Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia
Abstract
Objective:
Some 25%–30% of patients with schizophrenia have auditory verbal hallucinations that are refractory to antipsychotic drugs. Outcomes in studies of repetitive transcranial magnetic stimulation suggest the possibility that application of transcranial direct-current stimulation (tDCS) with inhibitory stimulation over the left temporo-parietal cortex and excitatory stimulation over the left dorsolateral prefrontal cortex could affect hallucinations and negative symptoms, respectively. The authors investigated the efficacy of tDCS in reducing the severity of auditory verbal hallucinations as well as negative symptoms.
Method:
Thirty patients with schizophrenia and medication-refractory auditory verbal hallucinations were randomly allocated to receive 20 minutes of active 2-mA tDCS or sham stimulation twice a day on 5 consecutive weekdays. The anode was placed over the left dorsolateral prefrontal cortex and the cathode over the left temporo-parietal cortex.
Results:
Auditory verbal hallucinations were robustly reduced by tDCS relative to sham stimulation, with a mean diminution of 31% (SD=14; d=1.58, 95% CI=0.76–2.40). The beneficial effect on hallucinations lasted for up to 3 months. The authors also observed an amelioration with tDCS of other symptoms as measured by the Positive and Negative Syndrome Scale (d=0.98, 95% CI=0.22–1.73), especially for the negative and positive dimensions. No effect was observed on the dimensions of disorganization or grandiosity/excitement.
Conclusions:
Although this study is limited by the small sample size, the results show promise for treating refractory auditory verbal hallucinations and other selected manifestations of schizophrenia.
Some 50%–70% of individuals with schizophrenia report auditory verbal hallucinations, even during treatment with antipsychotic medication. For 25%–30% of schizophrenia patients, such hallucinations are refractory to drug treatment, resulting in persistent distress, functional disability, and frequent loss of behavioral control. System models suggest that abnormal levels of regional cerebral excitation and inhibition may occur, but this has been difficult to study.
Evidence suggests that repetitive transcranial magnetic stimulation (rTMS), a noninvasive neurostimulation technique, could modulate cortical excitability to improve refractory auditory verbal hallucinations in schizophrenia. Neuroimaging studies have implicated left temporo-parietal hyperactivity during auditory hallucinations (1), and related therapeutic studies have shown reduced severity of hallucinations with low-frequency rTMS (putatively reducing cortical excitability) with the stimulation coil applied midway between T3 and P3 (using the 10-20 EEG international system). Meta-analyses have supported this approach, reporting a substantial effect size (d values, 0.515–0.88) of low-frequency rTMS on hallucinations (2–5). However, results have been inconsistent, with two recent studies reporting no effect (6, 7). While the systems involved in speech generation and perception are broad and involve frontal as well as temporo-parietal areas (8), only a few studies have examined low-frequency rTMS targeting these broader brain regions. While hyperactivity has been reported in Broca's area (9), its right homologue (10), Heschl's gyrus (11, 12), and the middle and superior temporal gyri (8, 13, 14), stimulation of these areas with rTMS was not found to be more effective than sham stimulation (15–19). It remains unclear whether the left temporo-parietal junction at T3–P3 is the optimal rTMS focus, since recent functional MRI (fMRI) studies cast doubt on the prominence of the temporal-parietal junction in treating these hallucinations and show great interindividual variability (6, 12, 13, 16, 19). Taken together, these results suggest some promise in the development of new neurostimulation approaches that will have a more effective impact on brain systems implicated in auditory verbal hallucinations.
In addition to temporal hyperactivity, hypoactivity in the prefrontal cortex, particularly in the dorsolateral and anterior cingulate regions, has been commonly described in schizophrenia (20, 21). Here, high-frequency rTMS stimulation (putatively increasing neuronal excitability) over the prefrontal cortex has shown some promise in improving negative symptoms (3, 22).
