Cortical Thinning in Psychopathy
Abstract
Objective:
Psychopathy is a personality disorder associated with severely antisocial behavior and a host of cognitive and affective deficits. The neuropathological basis of the disorder has not been clearly established. Cortical thickness is a sensitive measure of brain structure that has been used to identify neurobiological abnormalities in a number of psychiatric disorders. The authors assessed cortical thickness and corresponding functional connectivity in psychopathic prison inmates.
Method:
Using T1 MRI data, the authors computed cortical thickness maps in a sample of adult male prison inmates selected on the basis of psychopathy diagnosis (21 psychopathic inmates and 31 nonpsychopathic inmates). Using resting-state functional MRI data from a subset of these inmates (20 psychopathic inmates and 20 nonpsychopathic inmates), the authors then computed functional connectivity within networks exhibiting significant thinning among psychopaths.
Results:
Relative to nonpsychopaths, psychopaths had significantly thinner cortex in a number of regions, including the left insula and dorsal anterior cingulate cortex, the left and right precentral gyri, the left and right anterior temporal cortices, and the right inferior frontal gyrus. These neurostructural differences were not due to differences in age, IQ, or substance use. Psychopaths also exhibited a corresponding reduction in functional connectivity between the left insula and the left dorsal anterior cingulate cortex.
Conclusions:
Psychopathy is associated with a distinct pattern of cortical thinning and reduced functional connectivity.
Psychopathy, characterized by callous and impulsive antisocial behavior, is present in roughly a quarter of adult prison inmates and is associated with a disproportionately high incidence of violent crime and recidivism (1). The identification of neural correlates of the disorder could thus have profound implications for the clinical and legal management of psychopathic criminals as well as for our basic understanding of the biological substrates underlying human social behavior. However, the collection of neuroimaging data from psychopaths involves a number of logistical and methodological challenges—psychopaths are difficult to identify and recruit outside of the criminal justice system, and prison inmates are typically not easily accessible for neuroimaging studies. As a result of these barriers, imaging studies of psychopaths have commonly been beset by inadequate sample sizes, substandard subject assessments and classification, poorly matched comparison subjects, and (predictably) limited convergence of findings (2). In this study, we overcame these major limitations by using a mobile MRI scanner in a correctional facility. We collected MRI data from a relatively large sample of well-characterized prison inmates to identify structural and functional differences between psychopathic and nonpsychopathic offenders' brains.
Given the complex psychological profile of psychopaths, researchers have proposed a role for dysfunction in a number of cortical and subcortical regions (3, 4). In addition to well-documented deficits in basic emotional responsiveness, which perhaps suggest dysfunction in subcortical structures such as the amygdala (5) and the ventral striatum (6), psychopathy is also associated with abnormalities in higher-order cognitive functions, such as attentional control (7, 8), reversal learning (9), and linguistic processing (10). This constellation of deficits suggests dysfunction in frontotemporal cortical areas, particularly the orbitofrontal cortex, ventromedial prefrontal cortex, anterior cingulate cortex, insula, and anterior temporal cortex. Our aim in this study was to assess cortical brain structure and corresponding function in psychopathy.
In recent years, the measurement of cortical thickness has been proven as an effective means of identifying neural correlates of psychiatric disorders. For example, distinct regional patterns of cortical thinning have been associated with depression (11), posttraumatic stress disorder (12), autism (13), schizophrenia (14), and subclinical trait anxiety (15). Here, we demonstrate a characteristic pattern of cortical thinning in psychopathy, as well as a corresponding deficit in functional connectivity.
Method
Participants
Participants were adult male inmates recruited from a medium-security correctional facility in Wisconsin. Eligible inmates had to be under 45 years of age, have an IQ >70, and have no history of psychosis or bipolar disorder, and they could not currently be taking psychotropic medications. Informed consent was obtained both orally and in writing. During the consent procedure, it was emphasized to the inmates that their participation in the study was voluntary, that they may refuse participation at any time, and that their decision to participate would not be recorded in their prison file, nor would it in any way affect their status within the correctional system. To protect confidentiality, each inmate's data were associated with a unique numerical code unrelated to correctional system codes. Inmates were paid approximately $7 per hour for their participation. Of the 57 inmates who were invited to participate in the study (all previously assessed as psychopathic or nonpsychopathic, as described below), 52 provided consent and participated.
