Autoimmune Diseases and Severe Infections as Risk Factors for Schizophrenia: A 30-Year Population-Based Register Study
Abstract
Objective:
Autoimmune diseases have been associated with an increased risk of schizophrenia. It has been suggested that brain-reactive autoantibodies are part of the mechanisms behind this association. Furthermore, an increased permeability of the blood-brain barrier has been observed during periods of infection and inflammation. The authors therefore investigated whether autoimmune diseases combined with exposures to severe infections may increase the risk of schizophrenia
Method:
Nationwide population-based registers in Denmark were linked, and the data were analyzed in a cohort study using survival analysis. All analyses were adjusted for calendar year, age, and sex. Incidence rate ratios and accompanying 95% confidence intervals (CIs) as measures of relative risk were used.
Results:
A prior autoimmune disease increased the risk of schizophrenia by 29% (incidence rate ratio=1.29; 95% CI=1.18–1.41). Any history of hospitalization with infection increased the risk of schizophrenia by 60% (incidence rate ratio=1.60; 95% CI=1.56–1.64). When the two risk factors were combined, the risk of schizophrenia was increased even further (incidence rate ratio=2.25; 95% CI=2.04–2.46). The risk of schizophrenia was increased in a dose-response relationship, where three or more infections and an autoimmune disease were associated with an incidence rate ratio of 3.40 (95% CI=2.91–3.94). The results remained significant after adjusting for substance use disorders and family history of psychiatric disorders. Hospital contact with infection occurred in nearly 24% of individuals prior to a schizophrenia diagnosis.
Conclusions:
Autoimmune disease and the number of infections requiring hospitalization are risk factors for schizophrenia. The increased risk is compatible with an immunological hypothesis in subgroups of schizophrenia patients.
Schizophrenia has complex and multifactorial etiologies, with family history of psychiatric disorders as one of the main contributing risk factors. However, family history is unlikely to be a sufficient cause. Recently, immunological hypotheses have become increasingly prominent (1), and both autoimmune diseases and infections have been suggested to be causally linked to schizophrenia (2–6). The immune system can produce autoantibodies that react against the body's own tissue, inducing autoimmune diseases. Some autoantibodies that can cross-react with brain tissue have been associated with psychiatric and neurological disorders (7). The brain is protected by the blood-brain barrier (8), and in order for autoimmune diseases to induce a syndrome in the CNS, an insult that compromises this barrier might be required (9). There is a range of possible insults, including stress of various types as well as infections and inflammation. Insults that increase the permeability of the blood-brain barrier may permit the influx of brain-reactive antibodies or other immune components into the brain (7, 9, 10).
There are several lines of evidence linking adult-onset psychiatric disorders to autoantibodies (11, 12). In some autoimmune diseases, there is a high prevalence of neuropsychiatric symptoms, which are suspected to be caused by brain-reactive autoantibodies (10, 13, 14). Elevated autoantibody levels and increased autoantibody reactivity in patients with schizophrenia have been observed in some studies (8, 15), and CNS inflammation, together with blood-CSF barrier dysfunction, has also been observed (16). Animal studies have shown that if brain-reactive autoantibodies are present in the blood and agents that increase the permeability of the blood-brain barrier are administrated, there is an influx of autoantibodies into the brain and a subsequent development of a neuropsychiatric syndrome (17). This indicates that brain-reactive antibodies in the circulation might not have pathological consequences until there is a breach of blood-brain barrier integrity (7). Furthermore, it is known that cancer may increase the permeability of the blood-brain barrier, and studies have shown increased incidence of psychiatric symptoms prior to cancer diagnosis (18). The psychiatric symptoms associated with cancer might be in part caused by antibodies produced against the cancer, which can cross-react with brain tissue (19). These lines of evidence suggest that there is an increased risk of neuropsychiatric symptoms when the brain is exposed to inflammation.
To our knowledge, the hypothesis that autoimmune diseases combined with infections are risk factors for schizophrenia has not yet been investigated. In this study, we predicted that autoimmune disease and infection may interact and increase the risk of schizophrenia.
Method
Registers
Since 1968, the Danish Civil Registration System has assigned a unique individual identifier to all residents of Denmark, which provides information on a person's date of birth as well as gender (20). The civil registration number is used as a personal identifier in all national registers, enabling accurate linkage.
The Danish Psychiatric Central Register was computerized in 1969 and currently includes data on approximately 725,000 persons and 3.25 million contacts, with virtually complete coverage of the entire population of Denmark (21). This register contains data on all admissions to Danish psychiatric inpatient facilities, and since 1995, registration of psychiatric outpatient services has been included. There are no private psychiatric inpatient facilities in Denmark, ensuring that all psychiatric admissions are represented in the register.
