Ventral Striatum Activity in Response to Reward: Differences Between Bipolar I and II Disorders
Abstract
Objective
Little is known about the neurobiology of bipolar II disorder. While bipolar I disorder is associated with abnormally elevated activity in response to reward in the ventral striatum, a key component of reward circuitry, no studies have compared reward circuitry function in bipolar I and bipolar II disorders. Furthermore, associations among reward circuitry activity, reward sensitivity, and striatal volume remain underexplored in bipolar and healthy individuals. The authors examined reward activity in the ventral striatum in participants with bipolar I and II disorders and healthy individuals, the relationships between ventral striatal activity and reward sensitivity across all participants, and between-group differences in striatal gray matter volume and relationships with ventral striatal activity across all participants.
Method
Twenty healthy comparison subjects and 32 euthymic bipolar I (N=17) and bipolar II (N=15) patients underwent a neuroimaging reward paradigm during functional MRI scanning, structural scanning, and completed psychometric and clinical assessments.
Results
Region-of-interest analyses revealed significant ventral striatal activity in all participants during reward anticipation that was significantly greater in bipolar II patients compared with the other groups. Ventral striatal activity during reward anticipation correlated positively with reward sensitivity and fun seeking across all participants. Bipolar II patients had significantly greater left putamen volume than bipolar I patients, and left putamen volume correlated positively with left ventral striatal activity to reward anticipation in all participants.
Conclusions
Abnormally elevated ventral striatal activity during reward anticipation may be a potential biomarker of bipolar II disorder. These findings highlight the importance of adopting a dimensional approach in the study of neural mechanisms supporting key pathophysiological processes that may cut across psychiatric disorders.
Early reports have suggested that bipolar disorder type II was more benign than type I because individuals with bipolar II disorder never experience full-blown mania.
Studies have indicated that individuals with bipolar I or bipolar II disorder may be characterized by reward hypersensitivity, and this may underlie a higher presence of approach behaviors and a predisposition to hypomania or mania, emotional lability, and mood dysregulation (4). For example, studies with bipolar individuals have reported higher self-reported scores on measures of reward sensitivity (5, 6), an association between heightened reward sensitivity and a more severe illness course (7), deficits in learning paradigms requiring stopping or delaying responses to rewarding stimuli (8), and a tendency for rewarding life events to precipitate hypomanic or manic episodes (9, 10).
The ventral striatum is a core component of the neural circuitry underlying reward processing (11, 12) and is part of a larger corticolimbic reward circuit that includes the ventral prefrontal cortex (12, 13). Studies with bipolar I patients have reported elevated ventral striatal activity to socially rewarding stimuli (happy faces) in remitted individuals (14), elevated ventrolateral prefrontal cortical activity during reward anticipation during mania (15), and elevated ventral striatal and ventrolateral prefrontal cortical activity to reward anticipation during euthymia (16).
Together, these findings support a reward hypersensitivity model of bipolar disorder, although no studies to our knowledge have compared reward circuitry between bipolar types. Given the dimensional focus of the Research Domain Criteria (17), bipolar I and bipolar II disorder may be conceptualized as a spectrum of disorders in which reward hypersensitivity is a key dimension of pathology, but this remains unexamined. Furthermore, no studies to our knowledge have compared reward circuitry gray matter volume in individuals with bipolar I and bipolar II disorder, although some studies have reported greater ventral striatal volume in bipolar relative to healthy adolescents (18, 19).
Our aims in this study were to examine 1) activity and gray matter volume in reward-related neural circuitry in patients with bipolar I disorder, bipolar II disorder, and healthy comparison subjects and 2) the extent to which reward-circuitry activity during reward processing was associated with reward sensitivity across all participants. Given the key role of the ventral striatum in reward processing (11, 12), along with previous findings (16), our specific focus was the examination of ventral striatal activity, although we also wished to examine whole-brain response to reward. We focused on examining euthymic bipolar I and bipolar II patients to identify neuroimaging measures that were independent of mood state-related influences and in turn could potentially more accurately diagnose bipolar II disorder in patients across different mood states in the future. Previous findings allowed us to hypothesize the following:
| 1. | Bipolar patients would produce greater ventral striatal activity during reward processing than healthy comparison subjects. We also wished to determine whether ventral striatal activity during reward processing would differ between bipolar I and bipolar II patients. | ||||
| 2. | Across all study participants, the magnitude of reward sensitivity and ventral striatal activity to reward processing would be positively correlated. | ||||
| 3. | Bipolar patients would have greater striatal volume than healthy comparison subjects. We also wished to explore the relationship between striatal volume and activity during reward processing across all participants. | ||||
Method
Participants and Questionnaires
Euthymic bipolar I and bipolar II patients were recruited from a preexisting database of well-characterized patients participating in ongoing molecular genetic studies at Cardiff University and through local community mental health teams. Comparison subjects were recruited from the community using advertisements.