The Psychopathy Checklist–Revised (PCL-R) (1) was used to assess psychopathy. The PCL-R assessment involves a 60- to 90-minute interview and file review to obtain information used to assess 20 psychopathy-related items with ratings of 0, 1, or 2, depending on the degree to which each trait characterizes the individual. A substantial body of literature supports the reliability and validity of PCL-R assessments with incarcerated offenders (1). To evaluate interrater reliability, a second rater who was present during interviews provided independent PCL-R ratings for eight inmates; the intraclass correlation coefficient was 0.85. PCL-R factor 1 and 2 scores were computed according to procedures outlined in the PCL-R manual (1). Substance use disorders were assessed with the Structured Clinical Interview for DSM-IV Disorders (16).
Participant Groups
Participants were recruited for this study based on their PCL-R scores. Psychopathic inmates had PCL-R scores of 30 or greater, while nonpsychopathic inmates had PCL-R scores of 20 or less (1). (The use of these cut scores affords clear distinction between high and low levels of psychopathy but precludes the correlation of imaging data with PCL-R factor or facet scores, which would require a more continuous range of PCL-R scores.) The characteristics of the two groups are summarized in Table 1.
| Variable | Nonpsychopaths (N=31) | Psychopaths (N=21) | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 32.1 | 7.7 | 32.7 | 6.7 | 0.77 |
| IQa | 101.7 | 12.3 | 100.1 | 10.3 | 0.64 |
| Digit span backward score | 6.9 | 2.0 | 6.8 | 3.3 | 0.90 |
| Anxiety/negative affect scoreb | 11.0 | 9.3 | 14.0 | 8.6 | 0.25 |
| Psychopathy | |||||
| Psychopathy Checklist–Revised | |||||
| Total score | 13.5 | 4.0 | 31.8 | 1.7 | <0.001 |
| Factor 1 score | 4.6 | 2.2 | 11.8 | 1.7 | <0.001 |
| Factor 2 score | 7.1 | 3.2 | 17.2 | 1.4 | <0.001 |
| % | N | % | N | ||
| White | 93.5 | 29 | 76.2 | 16 | 0.19 |
| Right-handed | 93.5 | 29 | 95.2 | 20 | 0.99 |
| Substance abuse | |||||
| Alcohol | 38.7 | 12 | 57.1 | 12 | 0.26 |
| Cannabis | 25.8 | 8 | 52.4 | 11 | 0.08 |
| Cocaine | 12.9 | 4 | 33.3 | 7 | 0.10 |
| Stimulants | 6.5 | 2 | 19.0 | 4 | 0.21 |
| Opioids | 9.7 | 3 | 33.3 | 7 | 0.07 |
| Sedatives | 3.2 | 1 | 9.5 | 2 | 0.56 |
| Hallucinogens | 3.2 | 1 | 23.8 | 5 | 0.03 |
TABLE 1. Demographic and Clinical Characteristics of Psychopathic and Nonpsychopathic Participants in a Study of Cortical Thickness
MRI Data Collection
All MRI data were acquired using the Mind Research Network's Siemens 1.5-T Avanto Mobile MRI System with advanced SQ gradients (maximum slew rate=200 T/m/sec, vector summation=346 T/m/sec, rise time=200 μsec) equipped with a 12-element head coil. Head motion was limited by padding and restraint. All prisoners underwent scanning on correctional facility grounds.