The Danish National Hospital Registry contains records for all inpatients treated in Danish nonpsychiatric hospitals since 1977, and since 1995, it has included outpatient and emergency room contacts (22).
From 1977 to 1993, diagnostic information in these psychiatric and hospital registers was based on the Danish modification of ICD-8 (23), and from 1994 to 2006, the diagnostic system was based on the Danish modification of ICD-10 (24). All treatments in Danish hospitals are free of charge for all residents.
Study Population
All individuals born in Denmark between January 1, 1945, and December 31, 1996, and who were alive during the study period were included in our analyses. We conducted a cohort study that followed a total of 3,567,573 persons from the first day of 1977 until onset of the disorders of interest, death, emigration from Denmark, or the last day of 2006, whichever came first. During the study period (from 1977 to 2006), complete information on these individuals was available from the national registers. Information pertaining to autoimmune diseases and infections in individuals in this cohort was obtained from the Danish National Hospital Register. Cohort members were linked with the Danish Psychiatric Central Register and followed from their 10th birthday to establish whether they received a diagnosis of a schizophrenia spectrum disorder.
Assessment of Schizophrenia and Other Mental Illness
Persons with schizophrenia or schizophrenia-like psychoses (including schizotypal personality disorder [ICD-8: 295, 296.89, 297, 298.39, 301.83; ICD-10: F20–F29]), as diagnosed by the patient's treating psychiatrist, were included in the study. Date of illness onset was defined as the first day of the first hospital contact for a schizophrenia spectrum disorder, irrespective of other previous psychiatric diagnoses. Parental psychiatric history was identified from the Danish Psychiatric Central Register based on any psychiatric contact made by a patient's parents. Substance use disorders (ICD-8: 291, 294.30, 294.38, 303, 304; ICD-10: F10–F19) were identified from both the Danish Psychiatric Central Register and the Danish National Hospital Register.
Assessment of Autoimmune Disease and Infection
The time of onset of an autoimmune disease or infection was defined as the first day of the first hospital contact for one of these diagnoses as recorded in the Danish National Hospital Register. Each person could have a history of more than one autoimmune disease and more than one infection. Individuals were classified as having a positive history of one or more autoimmune diseases, as shown in Table 1 (described in detail in the recent study by Eaton et al. [25]), if they had an in- or outpatient hospital contact for the relevant diagnosis. When defining infection, we omitted all ICD-8 diagnoses with the modification code “suspected” or “not found” and similar codes in ICD-10. Further, we omitted AIDS/HIV (07983, B20–24). An individual's first registered infection was categorized as follows: sepsis infection (ICD-8: 038, ICD-10: A40–A41), hepatitis infection (ICD-8: 070, ICD-10: B15–B19), gastrointestinal infection (ICD-8: 000–009, 540, ICD-10: A00–A09, K35), skin infection (ICD-8: 035, 050–057, 110–111, 680–686, ICD-10: B00–B09, A46, L00–L08), respiratory infection (ICD-8: 460–486, ICD-10: J00–J18), pregnancy-related infection (ICD-8: 630, 635, 670, ICD-10: 023, 0264, 085–086, 098), urogenital infection (ICD-8: 612, 620, 622, 590, 59500–59501, ICD-10: N300, N518B, N70–N72, N76, N770D, N771B, N771L), CNS infection (ICD-8: 013, 02701, 03609, 04000–04399, 045–046, 05201, 05302, 05403, 05501, 05601, 062–065, 07199, 07202, 07501, 07929, 09049, 0949, 320–324, 392, 47400, ICD-10: DA022C, DA066, DA17, DA229C, DA321, DA390, DA504, DA514B, DA521A–B, DA548A,D, DA80–DA89, DB003–DB004, DB010–DB011, DB020–DB021, DB050–DB051, DB060, DB261–DB262, DB375, DB451, DB582, DB602, DE236A, DG0, DI02, DP352A), or other type of infection (i.e., the remaining infections within the general chapters ICD-8: 000–136 and ICD-10: A, B; together with ICD-8: 710 and ICD-10: M00). Time since the most recent infection was established by examining individuals with a first, second, or third infection. The latter group could have three or more infections, but only the third infection was included in the time-dependent analyses because of practical considerations.
