Resting Amygdala and Medial Prefrontal Metabolism Predicts Functional Activation of the Fear Extinction Circuit
Abstract
Objective:
Individual differences in a person's ability to control fear have been linked to activation in the dorsal anterior cingulate cortex, the ventromedial prefrontal cortex, and the amygdala. This study investigated whether functional variance in this network can be predicted by resting metabolism in these same regions.
Method:
The authors measured resting brain metabolism in healthy volunteers with positron emission tomography using [18F]fluorodeoxyglucose. This was followed by a 2-day fear conditioning and extinction training paradigm using functional MRI to measure brain activation during fear extinction and recall. The authors used skin conductance response to index conditioned responding, and they used resting metabolism in the amygdala, the dorsal anterior cingulate cortex, and the ventromedial prefrontal cortex to predict responses during fear extinction and extinction recall.
Results:
During extinction training, resting amygdala metabolism positively predicted activation in the ventromedial prefrontal cortex and negatively predicted activation in the dorsal anterior cingulate cortex. In contrast, during extinction recall, resting amygdala metabolism negatively predicted activation in the ventromedial prefrontal cortex and positively predicted activation in the dorsal anterior cingulate cortex. In addition, resting metabolism in the dorsal anterior cingulate cortex predicted fear expression (as measured by skin conductance response) during extinction recall.
Conclusions:
Resting brain metabolism predicted neuronal reactivity and skin conductance changes associated with the recall of the fear extinction memory.
Much has been learned about the brain network implicated in processing fear learning and its expression. Fear conditioning and extinction are believed to induce synaptic plasticity in the amygdala. Amygdala GABA-ergic neurotransmission modulates both acquisition of fear (1) and its extinction consolidation (2), suggesting that this structure has a critical function in both fear learning and its extinction. In addition to the amygdala, fear extinction and extinction recall engage the infralimbic cortex in rodents (3, 4), whereas the more dorsal prelimbic cortex is associated with fear expression (5). In humans, increased activation of the amygdala to conditioned and extinguished cues has been reported in several neuroimaging studies (6). Structural characteristics and functional reactivity in the ventromedial prefrontal cortex, the functional homologue to the rat infralimbic cortex, are associated with fear inhibition (7) and extinction recall (8–10). In support of this, high structural integrity of the white matter tracts between the amygdala and the ventromedial prefrontal cortex is associated with low levels of anxiety (11). Conversely, structural characteristics and functional reactivity in the dorsal anterior cingulate cortex, an apparent functional homologue to the rat prelimbic cortex, are likely critical for maintaining a balance between fear expression and inhibition.
Many of the rodent studies mentioned above show that functional variation in the infralimbic and prelimbic cortices is associated with variation in conditioned freezing. Human neuroimaging studies show substantial individual variation in the activation of the amygdala, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex during fear extinction. Individual differences in the activation of these brain regions correlate with psychophysiological indices of fear expression (9, 12). Moreover, the thickness of the ventromedial prefrontal cortex and the dorsal anterior cingulate cortex is associated with psychophysiological response during fear extinction (13). These data suggest that there are biological factors that influence the ability to express and control fear that predate exposure to fear-inducing stimuli.
Much regional cerebral activity seen in functional MRI (fMRI) experiments is disregarded (14) because the method relies on contrasting blood-oxygen-level-dependent (BOLD) signals between conditions. Neural reactivity induced by cue presentation, typically measured with the BOLD signal, accounts for only 1%–10% of the brain's energy consumption, a small portion of total neuronal activity (15–17). Positron emission tomography (PET) using [18F]fluorodeoxyglucose (FDG) provides a stable (18) estimate of total, as opposed to cue-induced, brain activity by quantifying regional cerebral resting metabolism (19, 20), which closely correlates with neuronal signaling (21). Higher metabolism in the amygdala and the dorsal anterior cingulate and lower resting metabolism in the ventromedial prefrontal cortex have been related to anxiety (22–24). One possibility is that functional brain reactivity may depend, to some extent, on resting regional brain activity. If so, resting activity assessed with PET should predict subsequent functional reactivity assessed with fMRI, specifically during fear extinction and extinction recall tasks.
