Resting-State Functional Connectivity in Treatment-Resistant Depression
Abstract
Objective:
The authors used resting-state functional connectivity MRI to evaluate brain networks in patients with refractory and nonrefractory major depressive disorder.
Method:
In a cross-sectional study, 28 patients with refractory major depression, 32 patients with nonrefractory major depression, and 48 healthy comparison subjects underwent scanning using a gradient-echo echo-planar imaging sequence on a 3-T MR system. Thirteen regions of interest that have been identified in the literature as relevant to mood regulation were selected as seed areas. A reference time series was extracted for each seed and used for voxel-wise correlation analysis with the rest of the brain. Voxel-based comparisons of z-value maps among the three groups were performed using one-way analysis of variance followed by post hoc t tests with age and duration of illness as covariates of no interest.
Results:
Relative to healthy comparison subjects, both patient groups showed significantly reduced connectivity in prefrontal-limbic-thalamic areas bilaterally. However, the nonrefractory group showed a more distributed decrease in connectivity than the refractory group, especially in the anterior cingulate cortex and in the amygdala, hippocampus, and insula bilaterally; in contrast, the refractory group showed disrupted functional connectivity mainly in prefrontal areas and in thalamus areas bilaterally.
Conclusions:
Refractory depression is associated with disrupted functional connectivity mainly in thalamo-cortical circuits, while nonrefractory depression is associated with more distributed decreased connectivity in the limbic-striatal-pallidal-thalamic circuit. These results suggest that nonrefractory and refractory depression are characterized by distinct functional deficits in distributed brain networks.
Substantial efforts have been made in the past decade to elucidate the neural basis of major depressive disorder. Structural and functional neuroimaging studies of patients with depression have revealed a complex neuropathophysiology involving regional deficits in the limbic-thalamo-prefrontal and limbic-striatal-pallidal-thalamic systems (1–9). About 30% of patients do not respond to standard antidepressant treatment and are classified as having refractory depression, while those who respond have nonrefractory depression (10). Little is known about how these two clinical subtypes differ at the neuronal level. We investigated the functional deficits in these two subtypes in the hope that noninvasive measurements might eventually make it possible to distinguish them at an early stage of clinical intervention. We recently (1) identified regional cerebral perfusion differences between these groups: the refractory group showed reduced perfusion in prefrontal and thalamic areas, while the nonrefractory group showed reduced perfusion in left frontal areas and increased perfusion in limbic-striatal areas. The effects of these regional alterations in resting perfusion on systems-level disturbances in distributed brain networks are of course impossible to predict. There is increasing evidence that neural networks are disrupted in depression (11–15) as well as in other neuropsychiatric conditions, such as Alzheimer's disease (16), schizophrenia (17), and acute psychological trauma (18). However, no study has yet determined whether patients with refractory and nonrefractory depression can be distinguished by differential functional integration within specific neural networks.
Resting-state functional connectivity MRI (fcMRI) (19) has been increasingly used to investigate the integration of neural networks at a resting state when no task is performed (20). Low-frequency (0.01–0.08 Hz) fluctuations of the blood-oxygen-level-dependent (BOLD) signal in the resting state are considered to be physiologically meaningful and related to spontaneous neural activity (21). While task-based functional MRI (fMRI) studies can assess disturbances in functional connectivity when patients perform a particular task, assessment of resting-state connectivity has different and potentially broader significance, because it requires minimal patient compliance, can be obtained under anesthesia, and is well suited for translation into the clinical realm (19). This technique has been successfully used to detect abnormal functional integration in major depressive disorder (22).
As different regional alterations have been observed in patients with refractory and nonrefractory depression (1), we hypothesized that different systems-level disturbances would be observed in distributed brain networks. Our purpose, therefore, was to use resting-state fcMRI to quantify functional connectivity in 28 patients with refractory depression, 32 patients with nonrefractory depression, and 48 healthy comparison subjects.