A qualified clinical psychologist (X.C.) administered the Mini-International Neuropsychiatric Interview (20) to confirm diagnosis and exclude participants with a recent history (<1 year) of substance abuse or dependence or borderline personality disorder. To quantify the potential presence of residual symptoms of depression and hypomania or mania, the Hamilton Depression Rating Scale (HAM-D) (21) and the Young Mania Rating Scale (22) were also administered. Euthymia was defined as the absence of episodes of depression, mania, or hypomania for 2 months before scanning, based on clinical interview, plus scores <10 on the HAM-D and the Young Mania Rating Scale.
Healthy comparison subjects were also interviewed using the Mini-International Neuropsychiatric Interview, the HAM-D, and the Young Mania Rating Scale. Healthy participants were excluded for the same criteria as patients as well as for any personal or family history of affective or psychotic disorders or current psychiatric disorder.
All participants completed a questionnaire (the BIS/BAS scales) assessing the strength of the behavioral inhibition and behavioral activation systems (23), which regulate aversive motives and inhibition of action in response to negative stimulation (inhibition system), and appetitive motives and preparation to move toward desirable stimuli (activation system). The behavioral activation system is divided into three scales—drive, fun seeking, and reward responsiveness—that account for different aspects of incentive sensitivity. Patients also completed the Hypomanic Checklist–32 (24), a screening tool for bipolar disorder that allows for a detailed description of hypomanic and manic episodes. Verbal IQ was assessed in all participants using the National Adult Reading Test (25).
Two bipolar I patients were excluded because of concerns that they were no longer euthymic on the day of the scan. The final sample included 20 healthy comparison subjects and 32 bipolar patients (17 with bipolar I disorder and 15 with bipolar II disorder). One healthy comparison subject and one bipolar I patient were excluded from the reward paradigm analyses because they had more than three missing trials, suggesting poor engagement or understanding of the task.
All participants gave written informed consent and were paid £20 for participating. The study was approved by the local National Health System research ethics committee.
Experimental Procedure
We employed a monetary reward-processing task that has been shown to activate the ventral striatum (16, 26). The task was back-projected so that participants could see it through a mirror mounted on the head coil. Participants guessed the numeric value of cards in order to win or avoid losing money. Each trial included an anticipation phase, during which participants waited for the outcome of their guess, followed by the actual outcome (see the supplementary material in the data supplement that accompanies the online edition of this article). Participants practiced the task before scanning.
Image Acquisition and Data Analysis
Participants performed the task during an optimized functional MRI (fMRI) data acquisition protocol. An anatomical brain scan was also obtained using a 3-T General Electric HDx MRI scanner. fMRI data processing was performed using FMRIB’s Software Library (www.fmrib.ox.ac.uk/fsl), and preprocessing the functional data followed standard methods (see the online data supplement). The task was modeled within the general linear modeling framework, with crosshair periods as baseline stimuli. The anticipation periods were divided into independent events representing the initial 2 seconds and the remaining anticipation time. Since we assumed the effect of reward anticipation to be more prominent at the beginning of the anticipation, because of potential habituation effects, all the analyses reported here refer to the initial 2 seconds of the anticipation phase.