To test this hypothesis, resting brain activity (metabolism) was measured using FDG PET at rest. Within 5 days of the PET scan, the same participants underwent a 2-day fear conditioning and extinction protocol in the fMRI scanner (see Figure S1A in the data supplement that accompanies the online edition of this article) to measure brain reactivity during fear extinction and its recall (9). We used skin conductance responses as an index of conditioned fear responses. Data analysis focused on three a priori regions of interest based on their involvement in fear conditioning and extinction: the amygdala, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex.
We hypothesized that 1) resting metabolism in the amygdala, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex would predict fear response as measured by skin conductance; 2) resting metabolism in the amygdala would predict functional activations in the ventromedial prefrontal cortex and in the dorsal anterior cingulate cortex during extinction training and recall; 3) metabolism in the ventromedial prefrontal cortex and in the dorsal anterior cingulate cortex would positively and negatively predict, respectively, extinction recall; and 4) given that functional activation in the ventromedial prefrontal cortex is associated with fear reduction, whereas activation in the dorsal anterior cingulate cortex is associated with fear expression, the metabolism ratio between these two regions would predict functional activation of the extinction network during learning and recall (9).
Method
Participants
We recruited 21 right-handed healthy volunteers (11 men) between 21 and 40 years old (mean=26 years [SD=5]) from the local community. MRI data from three participants were excluded because of excessive movement, yielding a final sample of 18 participants (eight men). After participants were given a complete description of the study and its procedures, written informed consent was obtained in accordance with the Partners HealthCare System Human Research Committee requirements. Data from this cohort have not been reported in any previous publications.
Fear Conditioning, Extinction, and Recall
The experimental protocol was identical to that of previous studies described by Milad et al. (9). Briefly, subjects were habituated to all task images (not to be confused with MR images) before conditioning, during which no shock was delivered. During fear conditioning, the participants were shown an image of a room (e.g., an office) containing an unlit lamp. The lamp was then lit for 6 seconds to one of three colors (blue, red, or yellow; see Figure S1A in the data supplement) during 32 trials. Two of the colors were followed by a highly annoying but not painful shock to the fingers at a 62.5% partial reinforcement schedule, whereas 16 trials of the third color (nonreinforced conditioned stimulus) were not followed by a shock. The intertrial interval, with no displayed images, varied between 12 and 18 seconds. No shocks were administered during the subsequent phases of the experiment. Shortly thereafter, during extinction training, the participants were shown images of a second room (e.g., a library) with a lamp that lit for 32 trials. Only one of the two reinforced cues (i.e., colors) was extinguished (extinguished conditioned stimulus, 16 trials), whereas the other was not presented (nonextinguished conditioned stimulus). Sixteen trials of the nonreinforced cue were also presented. On the second day of the experiment, we tested extinction recall. Participants were shown images of the library with the light turning on for 32 trials. Eight trials each of the extinguished and nonextinguished cues and 16 trials of the nonreinforced cue were presented. We measured skin conductance responses to the stimulus presentations throughout the fMRI experiment; see the Method section and Figure S1 in the online data supplement for details.
Data Preprocessing, Analysis, and Statistical Inferences
During all phases of the experiment, fMRI was performed with a Trio 3-T whole-body MRI system (Siemens Medical Systems, Iselin, N.J.) equipped for echo planar imaging with a 32-channel head coil. We used SPM8 ( www.fil.ion.ucl.ac.uk) to process PET and MRI data (for additional details on both fMRI and PET data collection, see the material in the online data supplement). For PET analysis, each participant's static FDG image was coregistered to individual high-resolution structural MR images. After spatial normalization of the structural images to the SPM8 International Consortium for Brain Mapping Montreal Neurological Institute (MNI) template, we applied the same normalization parameters to the PET images, which were then smoothed (8 mm full width at half maximum) and global mean normalized to 50.