Method
Participants
This study was approved by the local ethical committee, and written informed consent was obtained from all participants. The patients in the present analysis were part of a large cohort study of major depression in the Chinese population of Han nationality. Patients were recruited consecutively, and the diagnosis of major depressive disorder was made with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (23). Exclusion criteria were bipolar disorder, any history of major illness, previous psychiatric therapy, cardiovascular disease, age less than 18 or over 60 years, use of vasoactive medications, and alcohol or drug abuse. Originally 82 right-handed patients were recruited, of whom 22 were excluded by the above criteria. Finally, 60 patients were included in the study, none of whom had received antidepressant treatment before enrollment. Severity of depression was quantified using the 17-item Hamilton Depression Rating Scale (HAM-D) (24) and the Clinical Global Impressions (CGI) severity item (25). To be included in the study, patients had to have a HAM-D total score ≥18 and a CGI severity score ≥4 on the day of MR scanning.
After MR imaging, antidepressant treatment was started for all patients. Three classes of antidepressants were used: tricyclics, typical serotonin-norepinephrine reuptake inhibitors, and typical selective serotonin reuptake inhibitors. All antidepressants were empirically prescribed according to the clinical judgment of the treating psychiatrist. No patient was treated with ECT or had received ECT in the past. Refractory depression is defined as a poor response after at least two trials with antidepressants from different classes, with adequate dosages, duration (6 weeks for each trial), and compliance (26, 27). A poor response is defined as a reduction of <50% in HAM-D score with a minimum dosage of 150 mg/day of imipramine or the equivalent for 6 weeks. This outcome measure was chosen because it allows simple analyses that aid interpretation, particularly from a clinical perspective. Nonrefractory patients are those who had a reduction >50% in HAM-D score after treatment.
In addition, 48 right-handed healthy comparison subjects were recruited from the local area by poster advertisements. Comparison subjects were screened using the non-patient edition of the SCID to confirm the lifetime absence of a history of psychiatric or neurological illness and were interviewed to exclude any family history of psychiatric illness.
All participants were found by two experienced radiologists to have no abnormalities on conventional MRI.
MRI Scanning
Patients and comparison subjects underwent scanning using a GE Signa EXCITE 3-T MR system (GE Healthcare, Milwaukee) with an 8-channel phased array head coil. During scanning, participants were instructed to relax with their eyes closed without falling asleep; after the experiment, each participant confirmed not having fallen asleep during scanning. Participants were fitted with soft earplugs and positioned carefully in the coil with comfortable support. MR images sensitive to changes in BOLD signal (repetition time=2,000, echo time=30 msec, flip angle=90 degrees) were obtained with a gradient-echo echo-planar imaging (EPI) sequence. Five dummy scans were discarded to remove the impact of magnetization stabilization. The slice thickness was 5 mm (no slice gap) with a matrix size of 64×64 and a field of view of 240×240 mm2, resulting in a voxel size of 3.75×3.75×5 mm3. Each brain volume comprised 30 axial slices, and each functional run contained 200 image volumes.
Data Processing and Analysis
Preprocessing and statistical analysis of functional images were carried out using the SPM2 software package (www.fil.ion.ucl.ac.uk/spm/). For each participant, EPI images were slice-time corrected, realigned to the first image in the first series, and unwarped to correct for artifacts due to susceptibility-by-movement interaction. The resulting images were spatially normalized to the Montreal Neurological Institute (MNI) EPI template in SPM2, and each voxel was resampled to 3×3×3 mm3. The processed images were smoothed with an isotropic Gaussian kernel (full-width at half-maximum=8 mm). Functional connectivity was examined using the Resting-State fMRI Data Analysis Toolkit (REST) software package (http://resting-fmri.sourceforge.net) using a seed voxel correlation approach (28, 29). As structural and functional studies in patients with depression have revealed regional deficits in the limbic-thalamo-prefrontal and limbic-striatal-pallidal-thalamic systems (1–9, 11–15), we selected as seeds 13 areas that constitute these: the left and right hippocampus, insula, dorsal lateral prefrontal areas, amygdala, putamen, and thalamus and the anterior cingulate cortex. Using REST, after bandpass filtering (0.01–0.08 Hz) (21) and linear trend removal, a reference time series for each seed was extracted by averaging the fcMRI time series of voxels within each region of interest as defined in the WFU (Wake Forest University) PickAtlas (30, 31). The Ideal Filter was used for bandpass filtering by transforming the time series into the frequency domain by discrete Fourier transform, assigning a value of zero to the excluded frequency, and then transforming back to the time domain by inverse discrete Fourier transform. Thirteen correlation analyses were performed voxel-wise between each seed reference and the rest of the brain. Finally, the correlation coefficients in each voxel were transformed to z-value images using the Fisher r-to-z transformation to improve normality before averaging across subjects. Using SPM2, the impact of potential physiological artifacts such as cardiac or respiratory noise (32, 33) was minimized by regressing out components with high correlations with CSF or white matter or low correlations with gray matter.
