Disruption in the Balance Between Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder
Abstract
Objective:
Obsessive-compulsive disorder (OCD) is characterized by repetitive, ritualistic behaviors and thought patterns. Although patients with OCD report that these compulsive behaviors are unproductive and often senseless, they are unable to desist. This study investigated whether the urge to perform compulsive acts is mediated by a disruption in the balance between flexible, goal-directed action control and habitual behavior.
Method:
A total of 21 patients with OCD and 30 healthy comparison subjects participated in a set of tasks designed to assess relative goal-directed versus habitual behavioral control. In the training stage, participants were asked to respond to different pictured stimuli in order to gain rewarding outcomes. In the subsequent (instructed) outcome devaluation test and in a novel “slips-of-action” test, the authors assessed whether participants were able to flexibly adjust their behavior to changes in the desirability of the outcomes. The authors also used a questionnaire to test explicit knowledge of the relationships between stimuli, responses, and outcomes.
Results:
Patients with OCD showed no deficit in their ability to use feedback to respond appropriately to stimuli in the training stage. However, their knowledge of the outcomes of these responses was impaired relative to healthy comparison subjects, and patients were more prone to slips of action, indicating a deficit in goal-directed control and an overreliance on habits.
Conclusions:
This study provides the first experimental evidence for selective impairment in flexible and goal-directed behavioral control in patients with OCD. The impairment forces patients with OCD to rely instead on habits that can be triggered by stimuli regardless of the desirability of the consequences. Goal-directed actions are supported by orbitofronto-striatal circuitry, and the study findings are thus in line with findings from research that implicate dysfunction in this circuitry in the neuropathology of OCD.
A striking characteristic of obsessive-compulsive disorder (OCD) is the propensity toward excessive stereotyped behavior that is carried out to reduce the likelihood of adverse consequences (1). While patients with OCD report that these compulsive behaviors are excessive and typically ineffective, they have significant difficulty inhibiting these behaviors. This observation has led researchers to speculate that flexible, goal-directed action control is compromised in OCD and that compulsive acts are instead driven by maladaptive habits (2, 3).
According to dual-system theories (4, 5), actions can be supported by either a goal-directed or a habit system. When the goal-directed system exerts dominant control, actions are performed to achieve desirable goals or to avoid undesirable outcomes. Washing one's hands before preparing a meal, for example, may constitute a goal-directed action that is performed to avoid contamination. However, after multiple repetitions of this action, the habitual system can begin to exert dominant control over behavior (6–8), leading to greater efficiency but also to a loss of behavioral flexibility. This becomes apparent when a person commits a “slip of action,” such as thoughtlessly washing her hands upon entering the kitchen when her intention was to retrieve a set of keys. Here, the kitchen environment has directly triggered the habitual response of hand washing. We hypothesize that in OCD, persistent reliance on the habitual system leads to compulsive responding, such as repetitive hand washing. Habitual responses for undesirable outcomes can be induced in animals by lesioning the prelimbic cortex (9–11), suggesting that this area is crucially involved in goal-directed action control. More recently, functional MRI (fMRI) studies have provided correlational evidence that the ventromedial prefrontal cortex is similarly involved in goal-directed action control in humans (12, 13).
It has been suggested that in drug abusers, the dominance of habitual response control contributes toward the subjective “must do!” experience that commonly accompanies a compulsive urge (14). Based on behavioral parallels between habits and OCD compulsions, we hypothesized that a deficit in goal-directed action control, and a consequent overreliance on habit formation, may underlie compulsivity in OCD. Furthermore, there is consensus that dysfunction in the orbitofrontal and cingulate cortices and in the caudate nucleus plays an important role in OCD (2, 15, 16). These same regions have been implicated in goal-directed control (10, 12, 13, 17, 18) and habit learning (19–21). Therefore, impairment of this frontostriatal loop (22) in patients with OCD is likely to cause disruptions in the goal-directed system and cause an overreliance on habitual control.
To test this hypothesis, we employed a series of tasks, as depicted in Figure 1. During the initial training stage (Figure 1B), participants learned to respond to different stimuli in order to gain outcomes that would earn them points. A baseline of habitual behavior was established by using incongruent events on a subset of trials, which have been shown to elicit habitual responses in healthy participants (13, 23, 24) (Figure 1A; see Method section). After the training stage, we tested relative goal-directed versus habitual control. The first of three tests was an outcome devaluation test (Figure 1C), in which participants must use their knowledge of the relationship between their actions and the various outcomes to direct their choices to valuable outcomes (and away from devalued outcomes). Second, we administered a questionnaire that explicitly probed knowledge of the relationships between stimuli, responses, and outcomes. Finally, we used a novel slips-of-action test (Figure 1D), in which participants could respond to stimuli that signaled either still valuable or devalued outcomes. Here, the goal-directed and habitual systems compete for behavioral control, and this provides a more sensitive index of which system retains relative control. Responses to devalued outcomes, or slips of action, imply a lack of sensitivity to outcome value and are therefore indicative of the dominance of habitual response control. We predicted that overreliance on the habit system would cause patients with OCD to commit more slips of action than the comparison subjects.