An a posteriori analysis with time as the within-subject factor (the initial 2 seconds compared with the rest of the anticipation period) and group as the between-subjects factor revealed a significant time-by-group interaction on activity in the ventral striatum (F=4.54, df=2, 47, p<0.05), reflecting a reduction in the group differences as the anticipation period progressed. This result supported our a priori strategy of focusing our analyses on the initial 2 seconds of the anticipation period. We were also interested in all positive outcomes; therefore, our outcome variable included trials in which participants won after anticipating reward and trials in which participants avoided losing after anticipating punishment. Thus, our contrasts of interest were anticipation of reward relative to intertrial crosshair baseline and positive outcome relative to intertrial crosshair baseline. The full model also included events for anticipation of punishment and negative outcomes. The resulting functional images were converted to the standard space of the Montreal Neurological Institute (MNI) using FMRIB’s linear registration tool (FLIRT). Higher-level analysis was carried out using FMRIB’s local analysis of mixed effects (FLAME) (27).
Since our hypotheses were strongly focused on the ventral striatum, region-of-interest analyses were conducted within a mask of two 8-mm radius spheres (Figure 1) based on MNI coordinates (right: x=9, y=9, z=−8; left: x=−9, y=9, z=−8) from previous meta-analyses (28, 29). 3dClustSim within AFNI was used on the normalized images (voxel size, 2 mm isotropic) to determine that a corrected p<0.05 within this region of interest required a voxel-wise threshold of p<0.05 coupled with a cluster size criterion of 41 voxels. Subsequently, we conducted exploratory whole-brain analyses using a voxel-wise Z statistic >2 and a cluster-corrected threshold of p<0.05. In both region-of-interest and whole-brain analyses, we conducted a voxel-wise analysis of variance (ANOVA) to examine the main group factor, and we performed post hoc pairwise between-group comparisons as appropriate. Although not directly related to the objectives of this study, results of the three (group) by two (condition: reward anticipation relative to punishment anticipation) ANOVA and the analysis of the effect of group on punishment anticipation are presented in the online data supplement.
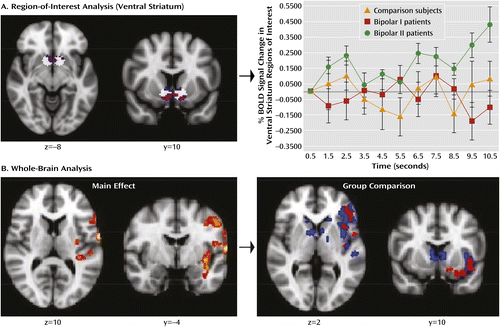
a These images are presented in radiological convention, with the left side of the image representing the right hemisphere and vice versa. The top row (A) shows the anatomical masks used in the regionally restricted analysis accounting for group differences in ventral striatal activity (in white), and the clusters of activity found to be increased in bipolar II patients relative to healthy comparison subjects (blue) and bipolar I patients (red). No other significant differences were observed. For display purposes, an estimation of the percentage of blood-oxygen-level-dependent (BOLD) signal change within the ventral striatum per group is presented on the right. Raw BOLD signal change in the voxels that showed significant activity during the initial 2 seconds of anticipation of reward for all participants (after regressing out the remainder of the anticipation period) was extracted using the Featquery tool in FMRIB’s Software Library. The first value of the time series was considered the baseline in order to calculate the percentage of BOLD signal change. The bottom row (B) shows the BOLD responses obtained from the whole-brain analysis when pooling all of the participants together (left) and the clusters showing significantly greater activity in bipolar II patients relative to healthy comparison subjects (blue) and bipolar I patients (red). No other significant differences were observed.
To test our second hypothesis, we examined relationships between ventral striatal activity (highest peak percentage blood-oxygen-level-dependent [BOLD] signal change within our predefined region of interest) during reward anticipation and outcome and behavioral activation system scores using correlation analyses. The relationships between ventral striatal activity and residual manic or depressive symptoms (HAM-D, Young Mania Rating Scale, and Hypomanic Checklist–32 scores) were also investigated. To avoid floor effects produced by the high frequency of zero scores on clinical measures in healthy comparison subjects, correlation analyses with these scales were performed only for bipolar patients. Given the nonnormal distribution of ventral striatal activity, nonparametric Spearman’s rho analyses were conducted.