For the patient data, individual z-value maps were analyzed with a random-effects one-sample t test to identify voxels showing a significant positive or negative correlation with the seed time series, the correlations being thresholded using a p<0.05 family-wise error correction for multiple comparisons. Voxel-based comparison of z-value maps among the three groups was performed using a design model of one-way analysis of variance with age and disease duration as covariates followed by post hoc two-sample t tests. The statistical significance of each region was estimated by distributional approximations from the theory of random Gaussian fields (34). In this method, clusters in smooth areas are shrunk while those in rough areas are expanded to account for differences in smoothness (34). Significance thresholds were set at 0.05 after family-wise error correction with an extent of more than five contiguous voxels. MNI coordinates were transformed to Talairach coordinates using mni2tal (http://imaging.mrc-cbu.cam.acuk/download/MNI2tal).
Results
Age, sex, and handedness were not significantly different between the patient groups and the comparison group. Depression severity (HAM-D score) was not significantly different between the refractory and nonrefractory groups, although the refractory group had the longer illness duration (Table 1) (p<0.05). Differences in HAM-D scores between male and female patients did not reach significance in either the refractory group (male: mean=23 [SD=4]; female: mean=22 [SD=3]) or the nonrefractory group (male: mean=24 [SD=3]; female: mean=24 [SD=4]), and depression severity was not correlated with age.
| Characteristic | Patients With Nonrefractory Depression (N=32) | Patients With Refractory Depression (N=28) | Healthy Comparison Subjects (N=48) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Female | 11 | 34.4 | 10 | 35.7 | 17 | 35.4 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 32 | 10 | 33 | 11 | 35 | 12 |
| Illness duration (months) | 22 | 18 | 193 | 120 | ||
| Hamilton Depression Rating Scale | ||||||
| Score before treatment | 23.0 | 4.7 | 23.3 | 4.1 | ||
| Score after treatment | 11.2 | 2.6 | 19.6 | 3.1 | ||
TABLE 1. Demographic and Clinical Characteristics of Patients With Nonrefractory and Refractory Depression and Healthy Comparison Subjects
Voxel-Based Analysis Results
Relative to the comparison group, both the nonrefractory and refractory groups showed significantly reduced connectivity within prefrontal-limbic-thalamic areas bilaterally (Table 2). The nonrefractory group showed the more distributed decrease in connectivity, especially in the anterior cingulate cortex and the left and right prefrontal cortex, hippocampus, insula, and amygdala (Table 2, Figure 1A), while in the refractory group decreased connectivity was mainly in prefrontal areas and the thalamus areas bilaterally (Table 2, Figure 1B). Direct comparison between the groups showed decreased connectivity in the nonrefractory compared with the refractory group within the left amygdala-anterior cingulate cortex-right insula-precuneus region (Table 2, Figure 1C). These findings were not correlated with illness duration or age.