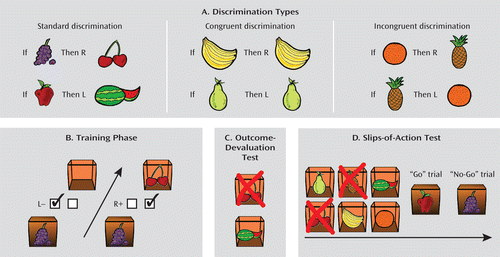
FIGURE 1. Instrumental Learning Task Descriptiona
a Panel A illustrates the three discrimination types: standard, congruent, and incongruent. Panel B illustrates the training phase. In this example from the standard discrimination, participants are presented with grapes on the outside of the box. If the incorrect (left) key is pressed, an empty box is revealed (and no points are earned). If the correct (right) key is pressed, participants are rewarded with cherries on the inside of the box (and points). Panel C illustrates the outcome devaluation test. In this example, participants are presented with two open boxes with a melon and cherries inside. The red cross (or X) superimposed on the cherries indicates that this fruit type is no longer worth any points. The correct response in this example would be to press the left key (which during training yielded the still-valuable melon outcome). Panel D illustrates the slips-of-action test. In this example, the initial instruction screen shows that the pineapple and cherries outcomes will now lead to the subtraction of points, as indicated by the red crosses. The other four outcomes are still valuable. Following the instruction screen, participants are presented with a rapid succession of the fruit stimuli (on the front door of the boxes) and are asked to press the correct keys (“Go”) when a stimulus signals the availability of a still-valuable outcome inside the box but to refrain from responding (“No-Go”) when the outcome inside the box has been devalued. In this particular example, participants should press the correct key when the apple stimulus is depicted on the front door (“Go”) but should refrain from responding to the grapes stimulus (“No-Go”).
Method
Participants
This study was approved by the Hertfordshire Research Ethics Committee. Patients were recruited from a specialist outpatient OCD clinic and were screened by the consultant psychiatrist (N.A.F.) using an extended clinical interview to ensure that they met DSM-IV-TR criteria for OCD and did not suffer from any current comorbid axis I disorders. Participants completed two other behavioral tasks, unrelated to the present study (25, 26), in a counterbalanced order in the same session. Analysis of these data is ongoing.
Twenty-one patients with OCD (13 women) and 30 healthy comparison subjects (18 women) participated in this study. Table 1 summarizes the groups' demographic and clinical characteristics. Groups were matched for gender, age, and verbal IQ as indicated by the National Adult Reading Test (27). As expected, the groups differed on scores of OCD symptom severity as measured by the Yale-Brown Obsessive Compulsive Scale (28) and the Obsessive-Compulsive Inventory–Revised (29). In keeping with a dimensional approach to OCD (30), patients were not categorized in terms of subtype (e.g., washing, checking). Patients with OCD showed higher levels of depression on the Montgomery-Åsberg Depression Rating Scale (MADRS; 31) and anxiety on the State-Trait Anxiety Inventory (32).
| OCD Patients | Comparison Subjects | Analysis | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | t | df | p |
| Age (years) | 43.10 | 2.52 | 41.80 | 2.31 | 0.373 | 49 | 0.711 |
| National Adult Reading Test score | 36.24 | 1.80 | 38.97 | 0.99 | 1.428 | 49 | 0.16 |
| Yale-Brown Obsessive Compulsive Scale score | 18.95 | 1.70 | 0.23 | 0.23 | 10.930 | 20.76 | <0.001 |
| Obsessive-Compulsive Inventory–Revised total score | 32.38 | 3.01 | 7.67 | 1.24 | 7.587 | 26.864 | <0.001 |
| State-Trait Anxiety Inventory state score | 48.00 | 12.91 | 30.87 | 6.63 | 6.210 | 49 | <0.001 |
| State-Trait Anxiety Inventory trait score | 57.00 | 12.50 | 35.47 | 9.05 | 7.317 | 49 | <0.001 |
| Montgomery-Åsberg Depression Rating Scale score | 13.05 | 2.16 | 1.53 | 0.38 | 5.242 | 21.22 | <0.001 |
TABLE 1. Demographic and Clinical Characteristics of Participants in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)
The average duration of illness was 18.25 years (SD=9.06). Nineteen of the 21 patients with OCD were taking psychotropic medication. Of these, 18 patients were taking selective serotonin reuptake inhibitors (SSRIs), and of these taking SSRIs, one was also taking the tricyclic antidepressant clomipramine and four were also taking antipsychotic medication. One patient was only taking the antipsychotic risperidone. Patients whose medication was augmented with antipsychotics represented refractory cases; all patients with OCD were free of comorbid disorders, and none of the comparison subjects was on medication.