Transcranial direct-current stimulation (tDCS) is a new noninvasive neurostimulation treatment (23) that is being used increasingly for the treatment of neurologic and psychiatric symptoms (24, 25). With tDCS, the cortical neuronal excitability is increased in the vicinity of the anode (analogous to high-frequency rTMS) and is reduced near the cathode (analogous to low-frequency rTMS) (26). The first studies investigating the effects of tDCS in humans focused on the motor cortex, where changes in cortical excitability can easily be monitored. The effects of tDCS on cortical excitability can be explained by neuronal membrane polarization shifts (subthreshold depolarization or hyperpolarization of resting membrane potential) and modifications of NMDA receptor efficacy (26), which result in prolonged synaptic efficacy changes (27). Electrophysiological studies show increased neuronal activity near anodal tDCS using somatosensory evoked potentials (28) and anodal (stimulation) and cathodal (inhibition) tDCS on visual cortex stimulation (27). Keeser et al. (29) reported that prefrontal anodal tDCS modulates resting-state functional connectivity in predicted functional networks located close to the primary stimulation site and in connected brain regions.
Thus, tDCS could be focused on two nodes of a cortical system, increasing tissue activity in one area and decreasing it in another. It could generate a more potent therapeutic action given these two local effects compared with other neurostimulation approaches, such as rTMS. Our aim in this study was to confirm our promising observations from two open cases (30) by assessing the efficacy of tDCS in refractory auditory verbal hallucinations. We also assessed the maintenance of the effect of tDCS on these hallucinations across a 3-month follow-up period. We hypothesized that a tDCS treatment with the cathode on the left temporo-parietal junction and the anode on the left dorsolateral prefrontal cortex can reduce the severity of auditory verbal hallucinations in schizophrenia patients. We also investigated the impact of tDCS on other schizophrenia symptoms in secondary exploratory outcome analyses.
Method
Thirty patients who met DSM-IV-TR criteria for schizophrenia were included in the study. All of them displayed refractory auditory verbal hallucinations, defined as the persistence of daily hallucinations without remission despite antipsychotic medication at an adequate dosage for at least 3 months. All patients were maintained on their treatment throughout the study period.
The study was approved by the Comité de Protection des Personnes of Sud-Est VI (Lyon, France), and all patients provided written informed consent. A randomized double-blind parallel-arm (raters, experimenters, and patients were blind to randomized treatment assignment) tDCS protocol was used in the study. Stimulation was done using an Eldith DC stimulator (www.neuroconn.de/dc-stimulator_plus_en/) with two 7×5 cm (35 cm2) sponge electrodes soaked in a saline solution (0.9% NaCl). Electrodes were placed on the basis of the international 10-20 electrode placement system. The anode was placed with the middle of the electrode over a point midway between F3 and FP1 (left prefrontal cortex: dorsolateral prefrontal cortex, assumed to correspond to a region including Brodmann's areas [BA] 8, 9, 10, and 46, depending on the patient) and the cathode located over a point midway between T3 and P3 (left temporo-parietal junction, assumed to correspond to a region including BA 22, 39, 40, 41, and 42, depending on the patient).
In accordance with recent studies of tDCS in other psychiatric or neurological illnesses (24, 25, 30), the stimulation level was set at 2 mA for 20 minutes. In line with our previous study using 1-Hz rTMS for auditory verbal hallucinations (31, 32), stimulation sessions were conducted twice a day on 5 consecutive weekdays. The twice daily sessions were separated by at least 3 hours. In sham stimulation, the chosen stimulation parameters were displayed, but in fact after 40 seconds of real stimulation (2 mA), only a small current pulse occurred every 550 msec (110 mA over 15 msec) through the remainder of the 20-minute period.
Outcome Measures
The primary outcome measure was the change over time in the severity of auditory verbal hallucinations, as assessed by an investigator blind to group assignment using the Auditory Hallucination Rating Scale (AHRS). Assessments were conducted at baseline (before the first tDCS session), after the 5 days of tDCS (acute effect), and 1 and 3 months after tDCS (maintenance effect).
An exploratory outcome measure was the severity of other schizophrenia symptoms as quantified by the Positive and Negative Syndrome Scale (PANSS). The effect of tDCS on overall schizophrenia symptoms was assessed using the total PANSS score and using a dimensional approach of PANSS (33) to distinguish five main dimensions of symptoms: positive, negative, depression, disorganization, and grandiosity/excitement.