Structural MRI data were processed with the fully automated segmentation procedure in Freesurfer (http://surfer.nmr.mgh.harvard.edu/; see the online data supplement). We focused on examining ventral striatal volume, including the putamen and nucleus accumbens, which have been shown to be contiguous ventral striatal regions in humans (30). Following subcortical segmentation, we compared the volumes of these regions between groups using ANOVA and pairwise comparisons, and we performed correlation analyses to examine relationships with peak ventral striatal activity (as for the above correlation).
Demographic and clinical characteristics were compared across groups using ANOVA followed by pairwise comparisons or chi-square tests as appropriate.
Results
Behavioral Responses and Demographic Characteristics
Post hoc analysis of the reaction time to the guessing cues that preceded the reward anticipation periods revealed no differences between groups, suggesting similar levels of engagement.
Table 1 summarizes participants’ demographic and clinical characteristics. Groups were matched for gender, age, and estimated verbal IQ (p>0.1 in all cases). Healthy comparison subjects had lower scores than both bipolar patient groups on residual mood symptoms measured by the HAM-D (F=6.18, df=2, 49, p<0.005) and the Young Mania Rating Scale (F=29.37, df=2, 49, p<0.005); the two bipolar groups had similar scores, although bipolar I patients tended to have higher Young Mania Rating Scale scores (p=0.07). The bipolar patient groups did not differ on the number of manic symptoms experienced during “highs” (Hypomanic Checklist–32), age at first mood episode, age at first bipolar diagnosis, or time from first mood episode until diagnosis. Slightly under half of the bipolar patients had identified a family history of bipolarity in first-degree relatives, the distribution of which did not differ between bipolar I and bipolar II disorder. However, among those not reporting a family history of bipolarity, 64% reported the presence of depression in at least one first-degree relative. No group differences were observed on any of the behavioral activation system scales, but both bipolar patient groups had greater behavioral inhibition system scores than healthy comparison subjects (F=11.61, df=2, 47, p<0.001) (data from two participants were missing for the BIS/BAS questionnaire).
| Characteristica | Healthy Comparison Subjects (N=20) | Bipolar I Patients (N=17) | Bipolar II Patients (N=15) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 42.30 | 5.99 | 42.82 | 7.31 | 40.53 | 8.09 |
| IQ | 115.73 | 7.73 | 114.52 | 8.02 | 113.10 | 6.91 |
| Hamilton Depression Rating Scale score | 0.60b | 0.94 | 3.88 | 3.87 | 2.67 | 2.94 |
| Young Mania Rating Scale score | 0.65b | 0.93 | 3.17 | 2.32 | 1.80 | 2.80 |
| Hypomania Checklist–32 score | 23.73 | 5.99 | 23.13 | 4.82 | ||
| Age at first mood episode (years) | 17.57 | 5.41 | 18.92 | 7.63 | ||
| Age at bipolar diagnosis (years) | 27.06 | 6.43 | 31.00 | 10.05 | ||
| Time from first mood episode to diagnosis (years) | 10.76 | 8.83 | 12.33 | 9.98 | ||
| BIS scale score | 16.61b | 4.95 | 22.50 | 2.70 | 22.43 | 4.12 |
| BAS-RR scale score | 15.00 | 2.56 | 16.44 | 2.58 | 16.00 | 2.60 |
| BAS-FS scale score | 10.06 | 2.94 | 11.69 | 2.08 | 11.21 | 2.81 |
| BAS-D scale score | 9.00 | 2.81 | 10.62 | 2.75 | 10.57 | 2.65 |
| N | % | N | % | N | % | |
| Family history of bipolar disorderc | 7 | 46 | 5 | 36 | ||
| Female | 13 | 65 | 11 | 64 | 9 | 60 |
| Taking mood stabilizersd | 12 | 75 | 10 | 67 | ||
| Taking antipsychoticse | 9 | 56 | 3 | 20 | ||
| Taking antidepressantsf | 6 | 37 | 6 | 40 | ||
| Comorbid anxiety | 5 | 29 | 4 | 27 | ||
Most bipolar patients were taking psychotropic medication; only one bipolar I patient and five bipolar II patients had been medication free for more than 2 months at the time of scanning. Bipolar I patients tended to be taking more antipsychotic medications than bipolar II patients, but the difference fell short of significance (χ2=4.29, p=0.07). The proportion of bipolar I and bipolar II patients with DSM-IV comorbid anxiety diagnoses did not differ.