Procedure
For a detailed task description, please see de Wit et al. (24). The instructions given to participants are reproduced in Table 2.
| Task | Verbal Instructions |
|---|---|
| Instrumental discrimination training | “In this game, you will get the chance to earn points by collecting fruit from inside a box on the screen by opening the box by pressing either the right 'm' or left 'z' key. If you press the correct key, the box will open to reveal a fruit inside, and points will be added to your total score. However, if you press the incorrect key, the box will be empty and no points will be added to your total. Your task is to learn which is the correct key to press. Sometimes it will be the left-hand one, and sometimes it will be the right-hand one. The picture on the front of the door should give you a clue about which is the correct response. The quicker you make the correct response, the more points will be added to your total. Your accumulated points will appear at the top of the screen. You should try to learn the types of fruits that are found inside the boxes following left-hand and right-hand responses because later on you will be asked to gather some types of fruits but not others.” |
| Outcome devaluation test | “Now two open boxes will appear on the screen with different fruits inside them. One fruit was earned by a left response in the first stage and the other by a right response. Although both the fruits were valuable previously, one of them is now devalued and earns no points, whereas the other is still valuable and gains points. The devalued fruit will have a cross on it. Please perform the button-press that previously led to the fruit that is still valuable.” |
| Slips-of-action test | “Once again, you will have the chance to earn points by pressing keys to open a box shown on the screen, only this time some of the fruits inside these boxes are no longer valuable. Your job is to press the correct key for each fruit shown on the box BUT withhold your key press if the fruit inside that box is devalued. Correct key presses will be rewarded and incorrect key presses will not be rewarded, but one point will be subtracted for every key press made to a box with a devalued fruit inside. This is a test and we won't show you your score until the very end. There will be six test sessions. At the beginning of each session we will show you the two fruits that are devalued during this test—these will have a cross on them. Then remember to press the keys quickly as the boxes will follow each other very rapidly! Good Luck!” |
TABLE 2. Task Instructions for Participants in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)a
Instrumental discrimination training.
On a computer screen, participants were presented with closed boxes with pictures of fruits on them. Their task was to learn by trial and error which key (the left “z” or the right “m”) opened each box to reveal another fruit prize inside, which added points to their total (see Figure 1B). The faster the correct response, the more points were earned. Participants were awarded 5 points for correct responses within 0–1 second; 4 points for 1–1.5 seconds; 3 points for 1.5–2 seconds; 2 points for 2–2.5 seconds; and 1 point for >2.5 seconds. Participants went through six blocks of 12 trials each. Three different (biconditional) discriminations were trained concurrently: congruent, standard, and incongruent (Figure 1A). In the standard discrimination, different fruits served as stimuli and outcomes. On trials of the congruent discrimination, the fruit icon on the outside of the box (stimulus) was identical to the fruit inside the box (outcome). Finally, in the incongruent discrimination, each fruit served as a stimulus in one component, but also as an outcome of the opposite response in the other component (e.g., orange as the stimulus signaled that pressing the right-hand key would reveal pineapple as an outcome, but when a pineapple appeared as the stimulus, the opposite response of pressing the left-hand key would reveal an orange as the outcome). Therefore, goal-directed learning is rendered disadvantageous because it causes opposing keys to become associated with the same fruit. In previous studies (13, 24), we showed that participants tend to rely on a (stimulus-response) habit strategy to solve the incongruent discrimination (as opposed to the standard and congruent discriminations). The incongruent discrimination therefore provided us with a baseline of habitual responding.
Instructed outcome devaluation test.
In the instructed outcome devaluation test (Figure 1 C), participants were presented with two open boxes on the screen with two different fruits inside; one fruit was previously earned by pressing the left-hand key and the other by pressing the right-hand key. One of the fruits was shown with a cross (an X) on it to indicate that it was no longer worth any points (devalued). Participants were instructed to press the key that had previously earned the still-valuable fruit. The test consisted of 12 trials—four trials from each of the three discriminations—and each outcome was devalued twice. No performance feedback was provided to participants during any of the test stages.
Questionnaire on response and outcome knowledge.
We used a questionnaire to assess the participants' explicit knowledge of the instrumental contingencies. There were six total questions, each with a response and outcome knowledge component. Participants were asked to indicate whether the right-hand or the left-hand response had been correct (“response knowledge”) and which fruit appeared inside the box following a correct response (“outcome knowledge”) for each fruit that had functioned as a discriminative stimulus (13, 23).
Slips-of-action test.