| Talairach Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Seed Area | Connected Location | Brodmann's Area | x | y | z | Voxel Size | pa |
| Comparison group > nonrefractory group | |||||||
| Anterior cingulate cortex | Left middle temporal gyrus | 7 | –42 | –63 | 6 | 43 | <0.001 |
| Left parietal cortex | 5 | –21 | –48 | 48 | 18 | 0.015 | |
| Right inferior frontal gyrus | 47 | 18 | 15 | –24 | 37 | <0.001 | |
| Left amygdala | Left cingulate cortex | 24 | 0 | 3 | 27 | 29 | 0.007 |
| Left frontal | Right insula | 13 | 42 | 15 | 18 | 25 | 0.008 |
| Right cingulate cortex | 32 | 9 | 30 | 27 | 39 | <0.001 | |
| Left cingulate cortex | 24 | –3 | 0 | 24 | 32 | 0.006 | |
| Left hippocampus | Cingulate cortex | 33 | 0 | 9 | 24 | 10 | 0.035 |
| Left putamen | –16 | 9 | 12 | 20 | 0.013 | ||
| Left parietal cortex | 40 | –24 | –45 | 54 | 46 | <0.001 | |
| Left insula | Precuneus | 7 | –18 | –48 | 48 | 33 | 0.005 |
| Right parietal cortex | 5 | 21 | –39 | 51 | 6 | 0.044 | |
| Left middle temporal gyrus | 38 | –36 | 0 | –15 | 35 | 0.002 | |
| Right occipital cortex | 18 | 51 | –21 | 6 | 9 | 0.039 | |
| Right cingulate cortex | 31 | 6 | –51 | 45 | 37 | <0.001 | |
| Left thalamus | Right inferior frontal gyrus | 45 | 30 | 27 | 9 | 36 | 0.001 |
| Right amygdala | Left cingulate cortex | 24 | –3 | –9 | 27 | 40 | <0.001 |
| Right insula | Right hippocampus | 30 | 0 | –24 | 35 | 0.001 | |
| Left insula | –39 | –21 | 24 | 42 | <0.001 | ||
| Right occipital cortex | 24 | –87 | 15 | 30 | 0.004 | ||
| Precuneus | 7 | –18 | –48 | 48 | 42 | <0.001 | |
| Right middle temporal gyrus | 39 | 30 | –57 | 30 | 21 | 0.011 | |
| Right putamen | Precuneus | 7 | –18 | –48 | 48 | 44 | <0.001 |
| Right thalamus | Cingulate cortex | 33 | 0 | 9 | 24 | 31 | 0.006 |
| Right hippocampus | Right inferior frontal gyrus | 45 | 45 | 18 | 12 | 17 | 0.016 |
| Right insula | 13 | 42 | 0 | 18 | 7 | 0.043 | |
| Left cingulate cortex | 23 | –3 | –15 | 30 | 20 | 0.013 | |
| Comparison group > refractory group | |||||||
| Left frontal | Precuneus | 7 | –27 | –51 | 51 | 19 | 0.01 |
| Right parietal cortex | 40 | 39 | –39 | 51 | 38 | <0.001 | |
| Left thalamus | Right insula | 13 | 30 | 24 | 9 | 5 | 0.051 |
| Right putamen | 17 | 7 | 11 | 15 | 0.022 | ||
| Right cingulate cortex | 32 | 3 | 30 | 51 | 18 | 0.014 | |
| Left middle frontal gyrus | 9 | –21 | 29 | 33 | 20 | 0.011 | |
| Left hippocampus | Left middle temporal gyrus | 37 | –54 | –57 | –27 | 19 | 0.013 |
| Right insula | Precuneus | 7 | 21 | –63 | 33 | 11 | 0.033 |
| Cingulate cortex | 31 | 15 | –45 | 24 | 26 | 0.007 | |
| Right putamen | Left middle frontal gyrus | 8 | –33 | 18 | 42 | 28 | 0.003 |
| Right inferior frontal gyrus | 9 | 36 | 9 | 24 | 13 | 0.034 | |
| Right thalamus | Right inferior frontal gyrus | 9 | 36 | 9 | 27 | 10 | 0.045 |
| Left middle frontal gyrus | 9 | –6 | 30 | 36 | 20 | 0.008 | |
| Left putamen | –18 | 12 | 9 | 19 | 0.009 | ||
| Right insula | 13 | 30 | 21 | 6 | 14 | 0.025 | |
| Refractory group > nonrefractory group | |||||||
| Left amygdala | Cingulate cortex | 24 | 0 | 18 | 27 | 37 | <0.001 |
| Right insula | Cingulate cortex | 31 | 15 | –45 | 24 | 26 | 0.007 |
| Precuneus | 7 | 21 | –63 | 33 | 12 | 0.033 | |
TABLE 2. Difference of Functional Connectivity Among Patients With Nonrefractory Depression, Patients With Refractory Depression, and Healthy Comparison Subjects
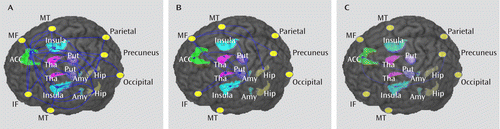
FIGURE 1. Difference of Functional Connectivity Map for Patients With Nonrefractory and Refractory Depression and Healthy Comparison Subjectsa
a All p values are <0.05, corrected. The blue lines show decreased functional connectivity. In panel A, patients with nonrefractory depression showed decreased connectivity relative to healthy comparison subjects mainly in limbic-striatal-pallidal-thalamic circuits, including the anterior cingulate cortex and the left and right prefrontal cortex, hippocampus, insula, and amygdala. In panel B, patients with refractory depression showed decreased connectivity relative to healthy comparison subjects mainly in thalamo-frontal circuits, including prefrontal and thalamus areas bilaterally. In panel C, direct comparison between the refractory and nonrefractory groups showed decreased connectivity in the left amygdala-anterior cingulate cortex-right insula-precuneus in the nonrefractory group. ACC=anterior cingulate cortex; Put=putamen; Tha=thalamus; Hip=hippocampus; Amy=amygdala; IF=inferior frontal gyrus; MF=middle frontal gyrus; MT=middle temporal gyrus.