Statistical Analysis
The demographic and clinical characteristics of the two groups were compared at baseline using Student's t tests, except for gender, which was assessed by the chi-square test. To compare the overall effect of treatment on auditory verbal hallucinations over time in the two groups, data from the full intent-to-treat sample were analyzed using a repeated-measures analysis of variance (ANOVA) with treatment as the intergroup factor and time as the intrasubject factor. Post hoc analyses were performed using Student's t tests for intergroup comparisons. The significance threshold was set at 0.05.
For the exploratory secondary outcome, intergroup comparisons were assessed using Cohen's d (effect size) followed by two-tailed Student's t tests immediately after the tDCS sessions. Analyses compared the percentage of variation in the scores between, before, and after treatment between the groups. The effect size estimate is slightly biased and is therefore corrected using a factor provided by Hedges and Olkin (34). An effect size is exactly equivalent to a z-score of a standard normal distribution. As suggested by Cohen (35), an effect size of 0.2 could be considered small, 0.5 medium, and 0.8 large.
Results
Thirty patients, all right-handed, were included in the study. Fifteen patients were randomly assigned to the active treatment group and 15 to the sham treatment group (Table 1; see also the CONSORT flow chart in the data supplement that accompanies the online edition of this article). At baseline, there was no statistically significant difference between groups on any variable (age, gender, education, medication, AHRS score, or PANSS scores). Treatment was well tolerated by all patients. All patients reported that they could not tell which group they had been allocated to, and all of them described a transient mild tingling or a slight itching sensation associated with the onset of stimulation.
| Active tDCS (N=15) | Sham tDCS (N=15) | |||
|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD |
| Age (years) | 40.4 | 9.9 | 35.1 | 7.0 |
| Education (years) | 10.8 | 2.9 | 10.6 | 2.8 |
| Antipsychotic dosage (mg/day, chlorpromazine equivalents) | 994 | 714 | 1,209 | 998 |
| Auditory Hallucination Rating Scale score | 28.3 | 3.5 | 27.1 | 6.9 |
| Positive and Negative Syndrome Scale | ||||
| Total score | 76.9 | 16.4 | 82.8 | 15.4 |
| Negative score | 16.2 | 5.0 | 20.5 | 6.5 |
| Positive score | 21.2 | 6.9 | 20.0 | 3.5 |
| Grandiosity/excitement score | 11.5 | 4.4 | 10.8 | 3.5 |
| Disorganization score | 15.1 | 3.9 | 15.7 | 4.8 |
| Depression score | 10.8 | 3.5 | 11.1 | 3.5 |
TABLE 1. Baseline Demographic and Clinical Characteristics of 30 Patients With Schizophrenia and Refractory Auditory Verbal Hallucinations Randomly Assigned to Receive Transcranial Direct-Current Stimulation (tDCS) or Sham Stimulationa
Auditory Verbal Hallucinations
Acute effect.
Compared with the sham condition, a large effect of tDCS on auditory verbal hallucinations was seen in the active group after 5 days of tDCS (d=1.58, p<0.001). The active group showed a mean improvement of 31% (SD=14.4) in AHRS score (from 28.3 [SD=4.1] to 19.9 [SD=5.8]), whereas the sham tDCS group had a mean reduction of 8% (SD=13.7) in AHRS score (from 27.2 [SD=6.9] to 25.1 [SD=7.7]) (Figure 1).
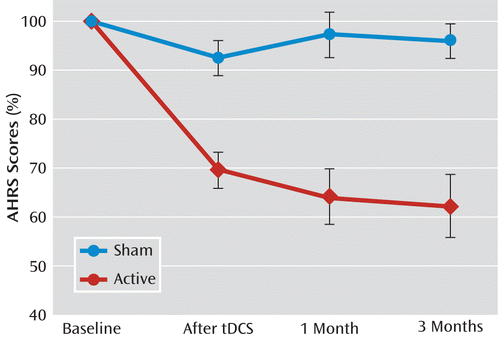
FIGURE 1. Effect of Active and Sham Transcranial Direct-Current Stimulation (tDCS) on the Severity of Auditory Verbal Hallucinationsa
a The graph illustrates the significant interaction between the mean percentage change in Auditory Hallucination Rating Scale (AHRS) score in the two groups across the four assessments (F=10.97, df=3, 84, p<0.0001). Post hoc analyses showed significant differences between groups at each postbaseline assessment: after tDCS, t=–4.45, p<0.001; 1 month after treatment, t=–4.48, p<0.001; 3 months after treatment, t=–4.58, p<0.001. Error bars indicate standard error.