Functional images were realigned, corrected for slice timing, coregistered with the structural image, normalized into MNI space using parameters obtained from the structural normalization process, and finally smoothed (8 mm full width at half maximum). After preprocessing, we modeled each participant's functional time series using a general linear model with regressors signifying the stimulus onsets and durations. Based on our previous studies (9, 13, 25–27), during extinction training, the extinguished cue was divided into the first four cue presentations (early extinction: reflective of recalling the fear memory), the subsequent eight cue presentations (mid extinction), and the last four cue presentations (late extinction: to capture the neural correlates of completed extinction). The context, the nonreinforced cue, and the cue offsets were also modeled. During extinction recall, separate regressors modeled the first four (early: reflective of extinction recall) extinguished and nonextinguished cues, the subsequent eight (late: reflective of reextinction) extinguished and nonextinguished cues, the context, the nonreinforced cue (the cue not paired with shock during conditioning), and the cue offsets. Signal drift, biorhythms, and motion artifacts were modeled using high-pass temporal filtering (128 seconds), an autoregressive AR-1 model, and six motion parameters (x, y, z, roll, pitch, and yaw). We identified the activated voxels in each experimental phase using a statistical model containing a boxcar function representing the contrasts of interest, convolved with the SPM8 canonical hemodynamic response function.
First-level contrast images were obtained for each participant and then modeled at the second level using a mixed linear model with subject and task factors as independent variables. To test for extinction processes, we contrasted early trials to late trials of the previously reinforced cue (extinguished conditioned stimulus) as defined above. To test for extinction recall the next day, we contrasted early extinguished cue trials with early nonextinguished cue trials while controlling for order effects with the adjacent trials of the nonreinforced cue. The rationale for trial grouping was based on our previous human studies (9, 13, 25–28) and the animal literature (3), which showed that ventromedial prefrontal cortex involvement in extinction recall is only evident during the early phase of extinction recall.
Statistical Inferences
For all analyses, clusters in the resulting SPM map that exceeded 10 voxels and were significant at p<0.05 (family-wise error corrected) were considered significant. Given the results of our previous studies in fear extinction and of studies conducted by other laboratories, we had strong unidirectional a priori hypotheses with respect to the functional reactivity of the ventromedial prefrontal cortex, the dorsal anterior cingulate cortex, and the amygdala. These regions of interest were the same as those used in the extraction of metabolic measurements from PET data (see below), with the exception of sphere size. Briefly, the left and right amygdala were defined according to the Anatomical Automatic Labeling atlas (29). The ventromedial prefrontal cortex was defined using an 8-mm sphere centered at MNI coordinates x=5, y=35, z=–13, which was selected based on our previous study of cortical thickness correlates of extinction memory in the ventromedial prefrontal cortex (15). The dorsal anterior cingulate cortex was defined by an 8-mm sphere centered at MNI coordinates x=2, y=22, z=29, which was selected based on our previous study of the involvement of the dorsal anterior cingulate cortex in conditioned fear (11). Within these a priori regions, clusters surviving small-volume correction (8-mm radius sphere, approximately 2,100 mm3) at p<0.05 (family-wise error corrected) were also considered significant.
Metabolism as a Predictor of BOLD fMRI and Skin Conductance Responses
We were interested in the roles of the amygdala, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex in the retention of extinction memory. Individual FDG metabolism measurements were calculated from four predefined regions of interest: the amygdala bilaterally, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex, defined as they were for the fMRI regions of interest above, except that for the dorsal anterior cingulate and ventromedial prefrontal cortex, the regions were 5-mm radius spheres. We calculated the average metabolism in these regions and the ventromedial prefrontal cortex/dorsal anterior cingulate cortex metabolism ratio for each individual. To correlate resting metabolism with fear during extinction learning and recall, we calculated skin conductance indices (see the Method section in the online data supplement) and entered them into a linear regression model with the FDG PET images. Resting metabolism measurements were also entered in linear regression models to investigate their respective influences on BOLD reactivity during extinction training and extinction recall.
Control Brain Region
We conducted an additional analysis to examine whether metabolism in the primary visual cortex (defined as Brodmann's area 17 in the Anatomical Automatic Labeling atlas [29]) predicted its own BOLD reactivity during the presentation of visual stimuli relative to baseline (with no visual stimuli) during extinction recall.