A high-resolution T1-weighted structural image was acquired for each subject using a four-echo magnetization-prepared rapid gradient-echo sequence (TR=2530 msec; TE=1.64, 3.5, 5.36, 7.22 msec; flip angle=7°, field of view=256×256 mm, matrix=128×128, slice thickness=1.33 mm, no gap, voxel size=1×1×1.33 mm, 128 interleaved sagittal slices). All four echoes were averaged into a single high-resolution image.
Resting-state functional images were collected while subjects lay still and awake, passively viewing a fixation cross. T2*-weighted gradient-echo echoplanar functional images (EPIs) were acquired with the following parameters: TR=2000 msec, TE=39 msec, flip angle=75°, field of view=24×24 cm, matrix=64×64, slice thickness=4 mm, gap=1 mm, voxel size=3.75×3.75×5 mm, 27 sequential axial oblique slices. Resting-state scans lasted 5.5 minutes (158 volumes). Resting-state functional MRI (fMRI) data were available for a subset of 20 psychopathic inmates and 20 nonpsychopathic inmates.
Measurement of Cortical Thickness
The averaged T1-weighted images were processed using FreeSurfer, version 5.0 (www.nmr.mgh.harvard.edu/freesurfer), as previously described (19). Briefly, the automated procedure includes skull-stripping, registration, intensity normalization, Talairach transformation, tissue segmentation, and surface tessellation. The pial surface of each hemisphere was computed by deforming the tessellated white matter surface outward toward the gray matter-CSF boundary. The distance between the white matter surface and pial surface yields an estimate of cortical thickness at each vertex. This method of estimating the thickness of the cortex has been validated histologically (20) and by manual measurement on MRI sections (21).
The reconstructed cortical surfaces for each participant were then aligned to produce an average cortical surface. A mapping was thus obtained between each vertex on the average surface and the corresponding vertex on the surface of each subject's cortical reconstruction. The cortical thickness maps for each subject were then resampled onto the average surface and smoothed with a 10-mm full width at half maximum Gaussian kernel.
Statistical Analysis of Cortical Thickness
For each group (psychopaths and nonpsychopaths), we computed a linear model of cortical thickness as a function of age at every vertex on the surface. Parametric maps derived from this between-group comparison of cortical thickness, while regressing out the effect of age, were overlain on the average cortical surface. Next, an automatic clustering algorithm was applied to segregate all points on the average cortical surface with an uncorrected p threshold of 0.01 into clusters and then to compute a cluster-wise p value correction by means of a Monte Carlo simulation to determine clusters with a corrected cluster-wise significance threshold of 0.05.
fMRI Data Processing
Preprocessing.
All fMRI data analysis was performed using AFNI (22) and FSL (www.fmrib.ox.ac.uk/fsl/). EPI volumes were slice time corrected using the first slice as a reference (sequential acquisition, Fourier interpolation) and motion corrected by rigid body alignment to the first EPI acquisition. Any subject with motion greater than 4 mm in any direction was excluded from further analysis. The first three volumes were omitted from the EPI time series, and the data were despiked to remove extreme time-series outliers. Time-series data were band-pass filtered (0.009 < F < 0.08) before spatial smoothing with a 4-mm full width at half maximum Gaussian kernel. EPI time-series data and high-resolution T1 images were normalized to the Talairach coordinate system using a 12-parameter linear warp, and the EPI data were resampled to 3-mm3 voxels for subsequent functional connectivity analyses. Normalized T1 anatomical images were segmented into gray matter, white matter, and CSF segments using FAST in FSL (23). White matter and CSF segments were used as masks to extract a representative time series from each tissue type.
Region of interest selection and correlation analysis.