Discussion
Using resting-state fMRI in a cohort of patients with well-characterized depression, studied before commencement of medication, we found altered functional connectivity mainly involving the frontal-subcortical circuits, which are strongly implicated in depression (35). Furthermore, we observed differences in functional connectivity related to treatment responsiveness, with the nonrefractory group showing a decrease mainly in the limbic-striatal-pallidal-thalamic circuits (Figure 1A), while the refractory group showed a decrease mainly in thalamo-cortical circuits (Figure 1B).
Convergent evidence from functional brain imaging, therapeutics, and lesion studies suggests that depression is associated with dysfunction in several functionally integrated pathways (36, 37). More specifically, a loss of top-down regulation, especially the loss of prefrontal cortex control over limbic regions, is thought to be at the root of the pathogenesis of emotional, behavioral, cognitive, and endocrine changes in depression (38, 39). Consistent with this hypothesis, reduced fronto-limbic connectivity has been reported in both task (40, 41) and resting-state (42) fMRI studies in patients with depression, although results have been inconsistent, with reports of both increased and decreased connectivity. Our results confirmed the decrease in connectivity involving the prefrontal cortex in a cohort of 60 patients with depression. Furthermore, this decreased connectivity was more widespread in the group with nonrefractory depression than in the group with refractory depression.
The limbic system has widespread connections to the prefrontal cortex, amygdala, and thalamus (43), and it plays a critical role in anxiety and depressive states (44) in addition to its contribution to learning and memory. In the patients with nonrefractory depression, connectivity was decreased among distributed limbic areas, particularly in the anterior cingulate cortex and in the prefrontal and insula regions bilaterally (Figure 1A). The same network of regions was identified in a recent meta-analysis of cortical-subcortical interactions in emotion processing (45). Thus, it may be that decreased connectivity in this network underlies emotional dysregulation in these patients. The insula is thought to mediate interpretation of sensory information from the body (interoception) that contributes to emotional states (46). Decreased connectivity in this circuit might therefore underlie such depressive symptoms as somatic complaints and negative bias in interpreting bodily feedback.
This decreased functional connectivity between prefrontal and limbic networks in the group with nonrefractory depression may also account for the inverse relationship of activation between prefrontal lobe and limbic regions reported in previous studies (1, 40). Prefrontal cortical-limbic connectivity serves as an inhibitory link between those regions and is reduced in depression (47). The consequent disinhibition might account for the overactivity of the limbic system in the group with nonrefractory depression. This in turn might stimulate the hypothalamic-pituitary-adrenal axis (48, 49), and consequent glucocorticoid oversecretion could contribute to loss of frontal lobe integrity (50). Such decreased connectivity has been reported to improve after 6 weeks of treatment with sertraline in responders (51) and may have a genetic basis, for example, in the 5-HTTLPR allele (52).