Each of the six test blocks started with a 10-second screen that showed the six different fruit outcomes from the initial training stage (see Figure 1D). Two of these fruit icons were marked with a cross indicating that collecting these fruits would result in subtraction of points. Following this screen, a series of closed boxes marked with fruit stimuli from the training phase were presented in quick succession. Participants could earn points by pressing the appropriate keys to open the boxes with valuable fruit outcomes inside. However, whenever they were presented with a stimulus for which the outcome inside was devalued, they could avoid losing points by refraining from pressing either key. Goal-directed action control was thus reflected in the selective responses to valuable as opposed to devalued outcomes. In contrast, if the habitual system exerted dominant control over behavior, it resulted in slips of action toward devalued outcomes. Each closed box was shown for 1 second and was replaced by another box with a different stimulus after a 1-second intertrial interval. Participants completed 144 trials over six blocks with each of the six stimuli presented four times per block in random order.
Data Analysis
Data were analyzed using repeated-measures analysis of variance (ANOVA). Where appropriate, data were investigated further using the Bonferroni correction, planned pairwise comparisons, and tests of simple effects. Probabilities involving repeated-measures factors are based on Greenhouse-Geisser sphericity corrections, and all significant interactions (set at p<0.05) are reported. For the training stage, outcome devaluation test, and questionnaire, data were analyzed for 30 healthy comparison subjects and 21 patients. However, only 20 participants from each group completed the slips-of-action test because this novel measure was added after 10 comparison subjects and one patient had already completed their participation.
Results
Table 3 summarizes the accuracy and reaction times for the standard, congruent, and incongruent discriminations. Table 4 summarizes the scores earned by participants (out of 2 possible) on the questionnaire that assessed explicit knowledge of the instrumental contingencies.
| Slips-of-Actionc | ||||||||
|---|---|---|---|---|---|---|---|---|
| Instrumental Discrimination Trainingb | Outcome-Devaluation Testb | Valued | Devalued | |||||
| Group and Discriminationa | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Comparison subjects | ||||||||
| Standard | ||||||||
| % | 76 | 17.56 | 71 | 31.54 | 75 | 16.76 | 49 | 25.72 |
| Reaction time (msec) | 1,987.91 | 297.90 | 4,962.14 | 778.10 | 721.36 | 8.00 | 743.34 | 14.24 |
| Congruent | ||||||||
| % | 82 | 11.30 | 90.00 | 22.36 | 79 | 16.27 | 23 | 18.90 |
| Reaction time (msec) | 1,717.97 | 207.95 | 3,784.23 | 809.33 | 699.65 | 13.47 | 691.82 | 25.19 |
| Incongruent | ||||||||
| % | 74 | 14.52 | 54 | 39.44 | 63 | 18.94 | 47 | 26.55 |
| Reaction time (msec) | 1,211.36 | 111.39 | 5,161.13 | 713.7 | 738.26 | 12.20 | 770.50 | 19.66 |
| Mean of discriminations | ||||||||
| % | 77 | 11.56 | 72 | 20.37 | 72 | 13.6 | 40 | 16.37 |
| Reaction time (msec) | 1,639.08 | 502.97 | 4,635.84 | 2,125.42 | 719.75 | 36.44 | 731.96 | 65.30 |
| OCD patients | ||||||||
| Standard | ||||||||
| % | 72 | 19.43 | 70 | 31.24 | 83 | 10.16 | 76 | 29.33 |
| Reaction time (msec) | 1,932.25 | 280.20 | 5,068.27 | 1,044.49 | 658.67 | 19.76 | 663.36 | 21.13 |
| Congruent | ||||||||
| % | 73 | 17.56 | 71 | 31.90 | 82 | 11.13 | 41 | 26.79 |
| Reaction time (msec) | 1,469.18 | 247.09 | 5,209.98 | 894.77 | 663.02 | 18.87 | 666.42 | 25.50 |
| Incongruent | ||||||||
| % | 69 | 19.96 | 39 | 38.38 | 67 | 16.95 | 74 | 22.61 |
| Reaction time (msec) | 1,855.70 | 326.47 | 4,281.89 | 667.53 | 673.92 | 19.33 | 656.04 | 25.92 |
| Mean of discriminations | ||||||||
| % | 72 | 15.67 | 60 | 18.80 | 77 | 7.70 | 64 | 19.18 |
| Reaction time (msec) | 1,752.38 | 597.16 | 4,853.05 | 2,111.57 | 664.87 | 82.99 | 661.96 | 101.67 |
TABLE 3. Results for the Standard, Congruent, and Incongruent Discriminations in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)
| Correct Responses on Questionnaires | ||||
|---|---|---|---|---|
| Response | Outcome | |||
| Group and Discrimination | Meana | SD | Meana | SD |
| Comparison subjects | ||||
| Standard | 1.73 | 0.52 | 1.37 | 0.81 |
| Congruent | 1.77 | 0.43 | 1.63 | 0.72 |
| Incongruent | 1.53 | 0.68 | 1.30 | 0.79 |
| Mean of discriminations | 1.68 | 0.41 | 1.43 | 0.66 |
| OCD patients | ||||
| Standard | 1.67 | 0.58 | 0.81 | 0.81 |
| Congruent | 1.67 | 0.66 | 0.90 | 0.89 |
| Incongruent | 1.33 | 0.66 | 0.62 | 0.67 |
| Mean of discriminations | 1.56 | 0.50 | 0.78 | 0.49 |
TABLE 4. Results of a Questionnaire on Response and Outcome Knowledge in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)
There was no significant difference between patients with OCD and comparison subjects in rate of learning (Figure 2), nor was there a significant group-by-discrimination (congruent/standard/incongruent) interaction. A nearly significant effect of discrimination was observed (F=2.96, df=2, 98, p=0.06), indicating a tendency toward differential learning rates that was dependent on discrimination type. Preplanned pairwise comparisons (using the Bonferroni correction) showed that participants performed better overall on the congruent discrimination (mean=78%) than on the incongruent discrimination (mean=72%, p<0.05), while performance on the standard discrimination (mean=74%) did not differ from performances on either the congruent or incongruent discriminations. There was neither a group difference in reaction time nor a group-by-discrimination interaction.
