overlays illustrate the convergence between regions in which patients showed overactivation (pink) and regions in which their activations correlated with their Global Assessment of Functioning Scale (GAF) scores (light blue); overlap is indicated in dark blue; p<0.05, false discovery rate corrected, extent threshold=50. The graphs on the right depict data for each individual by hormonal condition for PET (panel A) average rCBF from a 4-mm sphere centered at coordinates x=36, y=54, and z=4 and for fMRI (panel B) average BOLD signal from a 4-mm sphere centered at coordinates x=44, y=42, and z=28. Circles enclose the maximal voxel in activation-GAF score correlation maps within the dorsolateral prefrontal cortex mask, from which extracted values are plotted for each hormone condition separately. In the bottom panels, PET and fMRI scan overlays illustrate the convergence between regions in which patients showed overactivation (pink) and regions in which their activations correlated with their age at onset (light blue); overlap is indicated in dark blue; p<0.05, false discovery rate corrected, extent threshold=50. The graphs on the right show data for each individual by hormonal condition for PET (panel C) average rCBF from a 4-mm sphere centered on coordinates x=48, y=40, and z=20 and for fMRI (panel D) average BOLD signal from a 4-mm sphere centered on coordinates x=46, y=42, and z=24. Circles enclose the maximal voxel in activation-age at onset correlation maps within the dorsolateral prefrontal cortex mask, from which extracted values are plotted for each hormone condition separately.
Figure 3 also depicts post hoc correlational analyses between the GAF scores and the activation data separately for each of the three hormone conditions. For each imaging modality and each hormone condition, data were plotted for a 4-mm sphere around the voxel with the most robust GAF activation relationship within the independently derived dorsolateral prefrontal cortex region of interest. For both the PET and fMRI data sets, these voxels fell within the patients’ overactivated regions. Moreover, the correlations were similarly present in all of the hormone conditions.
Correlations Between Clinical Characteristics and Dorsolateral Prefrontal Cortex Activation
Both age at onset and duration of PMDD correlated with activation in the dorsolateral prefrontal cortex bilaterally (p<0.005, false discovery rate corrected), with earlier age at onset and longer duration associated with greater activations in this region. Neither age at onset nor duration of illness correlated with GAF scores; however, these two measures were highly correlated with each other (p<0.003 in all cases). To disambiguate the relationships between age at onset and duration with the neuroimaging results, we performed a stepwise regression analysis to determine the relative contributions of the two predictor variables in accounting for the dorsolateral prefrontal cortex findings. For both the PET and fMRI data, age at onset entered into the equation first and accounted for 48% and 37% of the variance, respectively. Including symptom duration in the model did not account for any additional variance in dorsolateral prefrontal cortex activation, indicating that age at onset was the primary predictor variable. Figure 3 depicts the relationship between age at onset and the neuroimaging data, both across- and within-hormone conditions, assessed the same way as the GAF scores.
The correlation between dorsolateral prefrontal cortex activation and the average pre- to postmenses change in negative symptom scores on the visual analogue scale (before study entry) was also significant (p<0.005, false discovery rate corrected); more severe pre- and postmenses changes in symptoms were associated with greater dorsolateral prefrontal cortex activation. Irritability was the predominant symptom that contributed to these correlations. In contrast, no significant correlations between symptoms and dorsolateral prefrontal cortex activation were observed during the hormone manipulation protocol (daily irritability ratings and Premenstrual Tension Syndrome Scale scores).
Discussion
Premenstrual dysphoric disorder is characterized by somatic symptoms and impairment in affective and cognitive processing. We examined neural recruitment in brain circuits relevant to these behaviors, and we tested the relationship between these findings and disability in this disorder. Our results indicate that under controlled hormonal conditions, patients with PMDD display abnormal patterns of activation during working memory. These abnormalities occurred in the absence of significant differences between comparison subjects and patients in plasma hormone levels, thus providing strong support for the presence of a differential neurophysiological response to cognitive challenge in patients with PMDD relative to comparison subjects under equivalent hormone conditions.
Additionally, correlations between the patients’ activations (2-back > 0-back) and several clinical characteristics, measures of disease burden, and prestudy symptoms were observed with both imaging modalities. These correlations occurred within the same circuitry where activation abnormalities were observed, particularly in the dorsolateral prefrontal cortex. The greater the abnormal dorsolateral prefrontal cortex overactivation, the greater the disability and disease burden (as evidenced by lower GAF scores, earlier age at onset, and greater prestudy menses-related change in negative affect). These findings demonstrate the clinical relevance of this neural network in the manifestations of and possibly the biological substrate of risk for PMDD.
Our findings of pathophysiology in PMDD and correlations of that pathophysiology with clinically relevant measures were confirmed with two different imaging platforms that assess related but distinct neurophysiological parameters of brain function: the PET imaging data provide a direct measurement of rCBF, whereas the fMRI data reflect changes in BOLD signal. Although correlation does not permit inference about causality, the fact that our two imaging data sets were highly convergent, both in anatomical distribution and with regard to consistency across hormone conditions, strengthens the assertion that dorsolateral prefrontal cortex function plays an important role in PMDD.