Results
Skin Conductance Responses During Extinction Training and Recall
During extinction training, a significant decline in skin conductance response was observed from the first four early extinguished cue trials to the last four late extinguished cue trials (see Figure S1B in the online data supplement), indicating that extinction learning had occurred. During extinction recall the next day, skin conductance responses to the extinguished cue were significantly lower than responses to the nonextinguished cue, indicating the recall (retention) of extinction learning (see Figure S1B, right, in the online data supplement).
fMRI Responses During Extinction Training and Recall
As stated previously, extinction training was divided into an early phase (reflecting recall of the acquired fear association) and a late phase (reflecting extinction learning). We observed amygdala deactivation from the early to late trials of the extinguished cue (MNI coordinates, x=–26, y=2, z=–16; t=3.42, df=17, p=0.019, family-wise error corrected), a finding that is consistent with reduced expression of the conditioned fear response as extinction proceeded. In contrast, we observed activation in the ventromedial prefrontal cortex (x=10, y=48, z=–8; t=3.37, df=17, p=0.022, family-wise error corrected; Figure 1) from early to late trials of the extinguished cue, consistent with its role in extinction learning. During extinction recall the next day, contrasting BOLD responses between the early extinguished and nonextinguished cue trials revealed activation in the ventromedial prefrontal cortex (x=10, y=42, z=–18; t=3.43, df=17, p=0.018, family-wise error corrected; Figure 1). This replicates earlier findings (8, 9) and is consistent with the role of the ventromedial prefrontal cortex in the recall of the previous day's extinction training. Significant activations and deactivations observed outside the a priori regions of interest are reported in Table S1 in the online data supplement. An additional analysis examined the responsiveness of the ventromedial prefrontal cortex during extinction recall to the early extinguished and nonextinguished cue trials relative to the fixation baseline. The results indicated that relative to baseline, the ventromedial prefrontal cortex was activated by the extinguished cue but deactivated by the nonextinguished cue (see Figure S2 in the online data supplement).
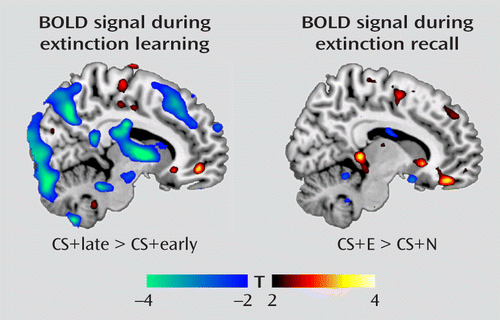
FIGURE 1. Blood-Oxygen-Level-Dependent (BOLD) Activations During Extinction Training and Extinction Recalla
a The BOLD responses during extinction training (left) represent activations to the conditioned stimulus (CS) during late extinction contrasted with early extinction. The recall contrast (right) represents activations to the extinguished cue (E) contrasted with activations to the nonextinguished cue (N). Activation maps are illustrated with a threshold of t>2.11, df=17, p<0.05 (uncorrected) and overlaid on a template structural MR image.
Regional Brain Metabolism as a Predictor of Skin Conductance Responses
In none of our a priori regions of interest did regional metabolism predict skin conductance responses during extinction learning. During extinction recall, however, dorsal anterior cingulate cortex metabolism positively predicted skin conductance response (x=8, y=32, z=26; t=5.21, df=17, p=0.015, family-wise error corrected, r=0.80; Figure 2), indicative of poorer recall of fear extinction.
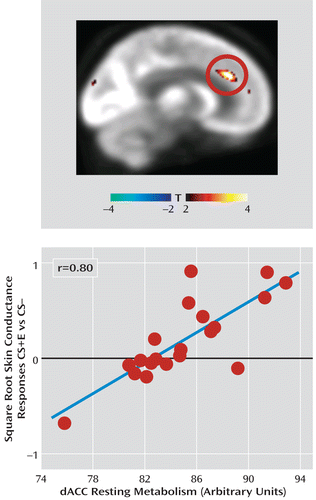
FIGURE 2. Resting Pretraining Metabolism in the Dorsal Anterior Cingulate Cortex (dACC) Predicts Differential Skin Conductance Responses During Extinction Recalla
a Whole brain correlation map showing that the dorsal anterior cingulate cortex metabolism was positively correlated with skin conductance responses to the extinguished cue (E) (minus the conditioned stimulus [CS]; r=0.80), indicative of spontaneously recovered fear. Correlated responses are illustrated with a threshold of t>2.11, df=17, p<0.05 (uncorrected) and overlaid on the average metabolism positron emission tomography maps from the study.