The cortical thickness analysis revealed a total of 13 areas of significant thinning among psychopaths (see the Results section). Among these 13 areas, three pairs of areas normally exhibit significant functional connectivity (within the pair): the right and left precentral gyri (24), the right and left temporal poles (25), and the left insula and left dorsal anterior cingulate cortex (26, 27). To calculate functional connectivity within each of these pairs, we first constructed seed regions of interest at the site of the maximum p value for cortical thinning in each area. We next constructed anatomical masks for the corresponding region within the pair (e.g., the seed in the right precentral gyrus was paired with a mask in the left precentral gyrus, and vice versa). The masks were created using the Harvard-Oxford anatomical atlas included in FSL (28). For the left dorsal anterior cingulate mask, the original anterior cingulate mask from the Harvard-Oxford atlas was edited in FSL to exclude the subgenual anterior cingulate. Functional connectivity was assessed by computing correlations within each mask for the mean time series derived separately from the corresponding seed region of interest. The mean time series was included in a generalized linear model with eight regressors of no interest, including six motion parameters (three translations and three rotations) obtained from the rigid body alignment of EPI volumes, the ventricular time series acquired by averaging across the CSF mask, the white matter time series acquired by averaging across the white matter mask, and a second-order polynomial to model baseline signal and slow drift. Voxel-wise correlation coefficients for each region of interest were converted to z-scores via Fisher's r-to-z transform, and the resulting z-score maps were entered into second-level statistical analyses.
Statistical analysis of correlation maps.
To compare functional connectivity between psychopathic and nonpsychopathic inmates, we performed unpaired two-sample t tests on the z-score maps derived from each seed/mask pair. Group difference maps were corrected for multiple comparisons using cluster-extent thresholding at an uncorrected p<0.005, α=0.05. Cluster extents were computed using Monte Carlo simulations implemented in the 3dClustSim program in AFNI. Group correlation maps were overlaid on the normalized mean anatomical image. All coordinates are reported in Talairach space.
Results
Psychopathic inmates had significantly thinner cortex than nonpsychopaths in a number of areas (Figure 1 and Figure 2, Table 2).
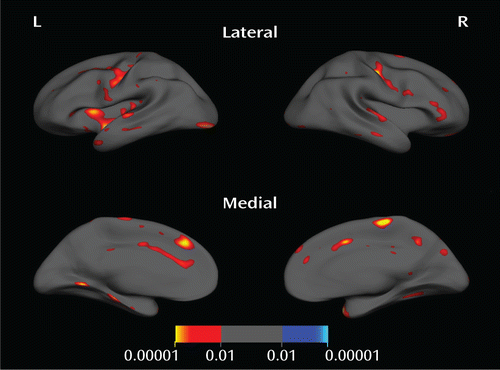
FIGURE 1. Areas of Significantly Thinner Cortex in Psychopathsa
a p<0.01, uncorrected. The color bar indicates p value.
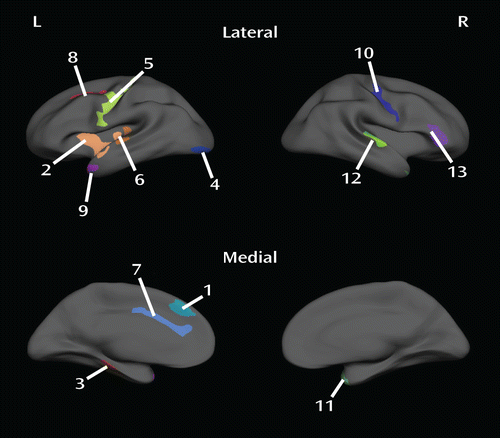
FIGURE 2. Clusters of Significantly Thinner Cortex in Psychopathsa
a p<0.05, corrected. Each color represents a distinct cluster. Numerals refer to cluster numbers in Table 2.