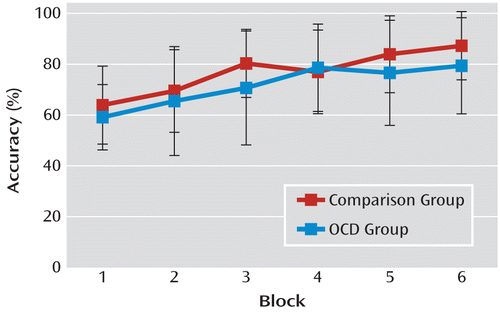
FIGURE 2. Response Accuracy Over the Course of Six Blocks in the Instrumental Discrimination Training for Participants in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)a
a Error bars denote standard deviations.
Instructed Outcome Devaluation Test
In line with our hypothesis, a significant group effect was observed (F=4.08, df=1, 49, p<0.05), with average values of 72% and 60% correct responses in the comparison and OCD patient groups, respectively, indicating that deployment of goal-directed knowledge was impaired in the patient group. We also observed a significant discrimination main effect (F=14.66, df=2, 98, p<0.001). Consistent with previous studies using this task, pairwise comparisons revealed that performance on the incongruent discrimination (mean=48%) was worse than on the congruent (mean=82%, p<0.0001) and standard (mean=71%, p=0.001) discriminations. Scores on the congruent and standard conditions did not differ significantly, and there was no discrimination-by-group interaction, illustrating that both groups performed worse on the incongruent discrimination relative to congruent and standard discriminations. Reaction times were not affected by group or discrimination type.
Slips-of-Action Test
A significant group-by-devaluation interaction (F=6.70, df=1, 38, p<0.05) was investigated with tests of simple effects. While there was no group difference in the level of responding for valuable outcomes, patients with OCD responded more often to stimuli associated with devalued outcomes than comparison subjects. These findings reveal that responses were under dominant habitual control in patients with OCD, thereby rendering their behavior insensitive to changes in outcome value (F=17.43, df=1, 38, p<0.001) (Figure 3). Separate group analyses revealed that while both groups showed a devaluation effect (i.e., overall fewer responses to stimuli with devalued than valued outcomes), this effect was much more pronounced in comparison subjects (F=3.61, df=1, 19, p<0.001) than in patients with OCD (F=8.69, df=1, 19, p<0.05).
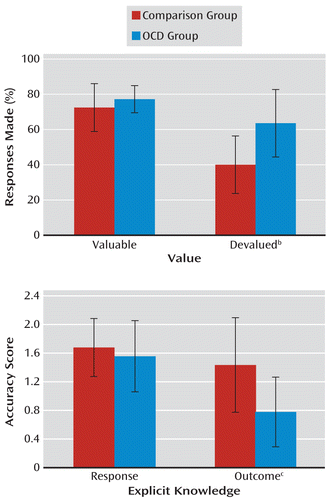
FIGURE 3. Performance on the Slips-of-Action Test and the Explicit Response and Outcome Questionnaire in a Study of Goal-Directed Behavior and Habit Learning in Obsessive-Compulsive Disorder (OCD)a
a The upper panel shows the percentage of responses made by the OCD and comparison groups in the slips-of-action test, and the lower panel shows the mean accuracy scores for the OCD and comparison groups on the explicit response and outcome questionnaire. Error bars denote standard deviations.
b While there was no group difference in percentage response to valuable outcomes, patients with OCD responded significantly more often for outcomes that were devalued relative to comparison subjects (F=17.43, df=1, 38, p<0.001).
c Groups did not differ in their knowledge of the correct responses from the training stage. Patients with OCD, however, showed significantly worse outcome knowledge relative to comparison subjects (F=14.92, df=1, 49, p<0.001).