Maintenance effect.
In the active tDCS group, AHRS score was reduced 36% (SD=21.8) at 1 month and 38% (SD=25.0) at 3 months, whereas in the sham tDCS group, AHRS score was reduced 3% (SD=18.3) at 1 month and 5% (SD=13.7) at 3 months (Figure 1).
The repeated-measures ANOVA showed a significant interaction between group and time (F=10.97, df=3, 84, p<0.0001). Post hoc analyses revealed significant differences between groups at all postbaseline assessments—after tDCS (t=–4.45, p<0.001), at 1 month (t=–4.48, p<0.001), and at 3 months (t=–4.58, p<0.001).
When the baseline point was excluded from the ANOVA, there was only a group effect (F=29.9, df=1, 28, p<0.0001). Time effect and group-by-time interaction were not statistically significant, suggesting that the tDCS effect on auditory verbal hallucinations is maintained from end of treatment to 3 months (Figure 1).
Although a decrease in AHRS score was observed for all patients in the active treatment group, no patient had a complete resolution of their hallucinations (i.e., an AHRS score of 0).
Other Schizophrenia Symptoms
A significant effect of tDCS on schizophrenia symptoms, as assessed by total PANSS score, was observed in the active treatment group (the score decreased from 76.9 [SD=16.4] to 66.9 [SD=15.0]) relative to sham treatment (a decrease from 82.8 [SD=15.4] to 80.5 [SD=12.0]) immediately after treatment (d=0.98; 95% CI=0.22–1.73, p=0.01) (Table 2).
| Active tDCS (N=15) | Sham tDCS (N=15) | ||||||
|---|---|---|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD | Cohen's d | 95% CI | pb |
| Auditory Hallucination Rating Scale score | 30.46 | 14.39 | 7.62 | 13.70 | 1.58 | 0.76 to 2.40 | <0.001 |
| Positive and Negative Syndrome Scale | |||||||
| Total score | 11.88 | 9.45 | 1.75 | 10.68 | 0.98 | 0.22 to 1.73 | 0.01 |
| Negative score | 11.93 | 13.56 | –5.57 | 17.96 | 1.07 | 0.30 to 1.84 | 0.01 |
| Positive score | 15.94 | 13.88 | 6.08 | 16.05 | 0.64 | –0.09 to 1.37 | 0.08 |
| Grandiosity/excitement score | 4.15 | 18.72 | 1.47 | 16.19 | 0.15 | –0.57 to 0.87 | 0.68 |
| Disorganization score | 6.82 | 14.60 | 0.89 | 15.19 | 0.39 | –0.34 to 1.11 | 0.28 |
| Depression score | 17.43 | 19.89 | –7.92 | 53.47 | 0.61 | –0.12 to 1.34 | 0.10 |
TABLE 2. Percent Decrease in Symptom Scores After Transcranial Direct-Current Stimulation (tDCS) or Sham Stimulation in 30 Patients With Schizophrenia and Refractory Auditory Verbal Hallucinationsa
According to the PANSS dimensional approach we used (33), a significant effect of active tDCS on the negative dimension was observed compared with sham treatment (d=1.07; 95% CI=0.30–1.84, p=0.01). The positive and depressive dimensions showed medium effect sizes (>0.5), although both fell short of statistical significance (positive dimension: d=0.64; 95% CI=–0.09 to 1.37, p=0.08; depressive dimension: d=0.61; 95% CI=–0.12 to 1.34, p=0.10). No effect on the dimensions of disorganization or grandiosity/excitement was observed (Table 2).