| Schizophrenia Spectrum Disorders in Persons Without Infections | Schizophrenia Spectrum Disorders in Persons With Infections | |||||
|---|---|---|---|---|---|---|
| Autoimmune Disease | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| Persons without autoimmune disease (reference) | 1.00 | Reference | 29,372 | 1.60 | 1.56–1.64 | 8,777 |
| Any autoimmune disease | 1.29 | 1.18–1.41 | 483c | 2.25 | 2.04–2.46 | 444c |
| Autoimmune disease with suspected presence of brain reactive antibodies | 1.48 | 1.31–1.68 | 244 | 2.56 | 2.25–2.89 | 243 |
| Autoimmune hepatitis | 2.75 | 1.38–4.83 | 10 | 8.91 | 6.50–11.84 | 43 |
| Autoimmune thyroiditis | 3 | 4.57 | 2.09–8.51 | 8 | ||
| Celiac disease | 2.11 | 1.09–3.61 | 11 | 2.47 | 1.13–4.61 | 8 |
| Guillain-Barré syndrome | 1.22 | 0.58–2.19 | 9 | 2.84 | 1.52–4.76 | 12 |
| Multiple sclerosis | 1.44 | 1.03–1.94 | 39 | 2.10 | 1.37–3.06 | 24 |
| Sjögren's syndrome | 2.07 | 0.82–4.20 | 6 | 4 | ||
| Systemic lupus erythematosus | 1.84 | 0.92–3.23 | 10 | 2.11 | 1.06–3.70 | 10 |
| Thyrotoxicosis (Graves' disease) | 1.94 | 1.47–2.49 | 56 | 2.47 | 1.68–3.49 | 29 |
| Type I diabetes | 1.27 | 1.04–1.53 | 104 | 2.04 | 1.68–2.44 | 109 |
| Other autoimmune diseases | 1.19 | 1.05–1.34 | 256 | 1.95 | 1.70–2.23 | 212 |
| Ankylosing spondylitis | 1.38 | 0.79–2.20 | 15 | 1.68 | 0.72–3.25 | 7 |
| Crohn's disease | 1.22 | 0.88–1.65 | 39 | 1.67 | 1.18–2.27 | 36 |
| Iridocyclitis | 1.32 | 0.87–1.91 | 25 | 1.99 | 1.21–3.06 | 18 |
| Juvenile arthritis | 1.00 | 0.52–1.71 | 11 | 1.77 | 0.95–2.97 | 12 |
| Psoriasis vulgaris | 1.37 | 1.01–1.80 | 47 | 2.77 | 2.07–3.63 | 49 |
| Seropositive rheumatoid arthritis | 0.75 | 0.51–1.06 | 28 | 2.15 | 1.52–2.95 | 35 |
| Ulcerative colitis | 1.22 | 0.97–1.51 | 80 | 1.65 | 1.24–2.14 | 52 |
| Autoimmune diseases with too few cases to calculate individual riskd | 1.59 | 1.13–2.17 | 36 | 2.21 | 1.53–3.07 | 32 |
TABLE 1. Incidence Rate Ratios of Schizophrenia Spectrum Disorders in Persons With Hospital Contact for Autoimmune Diseases and Infections in Denmark (1977–2006)a
Data Analyses
Data were analyzed using survival analysis. The relative risk of schizophrenia was estimated with a log linear Poisson regression model using the GENMOD procedure in SAS, version 9.2 (SAS Institute, Cary, N.C.). This method approximates a Cox regression. All analyses were adjusted for calendar year, age, and the interaction of age with sex. Age and calendar year and the occurrence of an infection, autoimmune disease, or substance use disorder as well as the psychiatric contacts among parents were treated as time-dependent variables (26), whereas all other variables were considered time independent. The p values and 95% confidence intervals (CIs) were based on likelihood ratio tests. Incidence rate ratio, which is the estimate of relative risk, was calculated using log-likelihood estimation. Synergy index was calculated using the method described by Andersson et al. (27). The population-attributable risk was estimated using methods described by Bruzzi et al. (28).
Results
From 1977 through 2006 (77 million person-years at risk), a total of 39,076 individuals had a psychiatric contact for a schizophrenia spectrum disorder. There were 927 persons diagnosed with one or more autoimmune diseases prior to a schizophrenia diagnosis, 9,221 persons with one or more infections prior to a schizophrenia diagnosis, and 444 persons diagnosed with both an autoimmune disease and an infection prior to a diagnosis of schizophrenia. In percentages, 23.6% of persons diagnosed with schizophrenia had a prior hospital contact for an infection, whereas only 2.4% of persons diagnosed with schizophrenia had a prior hospital contact for an autoimmune disease. Our analysis yielded a population-attributable risk, associated with hospital contacts for infections, of 9% (i.e., the fraction of the total number of schizophrenia cases that would not have occurred if the association with hospital contacts for infections was causal and could be eliminated).