| Cluster Number | Hemisphere | Location | Peak Vertex Talairach (x, y, z) | Cluster-wise p | Size (mm2) |
|---|---|---|---|---|---|
| 1 | Left | Medial superior frontal gyrus | –7.9, 32.3, 36.3 | 0.049 | 406.6 |
| 2 | Left | Anterior insula/superior temporal gyrus | –40.9, –10.4, –11.3 | 0.0001 | 1,589.5 |
| 3 | Left | Fusiform gyrus | –35.7, –18.6, –20.4 | 0.0065 | 594.1 |
| 4 | Left | Lateral occipital cortex | –26.9, –91.8, –6.5 | 0.0072 | 585.7 |
| 5 | Left | Precentral gyrus | –47.6, –8.3, 32.7 | 0.0001 | 1,566.4 |
| 6 | Left | Posterior insula/superior temporal gyrus | –61.0, –18.0, 2.7 | 0.0058 | 603.5 |
| 7 | Left | Dorsal anterior cingulate cortex | –5.3, 32.0, 16.9 | 0.0015 | 714.2 |
| 8 | Left | Lateral superior frontal gyrus/middle frontal gyrus | –23.9, –4.1, 40.8 | 0.018 | 498.3 |
| 9 | Left | Temporal pole | –46.3, 5.0, –27.1 | 0.030 | 453.9 |
| 10 | Right | Precentral gyrus | 31.8, –16.4, 41.5 | 0.0004 | 922.2 |
| 11 | Right | Temporal pole | 35.7, 14.2, –26.9 | 0.0023 | 754.3 |
| 12 | Right | Posterior superior temporal gyrus | 55.8, –19.3, 5.8 | 0.008 | 645.1 |
| 13 | Right | Inferior frontal gyrus | 43.9, 36.1, 2.5 | 0.0018 | 797.4 |
TABLE 2. Clusters of Significant Cortical Thinning in Psychopaths
The largest and most significant clusters were in the left insula, the left dorsal anterior cingulate cortex, the left and right precentral gyri, the left and right temporal poles, and the right inferior frontal gyrus. There were no areas where nonpsychopaths had significantly thinner cortex than psychopaths.
Given that psychopaths had a somewhat higher rate of substance use disorder than nonpsychopaths in our sample (Table 1), we performed a follow-up analysis to determine whether any of the observed differences in cortical thickness between psychopaths and nonpsychopaths could be due to differences in rates of substance use disorder. We grouped inmates on the basis of substance use disorder diagnosis: inmates with a substance use disorder diagnosis (N=26) compared with those without (N=26). Using the same statistical criteria as the psychopath versus nonpsychopath group analysis, we found no areas of significantly different cortical thickness between the two groups of inmates (i.e., no areas survived the cluster-wise threshold of p<0.05). We therefore conclude that the observed results are in fact due to different levels of psychopathy, rather than different rates of substance use disorder.
To determine whether the observed differences in cortical thickness were associated with corresponding reductions in functional connectivity, we assessed functional connectivity between three pairs of regions: the right and left precentral gyri, the right and left temporal poles, and the left insula and left dorsal anterior cingulate cortex. Each of these pairs of regions exhibited significantly thinner cortex in psychopaths, and each is known to exhibit significant functional connectivity in healthy subjects (24–27). Among these pairs of regions, only the left insula and left dorsal anterior cingulate cortex had significantly reduced functional connectivity in psychopaths relative to nonpsychopaths (Figure 3).
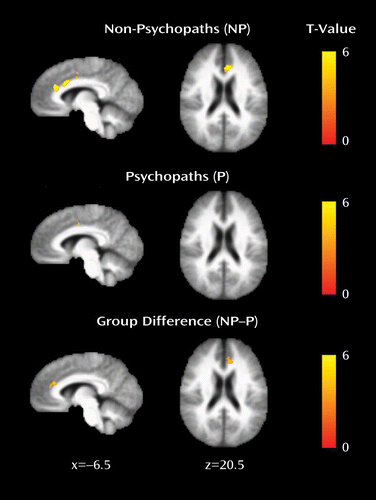
FIGURE 3. Reduced Functional Connectivity Between the Left Anterior Insula and the Left Dorsal Anterior Cingulate Cortex in Psychopathsa
a Mean left insula-dorsal anterior cingulate connectivity maps for nonpsychopaths and psychopaths are shown separately on the group mean anatomical image, thresholded at a cluster-corrected p of 0.05. Scale bars depict the uncorrected T-statistic. The group difference map indicates an area in the dorsal anterior cingulate where nonpsychopaths have significantly greater connectivity with the anterior insula than psychopaths (x=–12, y=30, z=20.5; cluster size=41 voxels).