Regional Brain Metabolism as a Predictor of BOLD Activations
We examined the association between resting amygdala metabolism and subsequent BOLD reactivity in the contrast between early and late trials of the extinguished cue during extinction training. We observed that left amygdala metabolism negatively predicted activation in the dorsal anterior cingulate cortex (x=8, y=14, z=26; t=3.74, df=17, p=0.035, family-wise error corrected; r=–0.68), whereas right amygdala metabolism positively predicted activation in the ventromedial prefrontal cortex (x=4, y=48, z=–18; t=3.87, df=17, p=0.020, family-wise error corrected; r=0.70) (Figure 3). Moreover, ventromedial prefrontal cortex metabolism positively predicted activation in the left amygdala (x=–22, y=–4, z=–26; t=3.80, df=17, p=0.035, family-wise error corrected; r=0.69) (Figure 4). Neither metabolism in the dorsal anterior cingulate cortex nor the metabolism ratio of the ventromedial prefrontal cortex to the dorsal anterior cingulate cortex significantly predicted BOLD responses during extinction training.
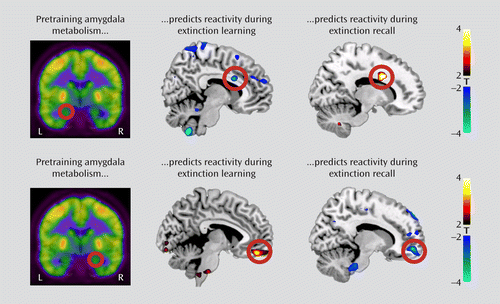
FIGURE 3. Resting Amygdala Metabolism and Functional Activations in the Dorsal Anterior Cingulate Cortex and Ventromedial Prefrontal Cortex During Extinction Training and Extinction Recalla
a Resting metabolism in the left and the right amygdala was extracted and used to predict blood-oxygen-level-dependent activations elicited during extinction training and extinction recall. Left amygdala metabolism (upper panel) negatively predicted dorsal anterior cingulate cortex responses during extinction training, but it positively predicted them during extinction recall. Right amygdala metabolism (lower panel) positively predicted ventromedial prefrontal cortex responses during extinction training, but it negatively predicted them during extinction recall. Correlated responses are illustrated with a threshold of t>2.11, df=17, p<0.05 (uncorrected) and overlaid on a template structural MR image.
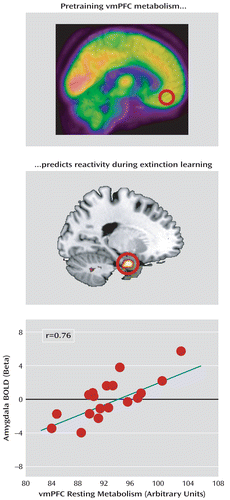
FIGURE 4. Resting Pretraining Ventromedial Prefrontal Cortex (vmPFC) Metabolism and Amygdala Functional Activity During Extinction Traininga
a Metabolism in the ventromedial prefrontal cortex was extracted and used to predict blood-oxygen-level-dependent (BOLD) activations during extinction training. Ventromedial prefrontal cortex metabolism positively predicted left amygdala responses (r=0.76). Correlated responses are illustrated with a threshold of t>2.11, df=17, p<0.05 (uncorrected) and overlaid on a template structural MR image.