The overall effect of a hospital contact for an infection, relative to no infection, increased the incidence rate ratio of schizophrenia to 1.60 (95% CI=1.56–1.64). The risk of schizophrenia among individuals with a hospital contact for an autoimmune disease, relative to no autoimmune disease, was also increased, with an incidence rate ratio of 1.45 (95% CI=1.36–1.55). Although autoimmune diseases were more frequent among women, there was no significant gender difference with regard to the association between autoimmune diseases and schizophrenia (women: incidence rate ratio=1.51; 95% CI=1.38–1.64; men: incidence rate ratio=1.39; 95% CI=1.26–1.54). Among those with a hospital contact for an autoimmune disease but not for an infection, the incidence rate ratio of schizophrenia was 1.29 (95% CI=1.18–1.41), as shown in Table 1.
For individuals with an autoimmune disease who also had a hospital contact for an infection, the incidence rate ratio of schizophrenia increased to 2.25 (95% CI=2.04–2.46). When autoimmune diseases were grouped by conditions with a suspected presence of antibodies that can react against the brain, we found that the incidence rate ratio of schizophrenia was further increased (incidence rate ratio=1.48; 95% CI=1.31–1.68), especially among individuals who also had a hospital contact for an infection (incidence rate ratio=2.56; 95% CI=2.25–2.89).
The synergy index (27) for the interaction of autoimmune diseases in general and infections was statistically significant (incidence rate ratio=1.40; 95% CI=1.13–1.74), showing that the effect was larger than what would be predicted under an additive model. However, the interaction was not statistically significant when a multiplicative interaction model was applied (incidence rate ratio=1.09; 95% CI=0.95–1.24).
The incidence rate ratio for schizophrenia increased with the number of each prior hospitalization for an infection, as shown in Table 2. Among persons with only one hospital contact for an infection, the incidence rate ratio was 1.39 (95% CI=1.35–1.43); individuals with two hospital contacts for an infection had an incidence rate ratio of 1.70 (95% CI=1.62–1.78); and three or more hospital contacts for an infection increased the incidence rate ratio of schizophrenia to 2.56 (95% CI=2.44–2.69). Each hospital contact for an infection increased the risk of schizophrenia significantly in a linear trend (p<0.00001) (Figure 1).
| General Population | No Substance Abuse | No Psychiatric Family Historyb | Psychiatric Family Historyb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoimmune Disease and Infection | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients | Incidence Rate Ratioc | 95% CI | Case Patients |
| Persons without a hospital contact for an autoimmune disease or infection (reference) | 1.00 | Reference | 29,372 | 1.00 | Reference | 22,730 | 1.00 | Reference | 16,041 | 1.00 | Reference | 5,515 |
| Autoimmune disease | 1.30 | 1.19–1.42 | 483 | 1.29 | 1.16–1.43 | 353 | 1.26 | 1.11–1.43 | 238 | 1.16 | 0.93–1.44 | 83 |
| One infection | 1.39 | 1.35–1.43 | 5,121 | 1.27 | 1.22–1.31 | 3,600 | 1.32 | 1.27–1.37 | 2,908 | 1.23 | 1.15–1.31 | 1,242 |
| One infection and autoimmune disease | 1.73 | 1.48–2.00 | 177 | 1.52 | 1.26–1.82 | 114 | 1.66 | 1.34–2.03 | 91 | 1.27 | 0.88–1.76 | 32 |
| Two infections | 1.70 | 1.62–1.78 | 1,789 | 1.46 | 1.37–1.54 | 1,171 | 1.59 | 1.49–1.69 | 1,007 | 1.41 | 1.28–1.55 | 470 |
| Two infections and autoimmune disease | 2.21 | 1.79–2.68 | 96 | 2.11 | 1.64–2.66 | 66 | 2.26 | 1.70–2.92 | 53 | 1.26 | 0.73–2.02 | 15 |
| Three or more infections | 2.56 | 2.44–2.69 | 1,867 | 1.99 | 1.87–2.11 | 1,070 | 2.33 | 2.18–2.49 | 997 | 1.94 | 1.77–2.12 | 554 |
| Three or more infections and autoimmune disease | 3.40 | 2.91–3.94 | 171 | 2.58 | 2.08–3.15 | 89 | 3.17 | 2.54–3.89 | 86 | 2.79 | 2.03–3.71 | 43 |
| Total | 39,076 | 29,193 | 21,421 | 7,954 | ||||||||
TABLE 2. Incidence Rate Ratios of Schizophrenia Spectrum Disorders Associated With Autoimmune Disease and Infection Among Persons With and Without Substance Use Disorders or Psychiatric Family Historya
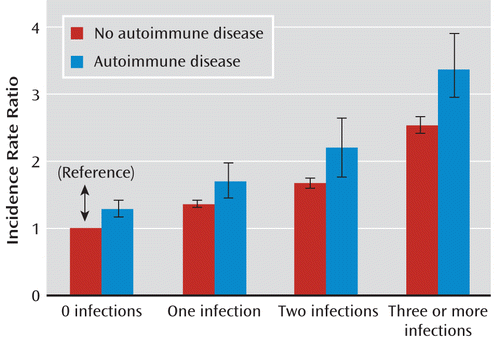
FIGURE 1. Incidence Rate Ratios of Schizophrenia Spectrum Disorders Associated With Autoimmune Disease and Infectiona
a The linear trend between the number of infections is significant (p<0.00001).