Discussion
We assessed the efficacy of tDCS administered to the left temporo-parietal junction (“inhibitory” cathodal tDCS) and to the left dorsolateral prefrontal cortex (“excitatory” anodal tDCS) in reducing the severity of refractory auditory verbal hallucinations in patients with schizophrenia. We also assessed the impact of this technique on other refractory schizophrenia symptoms.
In line with our hypothesis, we observed a significant reduction in severity of auditory verbal hallucinations after active tDCS relative to sham stimulation. After 10 active tDCS sessions over 5 days, we observed a 31% reduction in hallucination severity, compared with an 8% reduction after 10 sham sessions. The effect of tDCS on auditory verbal hallucinations seems to be maintained for at least 3 months. At the end of the trial, six patients (40%) could still be categorized as responders (defined as a >50% reduction in AHRS score), which has not been the case in rTMS studies (3–5). This long-lasting effect could not be explained by changes in medication, as all patients maintained the same medication regimen throughout the study period. Although the study did not take into account other possible confounding factors, such factors would be unlikely to have a significant effect on the results, as they would likely be counterbalanced between the active and sham tDCS groups by randomization. Moreover, the study of tDCS permits a highly effective sham treatment that allows double-blind sham-controlled experimental designs. In a comparative study, Gandiga et al. (36) found that tDCS and sham stimulation produced sensations of comparable quality, with minimal discomfort and duration. Neither healthy volunteers nor patients were able to distinguish between tDCS and sham sessions, underlining the effectiveness of this method for double-blind procedures. In the present study, there were no significant adverse events, and the patients could not identify which group they had been allocated to.
As expected, the beneficial effect of tDCS was not limited to auditory verbal hallucinations. We also observed an improvement in PANSS total score after treatment, especially in negative symptoms. Evidence suggests an association between negative symptoms and left dorsolateral prefrontal hypoactivity (21), and high-frequency rTMS applied over the dorsolateral prefrontal cortex has been reported to lead to improvement in negative symptoms in schizophrenia (3, 22, 37) as well as improvements in depressive symptoms in major depression (38). Taken together, these findings could explain the significant reduction of negative symptoms and the reduction in the depressive dimension associated with tDCS in the present study, suggesting that activation of the dorsolateral prefrontal cortex using noninvasive brain stimulation could correct hypofrontality or fronto-limbic imbalance (22).
One can hypothesize that the effects of tDCS on negative and depressive symptoms are a result of the anodal tDCS acting on frontal hypoactivity and the effects on auditory verbal hallucinations are a result of the cathodal tDCS at the temporo-parietal junction (covering a larger cortical area than the T3–P3 targeted with rTMS) acting on temporo-parietal hyperactivity. It is difficult, however, to draw any definitive conclusions about the efficacy of the anode or the cathode or both on the observed symptom improvement. The observed effect is probably a result of the combination of the local impacts of the two electrodes and their distant repercussions (29). For instance, the hypothesis of a dysfunctional fronto-temporal connectivity has frequently been mentioned in neuroimaging studies (20, 39) and in cognitive models (13, 32) to explain positive symptoms, especially auditory verbal hallucinations (20, 32). The effect of tDCS on auditory verbal hallucinations is probably a result of a global action of the two electrodes on the fronto-temporal network.
Our results suggest that tDCS, an easy-to-use, low-cost stimulation tool with few side effects (26, 29, 30, 40), by acting antagonistically on two distinct brain areas involved in the pathophysiology of schizophrenia, could constitute a new tool in the treatment of refractory symptoms. Further studies with larger samples and additional evaluations, such as functional evaluations (e.g., quality of life, social autonomy of patients) and imaging, are needed to confirm these promising results.