Ventral Striatal Activity
Region-of-interest analysis for reward anticipation revealed significant ventral striatal activity for the entire sample.
Region-of-interest analysis for positive outcome revealed a cluster of activity that approached but did not meet significance in the entire sample (p<0.05, 25 voxels). However, a main effect of group was observed (F=3.35, df=2, 47, p<0.05), with greater right ventral striatal activity in bipolar I patients than in bipolar II patients (p<0.05, 51 voxels). Bipolar I patients also exhibited significantly greater left ventral striatal activity than healthy comparison subjects, but this difference did not survive cluster-wise correction (p<0.05, 15 voxels).
Whole-Brain Analysis
For the entire sample, significant activity during reward anticipation was observed in the left superior temporal cortex, insula, putamen and claustrum, ventrolateral prefrontal cortex, and precentral gyrus (Figure 1). No significant overall group effect was observed in these regions. Planned pairwise comparisons did, however, reveal significantly greater activity in bipolar II patients relative to healthy comparison subjects and bipolar I patients in the left ventrolateral prefrontal cortex, insula, precentral gyrus, and middle and superior temporal cortex. Bipolar II patients also exhibited greater activity than healthy comparison subjects in the caudate nuclei bilaterally and the left dorsolateral prefrontal cortex (Figure 1 and Table 2). No significant differences were observed between bipolar I patients and healthy comparison subjects.
| Coordinates | |||||
|---|---|---|---|---|---|
| Comparison and Location | Brodmann’s Area | x | y | z | Z |
| Bipolar I > healthy comparison | |||||
| None | |||||
| Healthy comparison > bipolar I | |||||
| None | |||||
| Bipolar II > healthy comparison | |||||
| Superior temporal lobe | 41 | −52 | −20 | 8 | 3.54 |
| Dorsolateral prefrontal cortex | 13 | −44 | 28 | 4 | 3.38 |
| Posterior insula | 13 | −44 | −14 | 12 | 3.36 |
| Anterior insula | 13 | −32 | 24 | 8 | 3.18 |
| Ventrolateral prefrontal cortex | 47 | −44 | 34 | −4 | 3.11 |
| Healthy comparison > bipolar II | |||||
| None | |||||
| Bipolar I > bipolar II | |||||
| None | |||||
| Bipolar II > bipolar I | |||||
| Ventrolateral prefrontal cortex | 47 | −30 | 28 | −20 | 3.24 |
| Posterior insula | 13 | −44 | −14 | 12 | 3.20 |
| Middle temporal lobe | 21 | −36 | −12 | −10 | 3.16 |
| Orbitofrontal cortex | 11 | −30 | 38 | −16 | 3.08 |
Whole-brain analysis of activity to positive outcome did not yield any significant cluster of activity or differences between groups.
Ventral Striatal Activity, Reward Sensitivity, and Clinical Measures
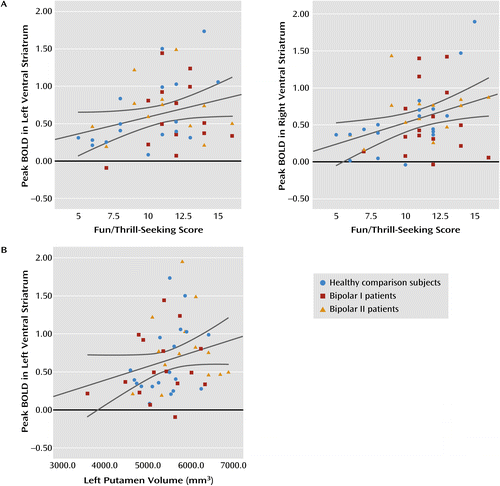
a These scatterplots depict the highest percentage blood-oxygen-level-dependent (BOLD) signal change from baseline during reward anticipation with the score in the behavioral activation system fun-seeking scale for the whole sample (healthy comparison subjects and bipolar I and II patients) and the gray matter volume in the left putamen.