In the analysis of persons with an autoimmune disease, the number of hospitalizations for infections also increased the incidence rate ratio of schizophrenia, in a dosage-like fashion. In persons with an autoimmune disease, the incidence rate ratio of schizophrenia was 1.73 (95% CI=1.48–2.00) for those with one infection, 2.21 (95% CI=1.79–2.68) for those with two infections, and 3.40 (95% CI=2.91–3.94) for those with three or more infections.
Persons with substance use disorders may have a compromised immune system or be prone to infections, and substance abuse is a frequent comorbidity among schizophrenia patients. Therefore, we conducted analyses excluding persons with substance use disorders, and the pattern of results was generally similar to that for the total study population (Table 2). Because persons with a family history of psychiatric disorders may have different tendencies toward autoimmune diseases and infections, we also conducted separate analyses for those persons with and without a family history of psychiatric disorders. Here too, a pattern similar to that for the total study population was observed (Table 2).
The incidence rate ratio of schizophrenia was increased among individuals with infection, irrespective of the site of the registered infection (Table 3). However, the highest incidence rate ratios of schizophrenia were observed among those who had prior hospital contact for hepatitis infection (incidence rate ratio=4.89; 95% CI=4.26–5.58) and among those who had prior contact for a hepatitis infection combined with prior contact for an autoimmune disease (incidence rate ratio=8.89; 95% CI=6.03–12.53). When a person with a sepsis infection also had an autoimmune disease, the risk of schizophrenia was also increased, with an incidence rate ratio of 4.98 (95% CI=2.49–8.73).
| Infection Only (No Autoimmune Disease) | Autoimmune Disease | |||||
|---|---|---|---|---|---|---|
| Infection | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| Sepsis | 1.95 | 1.47–2.51 | 55 | 4.98 | 2.49–8.73 | 10 |
| Hepatitis | 4.89 | 4.26–5.58 | 212 | 8.89 | 6.03–12.53 | 29 |
| Gastrointestinal | 1.32 | 1.26–1.39 | 1,847 | 1.82 | 1.46–2.24 | 83 |
| Skin | 1.71 | 1.62–1.80 | 1,427 | 2.14 | 1.69–2.66 | 74 |
| Pregnancy-related | 1.14 | 0.98–1.31 | 185 | 1.22 | 0.48–2.47 | 6 |
| Respiratory | 1.53 | 1.46–1.61 | 1,885 | 2.25 | 1.79–2.79 | 77 |
| Urogenital | 1.90 | 1.79–2.01 | 1,200 | 2.70 | 2.10–3.41 | 66 |
| CNS | 1.28 | 1.09–1.50 | 148 | 2.62 | 1.31–4.60 | 10 |
| Other | 1.70 | 1.62–1.78 | 1,818 | 1.99 | 1.60–2.43 | 89 |
| Persons without hospital contact for infection (reference) | 1.00 | 29,372 | 1.30 | 1.18–1.42 | 483 | |
TABLE 3. Incidence Rate Ratios of Schizophrenia Spectrum Disorders Among Persons With Infections According to the Infection Sitea
The temporal proximity of the infection to the schizophrenia diagnosis increased the risk of schizophrenia (Table 4). Those with a severe infection within a year of their schizophrenia diagnosis had an increased incidence rate ratio of 2.35 (95% CI=2.20–2.51). The incidence rate ratio decreased with time since the last infection, although it remained significantly increased even if the last infection occurred more than 15 years before the schizophrenia diagnosis (incidence rate ratio=1.42; 95% CI=1.35–1.48). For persons with hospital contact for both an autoimmune disease and an infection, the incidence rate ratio of schizophrenia showed the same pattern and increased with the temporal proximity of the infection to the schizophrenia diagnosis (incidence rate ratio=2.91; 95% CI=2.18–3.80), if the last infection occurred within a year of the schizophrenia diagnosis).