We then examined regional metabolism in relation to BOLD reactivity during extinction recall in the contrast between early extinguished and nonextinguished cue trials. In marked contrast to extinction training, left amygdala metabolism now positively predicted dorsal anterior cingulate cortex activation (x=16, y=12, z=30; t=4.27, df=17, p=0.032, family-wise error corrected; r=0.73), whereas right amygdala metabolism negatively predicted ventromedial prefrontal cortex activation (x=14, y=56, z=–10; t=3.86, df=17, p=0.021, family-wise error corrected; r=–0.69) (Figure 3). Scatterplots of the observed correlations are presented in Figure S3 in the online data supplement. The metabolism ratio of the ventromedial prefrontal cortex to the dorsal anterior cingulate cortex positively predicted pregenual anterior cingulate activation (x=–4, y=42, z=4; t=3.67, df=17, p=0.035, family-wise error corrected; r=0.68) (Figure 5); the rostral anterior cingulate cortex is a component of the ventromedial prefrontal cortex. Neither the ventromedial prefrontal cortex nor the dorsal anterior cingulate cortex metabolism alone predicted functional activations during extinction recall, including activations in their own regions; the latter also applied to the amygdala. In contrast, metabolism in the primary visual cortex control region did significantly predict its own functional reactivity in response to all visual stimuli presented during recall compared with nonvisual baseline measures (see Figure S4 in the online data supplement).
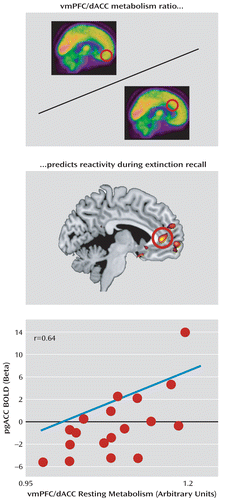
FIGURE 5. Resting Metabolic Ratio of the Ventromedial Prefrontal Cortex (vmPFC) to the Dorsal Anterior Cingulate Cortex (dACC) and Prefrontal Cortex Functional Activity During Extinction Recalla
a The ratio of ventromedial prefrontal cortex to dorsal anterior cingulate cortex metabolism was calculated and used to predict blood-oxygen-level-dependent activations elicited during extinction recall. The ventromedial prefrontal cortex-dorsal anterior cingulate cortex ratio was positively correlated to pregenual anterior cingulate cortex (pgACC) extinction recall responses (r=0.64). Correlated responses are illustrated with a threshold of t>2.11, df=17, p<0.05 (uncorrected) and overlaid on a template structural MR image.
Discussion
The unique feature of the research reported here is the use of a multimodal approach to examine the relationship between resting regional brain activity (i.e., metabolism) and subsequent psychophysiological and functional neuroimaging reactivity in the network of brain regions involved in fear extinction training and extinction recall. With regard to functional reactivity (fMRI) during extinction training and extinction recall, we largely replicated previous findings from our group and others (6, 8–10, 25). We also determined the extent to which resting metabolism obtained via FDG PET could predict later fMRI responses. The results revealed several interesting correlations. During extinction training, higher resting amygdala metabolism predicted deactivation in the dorsal anterior cingulate cortex and activation in the ventromedial prefrontal cortex. These associations are consistent with the critical involvement of the amygdala in the extinction learning process as opposed to its separate roles in fear learning and fear expression. However, the predictive relationships were reversed during extinction recall, such that higher resting amygdala metabolism now predicted activation of the dorsal anterior cingulate cortex and deactivation of the ventromedial prefrontal cortex. This result is consistent with a role for the ventromedial prefrontal cortex in inhibiting the amygdala's expression of the fear response during extinction recall and a role for the dorsal anterior cingulate cortex in promoting it. Electrophysiological data in the rat show that at least two separate populations of neurons exist in the amygdala—one that signals fear acquisition and expression and another that signals fear extinction (30). Our results, however, did not reveal that amygdala metabolism was predictive of extinction learning as measured by skin conductance response. Future studies with higher resolution to allow the parceling of amygdala subregions may reveal such a relationship. More animal studies, possibly employing optogenetic tools (29), are also needed to examine the interaction between different populations of amygdala neurons with different prefrontal regions during fear acquisition and extinction.