No significant relationships were observed between ventral striatal activity to positive outcome and any behavioral or clinical scores.
Striatal Gray Matter Volumes
Examination of striatal brain volumes indicated significant differences in the left putamen volume between groups (F=3.24, df=2, 48, p<0.05), with bipolar II patients showing enlargement relative to bipolar I patients (mean=5,834 mm3 [SD=648] and mean=5,278 mm3 [SD=701], respectively; p<0.05), as well as relative to healthy comparison subjects, although the latter difference fell short of significance (mean=5,420 mm3 [SD=512]; p=0.064). These analyses survived after controlling for intracranial volume. No differences were observed in the right putamen or accumbens volumes. Across the whole sample, left putamen volume was positively correlated with left ventral striatal activity during reward anticipation (rs=0.33, p<0.05) (Figure 2).
Antipsychotic Medication
As there was a trend toward more bipolar I than bipolar II patients taking antipsychotics, we repeated the above comparisons after excluding those patients taking this class of medication (bipolar I patients, N=8; bipolar II patients, N=12). Again, bipolar II patients had greater ventral striatal activity during reward anticipation than bipolar I patients (p<0.05, 53 voxels) and healthy comparison subjects (p<0.05, 185 voxels). The bipolar I > bipolar II difference in ventral striatal activity during positive outcome no longer reached significance. Similarly, the differences in whole-brain activity between bipolar groups during reward anticipation were not significant here, although bipolar II patients still showed greater activity than healthy comparison subjects in the left ventrolateral prefrontal cortex, insula, caudate, putamen, superior temporal cortex, and precentral gyrus.
Following the approach used in other studies (17, 31, 32), we converted antipsychotic medication into chlorpromazine equivalents (coded 0 for those not taking antipsychotics) and used this index as a covariate. Region-of-interest analyses revealed significantly greater ventral striatal activity in bipolar II patients during reward anticipation than in healthy comparison subjects (p<0.05, 172 voxels) and bipolar I patients (p<0.05, 60 voxels). During positive outcome, the bipolar I > bipolar II difference in ventral striatal activity did not reach significance (p<0.05, 33 voxels). As before, whole-brain analysis failed to replicate significant differences between bipolar I and bipolar II patients, but still revealed significantly greater activity in the left superior temporal cortex, left insula, left caudate, and left ventrolateral prefrontal cortex in bipolar II patients relative to healthy comparison subjects.
Covarying for antipsychotic medication, a nonsignificant main effect of group on left putamen volume was still observed (F=2.57, df=2, 48, p=0.08).
Discussion
Our main goal in this study was to compare ventral striatal activity during reward processing in euthymic bipolar I and bipolar II patients and healthy comparison subjects. While all participants exhibited ventral striatal activity during reward anticipation, bipolar II patients showed significantly greater activity than either bipolar I patients or healthy comparison subjects. During positive outcome, however, bipolar I patients showed significantly greater ventral striatal activity than bipolar II patients, but not healthy comparison subjects, although unlike reward anticipation, this difference became nonsignificant after controlling for potential effects of antipsychotic medication. Also supporting our hypotheses, the magnitude of ventral striatal activity during reward anticipation was positively correlated with the fun-seeking scale across all participants. Furthermore, bipolar II patients had enlarged left putamen volume relative to bipolar I patients, and a positive correlation was observed between left putamen volume and left ventral striatal activity during reward anticipation across the whole sample.
The importance of our findings is twofold. First, we demonstrate that bipolar II patients display elevated ventral striatal activity to reward anticipation not only relative to healthy comparison subjects but also relative to bipolar I patients. This difference between bipolar groups was not explained by differences in the severity of residual mood symptoms or antipsychotic medication load. Second, the pattern of ventral striatal activity observed across all participants during reward anticipation was positively associated with the fun/thrill-seeking component of the behavioral activation system (33). This latter finding concurs with previous research linking ventral striatal activity with the behavioral activation system (34) and with the dimensional approach to the study of psychiatric disorders advocated by the Research Domain Criteria (17), which focuses on dimensions of behavior and related neural circuitry function that cut across psychiatric diagnostic categories. Our finding that ventral striatal activity in response to positive outcome did not correlate with any of the behavioral activation system scales is in agreement with Gray’s model (35), in which this neurobiological system engages in preparation to obtain, but not to receive, reward.