| Infection Only (No Autoimmune Disease) | Autoimmune Disease | |||||
|---|---|---|---|---|---|---|
| Time Since Last Severe Infection | Incidence Rate Ratiob | 95% CI | Case Patients | Incidence Rate Ratiob | 95% CI | Case Patients |
| <1 year | 2.35 | 2.20–2.51 | 901 | 2.91 | 2.18–3.80 | 50 |
| 1 year | 1.89 | 1.75–2.04 | 670 | 2.85 | 2.09–3.77 | 44 |
| 2 years | 1.73 | 1.59–1.87 | 576 | 2.46 | 1.73–3.37 | 35 |
| 3–4 years | 1.66 | 1.56–1.77 | 1,020 | 2.42 | 1.86–3.08 | 61 |
| 5–9 years | 1.51 | 1.44–1.58 | 1,931 | 2.10 | 1.72–2.54 | 100 |
| 10–14 years | 1.46 | 1.38–1.53 | 1,524 | 2.31 | 1.83–2.87 | 76 |
| ≥15 years | 1.42 | 1.35–1.48 | 2,155 | 1.68 | 1.33–2.08 | 78 |
| Persons without hospital contact for infection (reference) | 1.00 | 29,372 | 1.29 | 1.18–1.41 | 483 | |
TABLE 4. Incidence Rate Ratios of Schizophrenia Spectrum Disorders Among Persons With Autoimmune Diseases According to the Time Since the Last Hospital Contact for an Infectiona
Discussion
In this national cohort study, we found a significant dose-response relationship between autoimmune disease, the number of severe infections, and the risk of schizophrenia. The results remained significant after excluding persons diagnosed with substance use disorders, and there were no important differences in the relative risk added in persons with or without a family history of psychiatric disorders. We found a statistically significant synergy between autoimmune diseases and infections with regard to the risk of schizophrenia, indicating that the effect was larger than what would be predicted under an additive model, even though the multiplicative interaction was not statistically significant.
Previous studies have shown that autoimmune diseases were more common prior to the diagnosis of schizophrenia (6) and that a general pattern of increased risk of a first episode of psychosis was present during the first years after the diagnosis of an autoimmune disease (25), suggesting a common pathogenic mechanism. In autoimmune diseases such as systemic lupus erythematosus, there is a high prevalence of neuropsychiatric symptoms in the affected individual, which might be caused by autoantibodies crossing the blood-brain barrier (13). This effect has been linked to the affinity of the antibody to the N-methyl-d-aspartate receptor in the brain (11, 14), a receptor that is central to current pathophysiological theories of schizophrenia (29). Interestingly, we found the highest risk of schizophrenia among individuals with autoimmune diseases with a suspected presence of antibodies that can react against brain tissue (7, 9, 30), and this was most pronounced for autoimmune hepatitis (30).
The number of severe infections increased the risk of schizophrenia significantly in a dose-response relationship, both independently and also by adding to the increased risk of schizophrenia in patients with an autoimmune disease. The pattern of schizophrenia risk increasing with the temporal proximity of the infection suggests that the results are unlikely to be due solely to detection bias but might be the result of a contemporary inflammatory process associated with autoimmune disease and/or infection. A possible help-seeking delay in individuals not yet diagnosed with schizophrenia could have also influenced the results, but this alternative would not explain the continued increase of risk if the last infection occurred more than 15 years previously.
The increased inflammation in both autoimmune diseases and infections may influence the brain through different pathways. One possible pathway is that increased permeability of the blood-brain barrier may make it possible for the brain to be affected by immune components, such as autoantibodies and cytokines. Our results might also be related to immunological reactivation of less severe infections acquired earlier in life or be the result of maternal infections, a known risk factor for schizophrenia (31) that could permanently alter the peripheral immune system of the fetus (32). Thus, our findings could also suggest a vulnerability to infections that may not be causal but an epiphenomenon in persons who later develop schizophrenia.