To investigate whether selective response suppression was directly related to symptom severity in the OCD patient group, we conducted Pearson correlational analyses on the difference scores (responses to stimuli for valuable outcomes minus responses to stimuli for devalued outcomes) and Yale-Brown Obsessive Compulsive Scale scores. We found a significant negative correlation (r=–0.56, p=0.01), indicating that OCD symptom severity predicted slips of action—failure to withhold responses toward devalued outcomes.
Finally, we investigated the significant devaluation by discrimination interaction (F=29.602, df=2, 76, p<0.001) using tests of simple effects. These tests confirmed that all participants responded fewer times when the outcome was devalued as opposed to still valuable on both congruent (F=119.53, df=1, 38, p<0.001) and standard (F=11.02, df=1, 38, p<0.01) discriminations. However, as predicted, outcome devaluation failed to affect the number of responses on incongruent trials, which tend to be solved by habit strategy. There was no three-way interaction.
Questionnaires of Response and Outcome Knowledge
All of the participants completed a questionnaire to test their explicit knowledge of responses and outcomes. The scores on the questionnaires could be 2, 1, or 0 for each of the discriminations. A significant main effect of discrimination (F=7.06, df=2, 98, p<0.01) was investigated using Bonferroni-corrected pairwise comparisons. Overall, participants' explicit knowledge of the congruent contingencies was better than knowledge of the standard and incongruent contingencies (p<0.05 in all cases). Crucially, there was an interaction between group and explicit knowledge (F=8.31, df=1, 49, p<0.01). Simple effects analyses revealed that while knowledge of the appropriate responses to the stimuli did not differ between comparison subjects and patients with OCD, knowledge of the associated outcomes was significantly worse in patients (F=14.915, df=1, 49, p<0.001) (Figure 3). Furthermore, the patients' outcome knowledge, and not response knowledge, was positively correlated with difference scores from the slips-of-action test (r=0.61, p<0.005), indicating that the failure to acquire outcome knowledge during the training stage was associated with habitual response control during the slips-of-action test.
Additional Analyses Controlling for Differences in Anxiety and Depression
Stress (33, 34) and anxiety (35) can cause impairments in goal-directed action control. To investigate whether anxiety contributed to the group differences observed, we used analyses of covariance (ANCOVAs) with state and trait anxiety scores from the State-Trait Anxiety Inventory (32) as covariates. As the OCD patient group had higher rates of depressive symptoms than comparison subjects, MADRS scores were included in separate ANCOVAs for each stage of the task. None of these effects was significant.
Discussion
Using an instrumental learning task, we present the first direct experimental evidence of a disruption in goal-directed action control in OCD. Healthy comparison subjects and patients with OCD were equally successful at using external feedback to guide instrumental choice between right and left responses, demonstrating that feedback learning was unaffected in OCD. To investigate the underlying learning mechanisms employed during the training stage, we first investigated goal-directed (action-outcome) learning using an instructed outcome devaluation test. The patients with OCD demonstrated weaker knowledge of the causal relationship between actions and their respective outcomes, suggesting a disturbance in goal-directed action control.
To investigate this possibility more directly, we developed a novel slips-of-action test in which the goal-directed system must compete with the habit system for control. Consistent with the habit hypothesis of OCD, patients showed a marked lack of sensitivity to devaluation. Furthermore, we found that symptom severity was predictive of poor performance on the slips-of-action test. We investigated the basis of this deficit using a response and outcome knowledge questionnaire. While knowledge of the correct responses to stimuli was intact in the OCD group, the patients showed a selective deficit in knowledge of the resulting outcomes. Furthermore, outcome—and not response—knowledge was found to predict performance on the slips-of-action test. Taken together, these findings suggest that failure to engage the goal-directed (action-outcome) system mediated slips of action in patients with OCD. We propose that a general impairment in goal-directed action control, with a consequent overreliance on habits, may contribute to the relatively inflexible behavior observed in patients with OCD and furthermore may play a part in the development of compulsivity.
It is evident that patients with OCD do not develop compulsions in every aspect of their lives. Rather, they develop avoidance compulsions related to specific obsessions. It may be critical that the goal of an avoidance response (e.g., hand washing) is not to obtain a tangible outcome but rather to bring about a nonevent (e.g., not contracting an illness). As this nonevent has a high likelihood of occurrence and can cause a generally reinforcing sense of relief, this might make avoidance behavior particularly sensitive to habit formation. The example of compulsive hand washing can be used to illustrate the theoretical overlap between habit formation and compulsivity in OCD. When probed, patients report that they are aware that hand washing has little bearing on whether or not they will contract the feared illness. However, in spite of this knowledge, the behavior persists. A lack of sensitivity to the direct outcomes of actions but preserved sensitivity to broader goals—such as relief from anxiety triggered by obsessions—might explain this phenomenon. This account can explain why patients with OCD have no deficit in their ability to perform the task to gain the broader outcome of earning points but show a lack of sensitivity to the more direct outcomes of their actions (which fruit they won in order to obtain those points). We postulate that the observed deficit constitutes a vulnerability factor for OCD, but the presence of obsessions is likely critical for compulsions to develop.