While the two bipolar patient groups and healthy comparison subjects did not differ on self-reported measures of reward sensitivity, bipolar II patients exhibited significantly greater ventral striatal activity during reward anticipation than in other groups. These findings suggest either greater sensitivity of reward neural circuitry activity than self-report measures in differentiating bipolar I and II patients or greater ventral striatal activity for a given level of self-reported fun and thrill seeking during reward anticipation in bipolar II patients than other groups. Either way, elevated ventral striatal activity during reward anticipation may be a potential neural mechanism for greater reward-seeking behavior and mood dysregulation in bipolar II patients. To better understand inconsistencies between findings for self-reported reward sensitivity and ventral striatal activity, future research should include other behavioral measures of reward sensitivity that may be more sensitive to group differences.
Our findings of significantly greater left putamen volume in bipolar II relative to bipolar I patients as well as a positive correlation between left putamen volume and left ventral striatal activity during reward anticipation suggest a structural neural basis for the between-group differences in ventral striatal activity. This finding will require further confirmation given the decrease in statistical significance after controlling for antipsychotic medication load. However, to our knowledge, these results represent the first demonstration of a potential structure-function relationship in reward circuitry in bipolar disorder.
Interestingly, bipolar I patients displayed significantly greater ventral striatal activity than bipolar II patients during positive outcome, suggesting that the former may have been more sensitive to reward receipt than the latter, although this difference did not remain after controlling for the potential effects of antipsychotics. Furthermore, the difference in ventral striatal activity during positive outcome between bipolar I patients and healthy comparison subjects did not meet the cluster-wise significance threshold. Together with the above findings, these may suggest different neural mechanisms for vulnerability to hypomanic or manic episodes in individuals with bipolar I and II disorder—that is, heightened sensitivity to reward anticipation in bipolar II disorder but to reward receipt in bipolar I disorder. Further research would help to clarify this point.
We observed some discrepancies between our results and a previous report (16) showing significantly greater ventral striatal activity during reward anticipation in euthymic bipolar I patients relative to healthy comparison subjects. Unlike the previous study, participants in our study were not led to believe that their remuneration was dependent on task performance. Instead, we asked participants to perform at their best and told them that performance would be compared across participants. Earlier studies have reported that social competition activates the ventral striatum (36). Our findings may suggest that the addition of this social reward component may be less effective at activating the ventral striatum than monetary reward during reward anticipation in bipolar I patients.
Exploratory whole-brain analyses revealed left frontotemporal and insula cortical activity during reward anticipation. These neural regions are associated with reward processing, in particular the ventrolateral prefrontal cortex (12). Between-group comparisons paralleled the pattern of differences in ventral striatal activity: bipolar II patients exhibited greater activity than other groups. While these patterns of between-group differences further support greater sensitivity to reward anticipation in bipolar II than in bipolar I patients or healthy comparison subjects, these results were less robust than the ventral striatal findings. It would therefore be useful to replicate these findings and further clarify to what extent the difference between bipolar patient groups may be related to antipsychotic medication.
Some limitations are noted. The majority of patients with bipolar disorder were receiving psychotropic medications. The potentially confounding effects of psychotropic medication in bipolar neuroimaging research have been discussed previously (37). Nevertheless, we were able to replicate our findings regarding the differences in ventral striatal activity after controlling for antipsychotic load (in chlorpromazine equivalents) and after excluding patients who were taking this medication class. Our findings in the ventral striatum for reward anticipation appeared to be robust enough to survive these additional analyses, but further replication is warranted, especially because the duration of medication treatment was not recorded during this study. No significant group differences in self-reported measures of reward sensitivity were observed, and our task does not produce a sensitive behavioral output to detect between-group differences in reward sensitivity. Future studies should include other behavioral self-reported measures of reward sensitivity.