Discussion
To our knowledge, this study is the first to examine thickness throughout the entire cortex in a sizable sample of stringently classified psychopaths (N=21 with a PCL-R score ≥30). Relative to nonpsychopathic prison inmates, psychopaths exhibited discrete areas of significant cortical thinning. The area of greatest difference in cortical thickness was the left insula. This finding aligns closely with results from a recent study showing that violent offenders exhibit gray matter volume reductions specifically in the left anterior insula (relative to nonoffenders matched for substance use disorder) and that severity of psychopathy is negatively correlated with left insula volume (29). In addition, a previous study of gray matter structure in psychopathy identified reductions in the insula, among other brain regions (30). These convergent results implicate thinning in the insula as a robust neural correlate of psychopathic behavior.
Interestingly, the insula is strongly interconnected with a second set of areas in which we also found significant cortical thinning among psychopaths: the dorsal anterior cingulate cortex and adjacent medial superior frontal gyrus. Studies of functional connectivity have identified a circuit comprising principally nodes in the anterior insula/frontal operculum and the dorsal anterior cingulate cortex/medial superior frontal gyrus (26, 27). Furthermore, the dorsal anterior cingulate cortex/medial superior frontal gyrus and the anterior insula/frontal operculum are the two areas of the brain with the highest numbers of von Economo neurons, a cell type that is specifically found in the great apes and especially humans (31, 32). Based on the functional and cytological links between these two areas, we see the corresponding cortical thinning in these areas as potential evidence for a compromised insula-dorsal anterior cingulate circuit in psychopathy. In strong support of this argument, we observed significantly lower functional connectivity between the left insula and left dorsal anterior cingulate among psychopaths. Although the function of this insula-dorsal anterior cingulate circuit remains to be fully elucidated, fMRI studies suggest a role in the flexible control of goal-directed behavior via signaling for top-down control (27, 33). It is noteworthy that behavioral and psychophysiological studies of psychopaths have previously demonstrated abnormalities in the flexibility of goal-directed attention (7, 8). Dysfunction in the insula-dorsal anterior cingulate circuit could conceivably be a neural correlate of this behavioral characteristic. Further work, particularly with measures of task-related functional connectivity in psychopaths, will be necessary to investigate this hypothesis more directly.
We also observed a robust difference in cortical thickness between psychopaths and nonpsychopaths in the precentral gyrus bilaterally. Although the precentral gyrus is primarily known as an area for motor planning and execution—and thus is not typically theorized to have any meaningful role in psychopathy—there is mounting evidence that it may indeed mediate functions germane to psychopathy. Recent studies have associated precentral gyrus activity with impulsivity in juvenile offenders (34), abstract emotional meaning (35), empathy for pain (36), and value signals during decision making (37). Moreover, thinning in the precentral gyrus has been identified in studies of individuals with psychopathic characteristics (38, 39). One study associated violence and antisocial personality disorder with thinning specifically in the precentral gyrus (38), while another found a thinner precentral gyrus in a community sample with relatively high psychopathy scores (39). In the present study, using a stringent and well-validated subject classification scheme, we demonstrate bilateral precentral thinning in psychopathic prison inmates.
Another set of areas where we found significant thinning bilaterally was the temporal pole and the superior temporal gyrus. This finding converges with functional imaging data showing reduced activity in the anterior superior temporal lobe in psychopaths during lexical decision making (10) and moral judgment (40), as well as with a recent voxel-based morphometry study showing a negative correlation between temporal pole gray matter volume and psychopathy severity in a large sample of prison inmates (41). In addition, one previous study found reduced thickness in the temporal poles bilaterally in a community sample with relatively high psychopathy scores (39), while an earlier voxel-based morphometry study found relatively specific volume reductions in the temporal poles bilaterally among incarcerated psychopaths relative to nonpsychopaths (42). However, the nonpsychopathic comparison group in the latter study was not matched for incarceration status, IQ, or substance use history. The present results and those of Ermer et al. (41), which fully account for these important variables, more definitively establish a link between psychopathy and thinning in the temporal pole.