The significant synergy index we found may indicate a biological interaction between the exposures (33, 34) but needs to be interpreted cautiously, since this interpretation of the synergy index rests on the assumption of no residual confounding from unmeasured or unknown risk factors (i.e., that there are no unmeasured or unknown risk factors for schizophrenia that are unevenly distributed across the exposure groups) (35). However, the hypothesis of a biological interaction between autoimmune diseases and infections is what led us to conduct this study and seems compatible with the results. The presence of brain-reactive autoantibodies in some autoimmune diseases may affect the risk of developing schizophrenia in persons exposed to infections and inflammations that increase the permeability of the blood-brain barrier (7, 12). A recent study on patients with treatment-resistant schizophrenia suggested the possible role of CNS pathology with dysfunction of the blood-CSF barrier and, in some cases, detection of autoantibodies in the CSF (16). The prime candidates for initiating autoimmunity in predisposed individuals are infections because they induce an inflammatory response and can lead to organ-specific diseases that sometimes target the CNS (10). These immune-mediated disorders may develop through a mechanism involving molecular mimicry (7, 14), which is particularly known from streptococcal infections, in which the immune system can produce brain-reactive antibodies. This cross-reaction can induce pediatric autoimmune neuropsychiatric disorders associated with streptococcal that are related to psychiatric disorders such as Tourette's syndrome, obsessive-compulsive disorder, and autism (10, 36). Furthermore, streptococcal infection has also been associated with inducing narcolepsy (37).
During the study period, severe infections occurred in nearly 24% of schizophrenia patients prior to their diagnoses, and the population-attributable risk associated with hospital contacts for infections was 9%. The association could be unspecific, but potentially it could be causal, and infections could therefore prove to be an important risk factor in some subgroups of schizophrenia patients. Only diagnoses with a hospital in- or outpatient contact were included, whereas less severe infections treated only by primary care practitioners or that were untreated would probably account for a larger number but never enter the national registers. However, one could argue against the generalization of the results to less severe infections, since severe infections might have a higher proportion of sepsis or inflammatory response and therefore are more likely to affect the brain. Even though the classification by site of the infection is crude, it is interesting that persons with both a sepsis diagnosis and an autoimmune disease had a fivefold increased risk of schizophrenia.
A recent study suggested that there is not one specific infectious agent that might be responsible for schizophrenia symptoms, but rather the resulting immune response affecting the CNS (38). Previous studies on adult infections and schizophrenia have mainly concentrated on selected infectious agents (38), mostly Toxoplasma gondii (4) and herpes simplex virus (5), which have been shown to increase the risk of schizophrenia. Furthermore, studies have also found an increased risk of schizophrenia in individuals with childhood CNS infections (3).
Schizophrenia, many autoimmune diseases, and infectious diseases have been associated with genetic markers in the human leukocyte antigens. We therefore examined the pattern of results for persons with a family history of psychiatric disorders to explore whether the results would be explained by a genetic vulnerability in these individuals and found no support for this alternative interpretation of the data. However, a family history may be too crude an indicator of variation in individual genes to exclude an effect of genetic stratification.
Schizophrenia patients have higher than normal levels of illicit drug use, and the tendency to acquire severe infections could be increased in people with a substance use disorder because of increased at-risk behavior and patient/doctor diagnostic delay. Furthermore, alcohol and drug abuse can suppress the immune system, increasing vulnerability to infection or immune activating effects resulting in autoimmunity (10). In addition, substance abuse also might increase the risk for the onset of schizophrenia through other pathways (39). Our analysis excluding persons with a substance use disorder did not change the general pattern, and the aforementioned hypothesis could therefore only explain certain parts of the results, although the highly elevated schizophrenia risk after hepatitis infection could be influenced by substance abuse.
Strengths and Limitations
Strengths of this study include the prospective design and use of the population-based nationwide registers in Denmark, ensuring a large study population where all exposures were recorded independently of the outcome and therefore were not subject to selection or recall bias. The extensive Danish registers allowed us to examine the combined effect of autoimmune diseases and infections on the risk of developing schizophrenia.
A limitation to our study is that we only examined the time up to the schizophrenia diagnosis, and since many autoimmune diseases have a clinical onset later than that for schizophrenia, the autoimmune disease could have already been present, resulting in an underestimation of the effect as a result of undiagnosed illness. Individuals not yet diagnosed with schizophrenia may have had unspecific psychiatric symptoms and possible initial misclassification that could have affected the results. Furthermore, diagnoses made before 1977 were not available for inclusion in the study, but a sensitivity analysis for the calendar period revealed no important bias. Additional sensitivity analyses of the number of infections when only one infection during the same hospital contact was counted did not significantly alter the results. It is also possible that medical treatment for infections and autoimmune diseases could influence the risk for schizophrenia. However, this seems unlikely considering the diversity of the diseases included in the study, which would not be dominated by treatment with steroids or other drugs suspected of inducing psychosis.