The lack of intraregional correlation between resting metabolism in the amygdala, the ventromedial prefrontal cortex, and the dorsal anterior cingulate cortex and these structures' functional reactivity during any phase of the experiment may appear counterintuitive. However, we did observe such a relationship in the visual cortex region; the higher resting metabolism in this region correlated with higher BOLD reactivity to visual stimuli during extinction recall. Results in this control brain region demonstrate that the method employed here is capable of detecting such an intraregional predictive relationship where it exists in the brain. Results in our a priori regions of interest suggest that a simple intraregional baseline activity-functional reactivity association model possesses insufficient explanatory value in the higher integrative cortical areas that are implicated in fear conditioning and extinction, which have more complex excitatory and inhibitory anatomical connections and functional roles.
It is also important to note that results in our a priori regions are consistent with several findings obtained with different experimental modalities. For example, in healthy subjects, the short allele of the serotonin transporter promoter polymorphism is associated with elevated threat-related reactivity of the amygdala (31), but baseline blood flow appears unaffected by this polymorphism (32). In social phobia, amygdala resting blood flow is normal (33), but amygdala reactivity is elevated. Thus, our data and those of others suggest that within a region, resting metabolism and functional reactivity are not necessarily linked. Rather, according to our results, resting activity in particular regions appears to be related to transient reactivity in functionally connected regions dependent on the experimental context. More studies are needed to examine the relationship between resting metabolism and functional reactivity across modalities within the human brain.
Given our previous studies showing opposite structural and functional correlations between the dorsal anterior cingulate cortex and the ventromedial prefrontal cortex during fear expression and extinction recall, we hypothesized that resting metabolic activity in these two regions would oppositely predict extinction recall performance. In support of this hypothesis, resting metabolism in the dorsal anterior cingulate cortex did predict skin conductance response during extinction recall, but resting metabolism in the ventromedial prefrontal cortex did not. Further investigation of this hypothesized opposite relationship is warranted.
Several caveats resulting from this study's multimodal design should be taken into account when interpreting the findings reported here. First, the inferred predictive value of regional brain metabolism for fear extinction and recall relies on stability over time. We recently demonstrated (28) that individual extinction recall responses in our psychophysiological paradigm are reliable over 12 weeks. With regard to metabolism, activity in cortical structures has been found to be more stable over time compared with subcortical structures (18). Given that the interval between the PET and fMRI phases of the experiment was no longer than 5 days (2 days on average), we expect the PET measures to be stable. However, PET and MRI data acquisition within the same experimental session is a potential next step (34). One study (35) indicated that resting-state functional connectivity may be used as a predictor of subsequent functional and behavioral responses, an exciting concept that complements the approach used here. Another caveat is that both PET and fMRI have limited spatial resolution and likely obscure finer anatomical details, especially in small structures such as the amygdala, which contains divisions that may exert opposing influences on behavior. The development of imaging techniques with greater spatial and temporal resolution should help to address this problem. Lastly, the correlation coefficients observed in this study may be inflated because of sample size and multiple comparisons, despite the statistical corrections employed; accordingly, they should be interpreted with caution.
Conclusions
The data reported here suggest that resting metabolism in the dorsal anterior cingulate cortex and in the ventromedial prefrontal cortex might predict the magnitude of fear learning and fear extinction in healthy individuals. As studies have shown that fear extinction is impaired in patients with some psychiatric disorders (36–39) and that functional activations of the dorsal anterior cingulate and the ventromedial prefrontal cortices are abnormal in the context of fear extinction in posttraumatic stress disorder (PTSD) (37, 40), studies delineating whether resting metabolism measurements predict the degree of successful extinction recall in anxiety disorders, and the degree of treatment response, are indicated. In support of this possibility, two twin studies (24, 41) found that elevated resting metabolism in the dorsal anterior cingulate cortex and functional responsivity are risk factors for developing PTSD. Furthermore, patients with PTSD have been found to display altered functional connectivity at rest in the subgenual anterior cingulate cortex and in the amygdala (42, 43). Pharmacological antianxiety treatment may decrease resting metabolism in the amygdala and increase metabolism in the ventromedial prefrontal cortex (22, 44). An additional question is whether regional brain metabolism can predict the success, or lack thereof, of cognitive-behavioral therapy for anxiety disorders, as has been found for functional activation (45). The ability to predict treatment response to therapy with neuroimaging tools might help guide treatment selection.
1. : Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 2006; 23:758–764Crossref, Medline, Google Scholar
2. : Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 2006; 86:123–132Crossref, Medline, Google Scholar
3. : Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002; 420:70–74Crossref, Medline, Google Scholar
4. : Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008; 33:56–72Crossref, Medline, Google Scholar
5. : Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 2009; 29:8474–8482Crossref, Medline, Google Scholar
6. : Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 2009; 4:e5865Crossref, Medline, Google Scholar
7. : Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron 2010; 66:949–962Crossref, Medline, Google Scholar
8. : Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci 2006; 26:9503–9511Crossref, Medline, Google Scholar
9. : Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007; 62:446–454Crossref, Medline, Google Scholar
10. : Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43:897–905Crossref, Medline, Google Scholar
11. : The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 2009; 29:11614–11618Crossref, Medline, Google Scholar
12. : Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 2011; 69:563–571Crossref, Medline, Google Scholar
13. : Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 2005; 102:10706–10711Crossref, Medline, Google Scholar
14. : Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci 2004; 27:489–495Crossref, Medline, Google Scholar
15. : Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001; 2:685–694Crossref, Medline, Google Scholar
16. : Brain work and brain imaging. Annu Rev Neurosci 2006; 29:449–476Crossref, Medline, Google Scholar
17. : Inhibition and brain work. Neuron 2007; 56:771–783Crossref, Medline, Google Scholar
18. : Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp 2000; 10:1–9Crossref, Medline, Google Scholar
19. : Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979; 6:371–388Crossref, Medline, Google Scholar
20. : The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res 1979; 44:127–137Crossref, Medline, Google Scholar
21. : Relation between physiological function and energy metabolism in the central nervous system. J Neurochem 1977; 29:13–26Crossref, Medline, Google Scholar
22. : A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology 2009; 34:390–398Crossref, Medline, Google Scholar
23. : Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 2010; 466:864–868Crossref, Medline, Google Scholar
24. : Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry 2009; 66:1099–1107Crossref, Medline, Google Scholar
25. : Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66:1075–1082Crossref, Medline, Google Scholar
26. : Context modulation of memory for fear extinction in humans. Psychophysiology 2005; 42:456–464Crossref, Medline, Google Scholar
27. : Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 2008; 42:515–520Crossref, Medline, Google Scholar
28. : Test-retest reliability during fear acquisition and fear extinction in humans. CNS Neurosci Ther (Epub ahead of print, Feb 16, 2011)Medline, Google Scholar
29. : Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011; 471:358–362Crossref, Medline, Google Scholar
30. : Amygdala intercalated neurons are required for expression of fear extinction. Nature 2008; 454:642–645Crossref, Medline, Google Scholar
31. : Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry 2010; 167:509–527Link, Google Scholar
32. : Baseline brain perfusion and the serotonin transporter promoter polymorphism. Biol Psychiatry 2010; 67:317–322Crossref, Medline, Google Scholar
33. : Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci 2009; 34:30–40Medline, Google Scholar
34. : Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med 2008; 14:459-465Crossref, Medline, Google Scholar
35. : Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA 2010; 107:355–360Crossref, Medline, Google Scholar
36. : Extinction memory is impaired in schizophrenia. Biol Psychiatry 2009; 65:455–463Crossref, Medline, Google Scholar
37. : Neurobiological basis for failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66:1075–1082Crossref, Medline, Google Scholar
38. : De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 2000; 109:290–298Crossref, Medline, Google Scholar
39. : Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 2010; 27:244–251Crossref, Medline, Google Scholar
40. : Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther 2011; 17:227–236Crossref, Medline, Google Scholar
41. : Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry 2011; 168:979–985Link, Google Scholar
42. : Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci 2009; 34:187–194Medline, Google Scholar
43. : Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand 2010; 121:33–40Crossref, Medline, Google Scholar
44. : Dose-dependent effects of the CRF(1) receptor antagonist R317573 on regional brain activity in healthy male subjects. Psychopharmacology 2010; 208:109–119Crossref, Medline, Google Scholar
45. : Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behavior therapy for post-traumatic stress disorder. Psychol Med 2008; 38:555–561Crossref, Medline, Google Scholar