Cortical thinning was also observed in the right inferior frontal gyrus of psychopaths, consistent with findings of a previous study (39). Growing evidence suggests a role for the inferior frontal gyrus in social cognition. Since the discovery of mirror neurons in the monkey area F5 (the homologue to the human inferior frontal gyrus) (43), the inferior frontal gyrus has been proposed as a potential neural substrate underlying empathy (44). Indeed, autism, a neurodevelopmental disorder featuring deficient empathy and impoverished interpersonal affection, is associated with a lack of “mirror” activity in the inferior frontal gyrus (45) as well as reduced thickness (46) and volume (47) of the inferior frontal gyrus. Perhaps the hallmark psychopathic traits of reduced affect and inability to experience empathy are related to thinning in the right inferior frontal gyrus.
Finally, we consider how our results relate to two leading neurobiological models of psychopathy. One prominent model proposes primary dysfunction in a circuit comprising the amygdala and the ventromedial prefrontal cortex (3). Although our cortical thickness analysis did not include subcortical structures such as the amygdala, we did find thinning in the anterior and superior temporal cortices, which overlie and densely interconnect with the amygdala (48, 49). With respect to the ventromedial prefrontal cortex, we found limited evidence of cortical thinning among psychopaths; a small area in the right gyrus rectus was significant at p<0.01 (uncorrected) but did not survive cluster-wise p<0.05 correction. Reduced thickness in the ventromedial prefrontal cortex may have been predicted on the basis of recent results from an overlapping inmate sample demonstrating significantly reduced functional connectivity in that structure in psychopaths (50). In addition, two previous structural imaging studies have associated psychopathy with reduced gray matter in areas adjacent to the ventromedial prefrontal cortex, including the frontopolar cortex and the orbitofrontal cortex (30, 39). The absence of any extensive difference in ventromedial prefrontal cortex thickness in the present study suggests that psychopathy may not reliably be associated with a substantial lack of tissue integrity in this structure, but rather that the ventromedial prefrontal cortex may be abnormally connected within the psychopathic brain.
A second prominent neuroanatomical model of psychopathy proposes dysfunction in a broader “paralimbic” network (4). Our data offer mixed support for this model; several areas of significant thinning were located within the paralimbic network (e.g., the insula, the dorsal anterior cingulate cortex, the temporal pole), while several areas were not (e.g., the precentral gyrus, the lateral occipital cortex, the superior frontal gyrus). Future research with multimodal imaging approaches will be necessary to more fully evaluate the extent to which either of these models accurately characterizes the neuropathological basis of psychopathy.