Numerous functional neuroimaging studies have shown that the orbitofrontal and ventromedial prefrontal cortices, and less consistently the caudate nucleus, are engaged when healthy volunteers perform goal-directed actions (12, 36). Importantly, dysfunction in this orbitofronto-striatal circuit has been consistently implicated both in many aspects of OCD symptomatology (37–39) and in aspects of cognitive flexibility and deficits in motor inhibition associated with the disorder (40). Furthermore, an fMRI investigation (13) implicated the ventromedial prefrontal cortex in goal-directed performance on our instructed outcome devaluation test. Our finding of impaired performance on this test by patients with OCD is therefore consistent with research implicating a dysfunction in this goal-directed corticostriatal pathway in OCD. The dysfunction forces patients to rely instead on a parallel, habitual system, which includes the putamen and possibly the sensorimotor cortex in humans (20, 41).
Although previous studies have provided evidence for abnormalities in implicit learning in OCD at both the behavioral and neural levels (42–44), this is the first study of habit formation as defined by Dickinson and colleagues (4, 5). The mechanisms underlying implicit learning and habits may well overlap, but research is needed to elucidate this. Critically, our data do not imply that habit formation is exaggerated in patients with OCD. Rather, we were able to show that a substantial goal-directed action control deficit tipped the balance toward reliance on habits. The question remains whether this imbalance offers an account of ego-dystonic behavior in OCD. In our study, patients often reported that they were aware of their impaired outcome knowledge and their reliance on habits (see Patient Perspective). It is therefore possible that egodystonic experience only arises after extensive behavioral repetition or only in the context of patients' specific obsessions.
We did not find evidence for superior learning of the standard discrimination relative to the incongruent one, which has been previously reported for young, healthy volunteers and which is thought to reflect the additional support of the goal-directed system in the standard discrimination (13). The fact that our healthy comparison subjects failed to show the congruency effect, possibly because the average age in the comparison group was higher (45), indicates that the congruency comparison cannot be used in our study as a reliable measure of outcome learning. However, the overall lack of a congruence effect does not bear on our robust demonstration of deficiencies in outcome knowledge in patients with OCD.
The majority of patients with OCD were taking SSRIs, and a small number were receiving antipsychotics. This represents a significant limitation of our study, as we cannot exclude the possibility of a medication effect. Some evidence from animal research has suggested that serotonin depletion in the orbitofrontal cortex can reduce sensitivity to outcome value (46, 47); therefore, it is possible that unmedicated patients would show an even more pronounced deficit. Nevertheless, in subsequent studies an appropriate clinical control group (e.g., drug addicts, pathological gamblers) or an unmedicated OCD patient group should be included to determine whether the observed deficit is specific to OCD.
In conclusion, patients with OCD showed no deficit in their ability to use feedback to guide instrumental learning. However, patients' knowledge of the outcomes following their responses was impaired, leading them to commit slips-of-action errors. These results indicate that patients' performance depended more strongly on habitual control at the expense of goal-directed control. We therefore propose that, as has been suggested for drug and gambling addiction, an imbalance between habitual and goal-directed control may underlie the urge to perform compulsive acts (14). Although additional research will be necessary to corroborate this account, in light of convergent neurobiological and behavioral evidence, we postulate that this imbalance may contribute to the compulsive behaviors typical of OCD.
“Mr. J” has lived with OCD for 31 years. His predominant symptoms include symmetry obsessions and compulsive urges to order, arrange, count, and check. Mr. J reports a fear of nonspecific disaster, which may cause him to lose possessions or people who are important to him if he does not perform his compulsive routines. The need to perform these compulsions is exacerbated by social contact, in person or via telephone. With a Yale-Brown Obsessive Compulsive Scale score of 28, he represents a severe case of OCD. Mr. J was interviewed about his performance on the instrumental learning task:
“I found it quite easy to learn the right buttons to press. I think I learned some of the fruits inside the boxes, but definitely not as well as I learned the buttons.” When asked why he found the responses easier than the outcomes, he said, “Well, with the buttons you're doing, so I can remember when I saw that fruit, I pressed that button. My hands knew what to do with those, but with the outcomes, it was much more difficult.” During the questionnaire, which probed response and outcome knowledge, Mr. J would close his eyes and mime pressing one of the buttons to aid his memory. When asked about the slips of action, he commented, “It was very quick. I tried to do it all at the start, but it was too much to do all at once, so I was messing everything up. After that, I just focused on getting the buttons right, as I knew I could do that on its own.”
1. : Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 2010; 35:591–604Crossref, Medline, Google Scholar
2. : Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000; 28:343–347Crossref, Medline, Google Scholar
3. : Cross-species models of OCD spectrum disorders. Psychiatry Res 2009; 170:15–21Crossref, Medline, Google Scholar
4. : Actions and responses: the dual psychology of behavior, in Spatial Representation: Problems in Philosophy and Psychology. Edited by Eilan NMcCarthy RABrewer B. Malden, Mass, Blackwell Scientific Publishing, 1993, pp 277–293Google Scholar
5. : Associative theories of goal-directed behaviour: a case for animal-human translational models. Psychol Res 2009; 73:463–476Crossref, Medline, Google Scholar
6. : Instrumental responding following reinforcer devaluation. Q J Exp Psychol 1981; 33:109–121Crossref, Google Scholar
7. : Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol 1982; 34B:77–98Crossref, Google Scholar
8. : Animal Intelligence: Experimental Studies. New York, Macmillan, 1911Crossref, Google Scholar
9. : The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 2005; 22:513–523Crossref, Medline, Google Scholar
10. : Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 2003; 13:400–408Crossref, Medline, Google Scholar
11. : The role of prelimbic cortex in instrumental conditioning. Behav Brain Res 2003; 146:145–157Crossref, Medline, Google Scholar
12. : Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 2007; 27:4019–4026Crossref, Medline, Google Scholar
13. : Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci 2009; 29:11330–11338Crossref, Medline, Google Scholar
14. : Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 2005; 8:1481–1489Crossref, Medline, Google Scholar
15. : White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry 2008; 165:1308–1315Link, Google Scholar
16. : Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23:563–586Crossref, Medline, Google Scholar
17. : Modulation of caudate activity by action contingency. Neuron 2004; 41:281–292Crossref, Medline, Google Scholar
18. : Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci 2008; 28:6750–6755Crossref, Medline, Google Scholar
19. : Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci 2004; 19:181–189Crossref, Medline, Google Scholar
20. : A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci 2009; 29:2225–2232Crossref, Medline, Google Scholar
21. : Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res 2003; 146:167–174Crossref, Medline, Google Scholar
22. : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
23. : Habitual versus goal-directed action control in Parkinson's disease. J Cogn Neurosci 2011; 23:1218–1229Crossref, Medline, Google Scholar
24. : Stimulus-outcome interactions during instrumental discrimination learning by rats and humans. J Exp Psychol Anim Behav Process 2007; 33:1–11Crossref, Medline, Google Scholar
25. : Reconciling the role of serotonin in behavioral inhibition and aversion: acute tryptophan depletion abolishes punishment-induced inhibition in humans. J Neurosci 2009; 29:11993–11999Crossref, Medline, Google Scholar
26. : The involvement of the orbitofrontal cortex in the experience of regret. Science 2004; 304:1167–1170Crossref, Medline, Google Scholar
27. : National Adult Reading Test (NART): Test Manual. Windsor, UK, NFER-Nelson, 1982Google Scholar
28. : The Yale-Brown Obsessive Compulsive Scale (Y-BOCS), part 1: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
29. : The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess 2002; 14:485–496Crossref, Medline, Google Scholar
30. : A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry 2005; 162:228–238Link, Google Scholar
31. : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
32. : State-Trait Anxiety Inventory (STAI). Palo Alto, Calif, Consulting Psychologists Press, 1985Google Scholar
33. : Stress prompts habit behavior in humans. J Neurosci 2009; 29:7191–7198Crossref, Medline, Google Scholar
34. : Socially evaluated cold pressor stress after instrumental learning favors habits over goal-directed action. Psychoneuroendocrinology 2010; 35:977–986Crossref, Medline, Google Scholar
35. : Anxiety and cognitive performance: attentional control theory. Emotion 2007; 7:336–353Crossref, Medline, Google Scholar
36. : Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex 2009; 19:483–495Crossref, Medline, Google Scholar
37. : Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 2008; 32:525–549Crossref, Medline, Google Scholar
38. : Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994; 51:62–70Crossref, Medline, Google Scholar
39. : Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; 35:26–37Crossref, Medline, Google Scholar
40. : Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 2007; 164:335–338Link, Google Scholar
41. : Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 2010; 35:48–69Crossref, Medline, Google Scholar
42. : Impaired procedural learning in obsessive-compulsive disorder and Parkinson's disease, but not in major depressive disorder. Behav Brain Res 2005; 157:253–263Crossref, Medline, Google Scholar
43. : Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry 2007; 61:330–336Crossref, Medline, Google Scholar
44. : Implicit sequence learning in obsessive-compulsive disorder: further support for the fronto-striatal dysfunction model. Biol Psychiatry 2005; 58:239–244Crossref, Medline, Google Scholar
45. : Habitual versus goal-directed action control in Parkinson's disease. J Cogn Neurosci 2010; 23:1218–1229Crossref, Medline, Google Scholar
46. : Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 2007; 17:18–27Crossref, Medline, Google Scholar
47. : Orbitofrontal cortex and amygdalar over-activity is associated with an inability to use the value of expected outcomes to guide behaviour in serotonin transporter knockout rats. Neurobiol Learn Mem 2010; 94:65–72Crossref, Medline, Google Scholar