In summary, we report significantly elevated ventral striatal activity in bipolar II patients compared with bipolar I patients and healthy comparison subjects, specifically during reward anticipation, and a significant positive correlation between ventral striatal activity to reward anticipation and fun/thrill seeking. These findings suggest that abnormally elevated ventral striatal activity during reward anticipation may be a biomarker of bipolar II disorder, as previously shown for bipolar I disorder, that could ultimately be used to help discriminate bipolar from unipolar depressed patients. Moreover, bipolar I and bipolar II patients may be distinguished by functional differences in ventral striatal activity during reward anticipation and outcome, which in turn may suggest different neural mechanisms predisposing to mood dysregulation across bipolar I and II disorders. Our findings are among the first to identify patterns of abnormal neural activity in bipolar II disorder that may be potential targets for therapeutic interventions for this disabling psychiatric disorder.
1 : Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 2011; 68:241–251Crossref, Medline, Google Scholar
2 : Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord 2011; 131:59–67Crossref, Medline, Google Scholar
3 : Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar II, minor bipolar disorders, and hypomania. J Affect Disord 2003; 73:133–146Crossref, Medline, Google Scholar
4 : Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clin Psychol Rev 2008; 28:1188–1205Crossref, Medline, Google Scholar
5 : Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J Psychopathol Behav Assess 2001; 23:133–143Crossref, Medline, Google Scholar
6 : The functioning of the behavioral activation and inhibition systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Pers Individ Dif 2007; 42:1323–1331Crossref, Google Scholar
7 : Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord 2008; 10:310–322Crossref, Medline, Google Scholar
8 : Severity of bipolar disorder is associated with impairment of response inhibition. J Affect Disord 2009; 116:30–36Crossref, Medline, Google Scholar
9 : Increases in manic symptoms after life events involving goal attainment. J Abnorm Psychol 2000; 109:721–727Crossref, Medline, Google Scholar
10 : A goal-striving life event and the onset of hypomanic and depressive episodes and symptoms: perspective from the behavioral approach system (BAS) dysregulation theory. J Abnorm Psychol 2007; 116:105–115Crossref, Medline, Google Scholar
11 : Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 2000; 84:3072–3077Crossref, Medline, Google Scholar
12 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26Crossref, Medline, Google Scholar
13 : A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 2003; 18:263–272Crossref, Medline, Google Scholar
14 : Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Crossref, Medline, Google Scholar
15 : Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp 2010; 31:958–969Crossref, Medline, Google Scholar
16 : Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord 2012; 14:249–260Crossref, Medline, Google Scholar
17 : Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
18 : Subcortical differences among youths with attention-deficit/hyperactivity disorder compared to those with bipolar disorder with and without attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2009; 19:31–39Crossref, Medline, Google Scholar
19 : Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. J Affect Disord 2007; 104:147–154Crossref, Medline, Google Scholar
20 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
21 : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
22 : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
23 : Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol 1994; 67:319–333Crossref, Google Scholar
24 : The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord 2005; 88:217–233Crossref, Medline, Google Scholar
25 : Verbal fluency: a NART-based equation for the estimation of premorbid performance. Br J Clin Psychol 1992; 31:327–329Crossref, Medline, Google Scholar
26 : Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry 2009; 166:64–73Link, Google Scholar
27 : General multilevel linear modeling for group analysis in fMRI. Neuroimage 2003; 20:1052–1063Crossref, Medline, Google Scholar
28 : Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex 2008; 18:2735–2747Crossref, Medline, Google Scholar
29 : Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2006; 16:1508–1521Crossref, Medline, Google Scholar
30 : Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. J Comp Neurol 2003; 460:345–367Crossref, Medline, Google Scholar
31 : Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord 2008; 10:916–927Crossref, Medline, Google Scholar
32 : Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry 2010; 67:414–421Crossref, Medline, Google Scholar
33 : The measurement of individual differences in behavioral inhibition and behavioral activation systems: a comparison of personality scales. Pers Individ Dif 2003; 34:999–1013Crossref, Google Scholar
34 : Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage 2009; 46:1148–1153Crossref, Medline, Google Scholar
35 : Personality and reinforcement in associative and instrumental learning. Pers Individ Dif 1995; 19:47–71Crossref, Google Scholar
36 : Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proc Natl Acad Sci USA 2011; 108:16044–16049Crossref, Medline, Google Scholar
37 : Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry 2008; 165:313–320Link, Google Scholar