1. : The Hare Psychopathy Checklist–Revised, 2nd ed. Toronto, Multi-Health Systems, 2003Google Scholar
2. : Investigating the neural correlates of psychopathy: a critical review. Mol Psychiatry 2011; 16:792–799Crossref, Medline, Google Scholar
3. : The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci 2008; 363:2557–2565Crossref, Medline, Google Scholar
4. : A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res 2006; 142:107–128Crossref, Medline, Google Scholar
5. : Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2005; 62:799–805Crossref, Medline, Google Scholar
6. : Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 2010; 13:419–421Crossref, Medline, Google Scholar
7. : Stroop tasks reveal abnormal selective attention among psychopathic offenders. Neuropsychology 2004; 18:50–59Crossref, Medline, Google Scholar
8. : Attention moderates the processing of inhibitory information in primary psychopathy. J Abnorm Psychol 2009; 118:554–563Crossref, Medline, Google Scholar
9. : Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J Abnorm Psychol 2006; 115:552–558Crossref, Medline, Google Scholar
10. : Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Res 2004; 130:27–42Crossref, Medline, Google Scholar
11. : Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA 2009; 106:6273–6278Crossref, Medline, Google Scholar
12. : Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry 2009; 66:1373–1382Crossref, Medline, Google Scholar
13. : A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry 2009; 66:320–326Crossref, Medline, Google Scholar
14. : Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 2011; 68:871–880Crossref, Medline, Google Scholar
15. : Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord 2011; 134:315–319Crossref, Medline, Google Scholar
16. : Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
17. : Shipley Institute of Living Scale: Revised Manual. Los Angeles, Western Psychological Services, 1986Google Scholar
18. : Factor dimensions in A and R, in Basic Readings on the MMPI in Psychology and Medicine. Edited by Welsh GSDahlstrom WG. Minneapolis, University of Minnesota Press, 1956, pp 264–281Google Scholar
19. : Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97:11050–11055Crossref, Medline, Google Scholar
20. : Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 2002; 58:695–701Crossref, Medline, Google Scholar
21. : Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 2003; 60:878–888Crossref, Medline, Google Scholar
22. : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
23. : Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001; 20:45–57Crossref, Medline, Google Scholar
24. : Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34:537–541Crossref, Medline, Google Scholar
25. : Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 2009; 45:614–626Crossref, Medline, Google Scholar
26. : Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007; 104:11073–11078Crossref, Medline, Google Scholar
27. : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356Crossref, Medline, Google Scholar
28. : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(suppl 1):S208–S219Crossref, Medline, Google Scholar
29. : Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry 2011; 68:1039–1049Crossref, Medline, Google Scholar
30. : Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage 2008; 40:1202–1213Crossref, Medline, Google Scholar
31. : A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA 1999; 96:5268–5273Crossref, Medline, Google Scholar
32. : A new type of special cells of the cingulate and insular lobes. Z Ges Neurol Psychiatr 1926; 100:707–712Google Scholar
33. : Saliency, switching, attention, and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667Crossref, Medline, Google Scholar
34. : Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci USA 2011; 108:11241–11245Crossref, Medline, Google Scholar
35. : A role for the motor system in binding abstract emotional meaning. Cereb Cortex (Epub ahead of print, Mar 14, 2012)Crossref, Medline, Google Scholar
36. : Psychopathy and the mirror neuron system: preliminary findings from a non-psychiatric sample. Psychiatry Res 2008; 160:137–144Crossref, Medline, Google Scholar
37. : Role of rodent secondary motor cortex in value-based action selection. Nat Neurosci 2011; 14:1202–1208Crossref, Medline, Google Scholar
38. : Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry 2007; 164:1418–1427Link, Google Scholar
39. : Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry 2009; 14:561–562Crossref, Medline, Google Scholar
40. : Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol 2010; 119:863–874Crossref, Medline, Google Scholar
41. : Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol (Epub ahead of print, Dec 12, 2011)Medline, Google Scholar
42. : Gray matter changes in right superior temporal gyrus in criminal psychopaths: evidence from voxel-based morphometry. Psychiatry Res 2008; 163:213–222Crossref, Medline, Google Scholar
43. : Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 1996; 3:131–141Crossref, Medline, Google Scholar
44. : Imitation, empathy, and mirror neurons. Annu Rev Psychol 2009; 60:653–670Crossref, Medline, Google Scholar
45. : Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci 2006; 9:28–30Crossref, Medline, Google Scholar
46. : Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 2006; 16:1276–1282Crossref, Medline, Google Scholar
47. : Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biol Psychiatry 2010; 68:1141–1147Crossref, Medline, Google Scholar
48. : Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 1984; 230:465–496Crossref, Medline, Google Scholar
49. : Temporal neocortical afferent connections to the amygdala in the rhesus monkey. Brain Res 1976; 115:57–69Crossref, Medline, Google Scholar
50. : Reduced prefrontal connectivity in psychopathy. J Neurosci 2011; 31:17348–17357Crossref, Medline, Google Scholar