In contrast, we observed no correlations between the symptoms present during each of the three hormone conditions—a negative result that may be consistent with the possibility of a persistent neurobiological diathesis that underlies PMDD. However, this lack of correlation must be considered in light of the fact that these symptoms did not arise in response to naturally fluctuating hormones, as occurs in PMDD, but instead arose in the context of controlled hormonal manipulation. Moreover, this observation requires studies with larger sample sizes, and we cannot rule out the possibility that the pathophysiology could be a sequela of a chronic recurrent mood disorder. Since PMDD symptoms correspond to fluctuating hormone levels, suggesting that state characteristics operate in conjunction with more enduring neural vulnerabilities, future research should explore the interactions between individual hormone conditions and PMDD. Since the dorsolateral prefrontal cortex is a known target for gonadal hormones, as demonstrated by a considerable body of research documenting hormonal modulation of dorsolateral prefrontal cortex function in animals and humans (8, 28–31), this region and other nodes modulated by the prefrontal cortex may show hormone-specific changes and interactions not observable with our current sample size. Indeed, seminal work by Goldman et al. (32–34) demonstrated that the dorsolateral prefrontal cortex in nonhuman primates comes online with a time course similar to that of the pubertal surge in gonadal hormones, a developmental epoch that also may have relevance to the genesis of PMDD and should be carefully studied in this light. In sum, by identifying the prefrontal cortex, specifically the dorsolateral prefrontal cortex, as key in the pathophysiology of PMDD, our research provides insight into potential neural mechanisms contributing to this disorder.
1 : Premenstrual syndrome. Lancet 2008; 371:1200–1210Crossref, Medline, Google Scholar
2 : Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry 2012; 169:465–475Link, Google Scholar
3 : Estimating direct and indirect costs of premenstrual syndrome. J Occup Environ Med 2005; 47:26–33Crossref, Medline, Google Scholar
4 : The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 2003; 28(suppl 3):1–23Google Scholar
5 : Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol 1988; 158:5–11Crossref, Medline, Google Scholar
6 : Mood, sexuality, hormones, and the menstrual cycle, II: hormone levels and their relationship to the premenstrual syndrome. Psychosom Med 1983; 45:503–507Crossref, Medline, Google Scholar
7 : Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338:209–216Crossref, Medline, Google Scholar
8 : Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA 1997; 94:8836–8841Crossref, Medline, Google Scholar
9 : Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 2007; 104:2465–2470Crossref, Medline, Google Scholar
10 : Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci 2005; 25:9309–9316Crossref, Medline, Google Scholar
11 : Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA 2005; 102:16060–16065Crossref, Medline, Google Scholar
12 : Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 2008; 108:87–94Crossref, Medline, Google Scholar
13 : Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry 2011; 69:374–380Crossref, Medline, Google Scholar
14 : Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 2002; 59:851–858Crossref, Medline, Google Scholar
15 : Menstrual phase-independent retrieval deficit in women with PMS. Biol Psychiatry 1995; 38:369–377Crossref, Medline, Google Scholar
16 : Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol 2006; 27:210–216Crossref, Medline, Google Scholar
17 : Prospective assessment of menstrually related mood disorders. Am J Psychiatry 1984; 141:684–686Link, Google Scholar
18 : Some correlates of prospectively defined premenstrual syndrome. Am J Psychiatry 1988; 145:491–494Link, Google Scholar
19 : Measuring amount of symptom change in the diagnosis of premenstrual syndrome. Psychol Assess 1989; 1:277–283Crossref, Google Scholar
20 : Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Women Ment Health 2006; 9:41–49Crossref, Medline, Google Scholar
21 : Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord 1986; 10:127–135Crossref, Medline, Google Scholar
22 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
23 : Radioimmunoassay of plasma progesterone. J Clin Endocrinol Metab 1971; 32:619–624Crossref, Medline, Google Scholar
24 : Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand 1980; 62:177–190Crossref, Medline, Google Scholar
25 : Reliability and validity of DSM-IV axis V. Am J Psychiatry 2000; 157:1858–1863Link, Google Scholar
26 : Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 1999; 9:20–26Crossref, Medline, Google Scholar
27 : Cytoarchitectonic definition of prefrontal areas in the normal human cortex, II: variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 1995; 5:323–337Crossref, Medline, Google Scholar
28 : Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci 2006; 26:2571–2578Crossref, Medline, Google Scholar
29 : Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci 2010; 30:12770–12776Crossref, Medline, Google Scholar
30 : Oestrogen receptor alpha localization in the prefrontal cortex of three mammalian species. J Neuroendocrinol 2008; 20:893–903Crossref, Medline, Google Scholar
31 : The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct 2006; 2:8Crossref, Medline, Google Scholar
32 : Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkey. Science 1974; 186:540–542Crossref, Medline, Google Scholar
33 : Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behav Neurosci 1989; 103:1287–1295Crossref, Medline, Google Scholar
34 : Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain Res 1978; 143:233–249Crossref, Medline, Google Scholar