In addition, we did not include other risk factors for infections, such as social class or other environmental factors. Since infections must have occurred prior to the schizophrenia diagnosis, the social status of the parent was likely to be most influential. However, Byrne et al. (40) found little evidence that family socioeconomic status was consistently associated with increased risk of schizophrenia in Denmark.
1. : Immune system and schizophrenia. Curr Immunol Rev 2010; 6:213–220Crossref, Medline, Google Scholar
2. : Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol 2005; 83:9–17Crossref, Medline, Google Scholar
3. : Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am J Psychiatry 2008; 165:59–65Link, Google Scholar
4. : Are some cases of psychosis caused by microbial agents? a review of the evidence. Mol Psychiatry 2008; 13:470–479Crossref, Medline, Google Scholar
5. : Selected infectious agents and risk of schizophrenia among US military personnel. Am J Psychiatry 2008; 165:99–106Link, Google Scholar
6. : Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006; 163:521–528Link, Google Scholar
7. : Losing your nerves? maybe it's the antibodies. Nat Rev Immunol 2009; 9:449–456Crossref, Medline, Google Scholar
8. : Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J Neuroimmunol 2003; 141:155–164Crossref, Medline, Google Scholar
9. : Autoantibody-mediated disorders of the central nervous system. Autoimmunity 2008; 41:55–65Crossref, Medline, Google Scholar
10. : Autoantibodies associated with psychiatric disorders. Curr Neurovasc Res 2006; 3:149–157Crossref, Medline, Google Scholar
11. : Antibodies and neuronal autoimmune disorders of the CNS. J Neurol 2010; 257:509–517Crossref, Medline, Google Scholar
12. : Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 2011; 258:686–688Crossref, Medline, Google Scholar
13. : Neuroimmunopathology in a murine model of neuropsychiatric lupus. Brain Res Rev 2007; 54:67–79Crossref, Medline, Google Scholar
14. : Molecular mimicry: anti-DNA antibodies bind microbial and nonnucleic acid self-antigens. Curr Top Microbiol Immunol 2005; 296:137–151Medline, Google Scholar
15. : Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res 2008; 158:83–86Crossref, Medline, Google Scholar
16. : Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res 2010; 44:321–330Crossref, Medline, Google Scholar
17. : Cognition and immunity: antibody impairs memory. Immunity 2004; 21:179–188Crossref, Medline, Google Scholar
18. : Psychiatric disorder as a first manifestation of cancer: a 10-year population-based study. Int J Cancer 2009; 124:2917–2922Crossref, Medline, Google Scholar
19. : Psychiatric manifestations of paraneoplastic disorders. Am J Psychiatry 2010; 167:1039–1050Link, Google Scholar
20. : The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 2006; 53:441–449Medline, Google Scholar
21. : The Danish Psychiatric Central Register. Dan Med Bull 1997; 44:82–84Medline, Google Scholar
22. : The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull 1999; 46:263–268Medline, Google Scholar
23.
24.
25. : Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord 2010; 12:638–646Crossref, Medline, Google Scholar
26. : Statistical Models in Epidemiology. New York, Oxford University Press, 1993Google Scholar
27. : Calculating measures of biological interaction. Eur J Epidemiol 2005; 20:575–579Crossref, Medline, Google Scholar
28. : Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 1985; 122:904–914Crossref, Medline, Google Scholar
29. : Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev 2011; 35:848–879Crossref, Medline, Google Scholar
30. : High prevalence of autoantibodies against phosphoglycerate mutase 1 in patients with autoimmune central nervous system diseases. J Neuroimmunol 2010; 219:105–108Crossref, Medline, Google Scholar
31. : Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280Link, Google Scholar
32. : Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 2009; 204:313–321Crossref, Medline, Google Scholar
33. : Biologic synergism and parallelism. Am J Epidemiol 1997; 145:661–668Crossref, Medline, Google Scholar
34. : Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 2008; 34:1066–1082Crossref, Medline, Google Scholar
35. : Basic problems in interaction assessment. Environ Health Perspect 1993; 101:59–66Crossref, Medline, Google Scholar
36. : Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998; 155:264–271Abstract, Google Scholar
37. : Anti-tribbles homolog 2 (TRIB2) autoantibodies in narcolepsy are associated with recent onset of cataplexy. Sleep 2010; 33:869–874Crossref, Medline, Google Scholar
38. : The association of infectious agents and schizophrenia. World J Biol Psychiatry 2010; 11:739–743Crossref, Medline, Google Scholar
39. : Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328Crossref, Medline, Google Scholar
40. : Parental socio-economic status and risk of first admission with schizophrenia: a Danish National Register based study. Soc Psychiatry Psychiatr Epidemiol 2004; 39:87–96Crossref, Medline, Google Scholar