1. : A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176–179Crossref, Medline, Google Scholar
2. : Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry 2007; 68:416–421Crossref, Medline, Google Scholar
3. : Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res 2009; 108:11–24Crossref, Medline, Google Scholar
4. : Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res 2010; 118:201–210Crossref, Medline, Google Scholar
5. : Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? a meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010; 71:873–884Crossref, Medline, Google Scholar
6. : Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? negative results from a large randomized controlled trial. Biol Psychiatry 2011; 69:450–456Crossref, Medline, Google Scholar
7. : A pilot double-blind sham-controlled trial of repetitive transcranial magnetic stimulation for patients with refractory schizophrenia treated with clozapine. Psychiatry Res 2011; 188:203–207Crossref, Medline, Google Scholar
8. : Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry 2011; 168:73–81Link, Google Scholar
9. : Increased blood flow in Broca's area during auditory hallucinations in schizophrenia. Lancet 1993; 342:703–706Crossref, Medline, Google Scholar
10. : Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain 2008; 131:3169–3177Crossref, Medline, Google Scholar
11. : Activation of Heschl's gyrus during auditory hallucinations. Neuron 1999; 22:615–621Crossref, Medline, Google Scholar
12. : The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage 2005; 27:644–655Crossref, Medline, Google Scholar
13. : Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br J Psychiatry 2011; 198:277–283Crossref, Medline, Google Scholar
14. : Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033–1038Crossref, Medline, Google Scholar
15. : Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport 2004; 15:1669–1673Crossref, Medline, Google Scholar
16. : Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex 2007; 17:2733–2743Crossref, Medline, Google Scholar
17. : Right prefrontal rTMS treatment for refractory auditory command hallucinations: a neuroSPECT assisted case study. Psychiatry Res 2002; 116:113–117Crossref, Medline, Google Scholar
18. : A sham-controlled trial of left and right temporal rTMS for the treatment of auditory hallucinations. Psychol Med 2009; 40:541–546Crossref, Medline, Google Scholar
19. : Effects of bilateral repetitive transcranial magnetic stimulation on treatment resistant auditory-verbal hallucinations in schizophrenia: a randomized controlled trial. Schizophr Res 2009; 114:172–179Crossref, Medline, Google Scholar
20. : Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 2002; 51:1008–1011Crossref, Medline, Google Scholar
21. : Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 2000; 57:471–480Crossref, Medline, Google Scholar
22. : Theta burst stimulation in the negative symptoms of schizophrenia and striatal dopamine release: an iTBS-[11C]raclopride PET case study. Schizophr Res 2011; 131:264–265Crossref, Medline, Google Scholar
23. : The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res Rev 2007; 56:346–361Crossref, Medline, Google Scholar
24. : Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol 2009; 219:14–19Crossref, Medline, Google Scholar
25. : A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol 2008; 11:249–254Crossref, Medline, Google Scholar
26. : Transcranial direct current stimulation: state of the art 2008. Brain Stimul 2008; 1:206–223Crossref, Medline, Google Scholar
27. : Transcranial direct current stimulation and the visual cortex. Brain Res Bull 2006; 68:459–463Crossref, Medline, Google Scholar
28. : Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol 2004; 115:456–460Crossref, Medline, Google Scholar
29. : Prefrontal transcranial direct current stimulation changes activity of resting-state networks during functional magnetic resonance imaging. J Neurosci 2011; 31:15284–15293Crossref, Medline, Google Scholar
30. : Efficacy and safety of bifocal tDCS as an interventional treatment for refractory schizophrenia. Brain Stimul (Epub ahead of print, Apr 24, 2011)Medline, Google Scholar
31. : Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry 2005; 57:188–191Crossref, Medline, Google Scholar
32. : Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res 2006; 81:41–45Crossref, Medline, Google Scholar
33. : Five factor model of schizophrenia: replication across samples. Schizophr Res 1995; 14:229–234Crossref, Medline, Google Scholar
34. : Statistical Methods for Meta-Analysis. New York, Academic Press, 1985Google Scholar
35. : Statistical Power Analysis for the Behavioral Sciences. New York, Academic Press, 1969Google Scholar
36. : Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 2006; 117:845–850Crossref, Medline, Google Scholar
37. : Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry 2010; 71:411–418Crossref, Medline, Google Scholar
38. : Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67:507–516Crossref, Medline, Google Scholar
39. : Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 2010; 117:21–30Crossref, Medline, Google Scholar
40. : Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 2007; 72:208–214Crossref, Medline, Google Scholar