The finding of decreased functional connectivity in the group with nonrefractory depression relative to the group with refractory depression (Figure 1C) is surprising, as one might have expected more impaired connectivity in the latter. However, this finding is not implausible in light of previous neuroimaging studies suggesting that functional alterations may be specifically present in nonrefractory patients. For example, in an investigation using arterial spin-labeling MRI (1), we found that patients with nonrefractory depression but not those with refractory depression showed altered perfusion in the limbic system relative to healthy comparison subjects. One possibility is that alterations in nonrefractory patients are localized within the limbic system, which is also the target of standard antidepressants (53), whereas alterations in refractory patients are expressed in a thalamo-cortical circuit, which may be less sensitive to antidepressant medication (53). This would explain why pharmacological treatment is effective in only one clinical group even though both groups show altered brain functioning.
Despite this at-first-sight surprising result of direct comparison between the refractory and nonrefractory groups, the comparisons between each patient group and healthy comparison subjects appeared to suggest more disrupted alterations of functional connectivity in the refractory than in the nonrefractory group in prefrontal areas and in the thalamus areas bilaterally (Figure 1B). This is consistent with results of previous studies (54–56) suggesting greater disruption within thalamo-frontal circuits in refractory depression relative to nonrefractory depression. For example, more severe frontal deficits are reported in patients with late-onset depression associated with frontal vascular disease (57), who have higher rehospitalization rates and treatment resistance (58). Also, therapeutic intervention targeting frontal areas has been reported to be useful in refractory patients (59, 60) and to be correlated with clinical improvement (61). Finally, increased thalamic metabolism has been reported in remitted depressed patients after tryptophan depletion but not after sham depletion (62). Abnormal functional connectivity between thalamus and medial prefrontal regions has also been found to be associated with refractoriness (22). These findings, together with the results of our investigation, suggest that refractory depression may be mainly associated with disrupted connectivity in thalamo-cortical circuits. This may partly explain why patients with refractory depression are refractory to standard antidepressants but respond well to treatments targeting frontal areas (59–61).
Several study limitations should be considered when interpreting these results. First, the data are cross-sectional; whether these altered neural networks change dynamically after therapy remains to be established in longitudinal studies. Second, patients were treated with a drug belonging to one of three different classes with heterogeneous pharmacological profiles. This heterogeneity limits the translational value of our results since the same patient may show a poor response to one drug class and a good response to another. Future studies aimed at informing clinical intervention will benefit from the investigation of a single drug, or at least drugs with the same pharmacological profile. Finally, the refractory group had a greater illness duration than the nonrefractory group. Although we used illness duration as a covariate in the statistical analysis, we cannot exclude the possibility that our results were influenced by this variable. Again, a longitudinal approach would allow examination of whether and how these altered neural networks change with the development of the illness.
1. : Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology 2009; 251:476–484Crossref, Medline, Google Scholar
2. : The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607–615Crossref, Medline, Google Scholar
3. : Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry 2001; 158:1321–1323Link, Google Scholar
4. : Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry 2001; 50:159–170Crossref, Medline, Google Scholar
5. : Cerebral perfusion correlates of depressed mood. Br J Psychiatry 1997; 170:77–81Crossref, Medline, Google Scholar
6. : Volume deficits of subcortical nuclei in mood disorders: a postmortem study. Eur Arch Psychiatry Clin Neurosci 2005; 255:401–412Crossref, Medline, Google Scholar
7. : Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004; 161:598–607Link, Google Scholar
8. : Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry 2005; 162:1706–1712Link, Google Scholar
9. : Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res 2008; 164:114–122Crossref, Medline, Google Scholar
10. : Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression: systematic review. Br J Psychiatry 2002; 181:284–294Crossref, Medline, Google Scholar
11. : 5-HTT genotype effect on prefrontal-amygdala coupling differs between major depression and controls. Psychopharmacology (Berl) 2009; 205:261–271Crossref, Medline, Google Scholar
12. : Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66:407–414Crossref, Medline, Google Scholar
13. : Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry 2008; 65:179–188Crossref, Medline, Google Scholar
14. : Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord 2010; 122:76–85Crossref, Medline, Google Scholar
15. : Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 2009; 63:754–761Crossref, Medline, Google Scholar
16. : Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004; 101:4637–4642Crossref, Medline, Google Scholar
17. : Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 2009; 166:196–205Link, Google Scholar
18. : High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci USA 2009; 106:15412–15417Crossref, Medline, Google Scholar
19. : Clinical applications of resting state functional connectivity. Front Syst Neurosci 2010; 4:19Google Scholar
20. : Brain work and brain imaging. Annu Rev Neurosci 2006; 29:449–476Crossref, Medline, Google Scholar
21. : Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 2001; 22:1326–1333Medline, Google Scholar
22. : Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62:429–437Crossref, Medline, Google Scholar
23. : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press, 1997Google Scholar
24. : A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988; 45:742–747Crossref, Medline, Google Scholar
25. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
26.
27. : Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 2007; 52:46–54Crossref, Medline, Google Scholar
28. : Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA 1998; 95:8939–8944Crossref, Medline, Google Scholar
29. : Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 1994; 2:56–57Crossref, Google Scholar
30. : Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000; 10:120–131Crossref, Medline, Google Scholar
31. : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
32. : Localization of cardiac-induced signal change in fMRI. Neuroimage 1999; 9:407–415Crossref, Medline, Google Scholar
33. : On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magn Reson Imaging 2002; 20:575–582Crossref, Medline, Google Scholar
34. : An improved theoretical p value for SPMs based on discrete local maxima. Neuroimage 2005; 28:1056–1062Crossref, Medline, Google Scholar
35. : Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull 1992; 28:261–274Medline, Google Scholar
36. : Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 2005; 57:1079–1088Crossref, Medline, Google Scholar
37. : Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65:193–207Crossref, Medline, Google Scholar
38. : Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience 2009; 164:300–330Crossref, Medline, Google Scholar
39. : Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009; 33:699–771Crossref, Medline, Google Scholar
40. : Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 2002; 51:693–707Crossref, Medline, Google Scholar
41. : Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol 2008; 11:255–260Crossref, Medline, Google Scholar
42. : Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 2009; 171:189–198Crossref, Medline, Google Scholar
43. : Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002; 159:1112–1118Link, Google Scholar
44. : The relationship of regional cerebral blood flow with subtypes of major depression. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:537–546Crossref, Medline, Google Scholar
45. : Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 2008; 42:998–1031Crossref, Medline, Google Scholar
46. : How do you feel? interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Medline, Google Scholar
47. : Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol 2009; 12:11–22Crossref, Medline, Google Scholar
48. : Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 1999; 408:365–377Crossref, Medline, Google Scholar
49. : Intracerebroventricular corticotropin-releasing factor increases limbic glucose metabolism and has social context-dependent behavioral effects in nonhuman primates. Proc Natl Acad Sci USA 2002; 99:15749–15754Crossref, Medline, Google Scholar
50. : Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. J Clin Endocrinol Metab 2005; 90:3262–3267Crossref, Medline, Google Scholar
51. : Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology 2005; 30:1334–1344Crossref, Medline, Google Scholar
52. : 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005; 8:828–834Crossref, Medline, Google Scholar
53. : Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 1998; 43:547–573Crossref, Medline, Google Scholar
54. : Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res 2006; 146:43–51Crossref, Medline, Google Scholar
55. : Improved anatomic delineation of the antidepressant response to partial sleep deprivation in medial frontal cortex using perfusion-weighted functional MRI. Psychiatry Res 2006; 146:213–222Crossref, Medline, Google Scholar
56. : 99mTc-HMPAO SPECT study of cerebral perfusion after treatment with medication and electroconvulsive therapy in major depression. J Nucl Med 2007; 48:1273–1278Crossref, Medline, Google Scholar
57. : Hypofrontality and microvascular dysregulation in remitted late-onset depression assessed by functional near-infrared spectroscopy. Neuroimage 2005; 26:234–242Crossref, Medline, Google Scholar
58. : Response of patients with major depression and silent cerebral infarction to antidepressant drug therapy, with emphasis on central nervous system adverse reactions. Stroke 1996; 27:2040–2042Crossref, Medline, Google Scholar
59. : Effects of antidepressant treatment with rTMS and fluoxetine on brain perfusion in PD. Neurology 2006; 66:1629–1637Crossref, Medline, Google Scholar
60. : Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann Clin Psychiatry 2005; 17:153–159Crossref, Medline, Google Scholar
61. : Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg 2003; 99:1010–1017Crossref, Medline, Google Scholar
62. : Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 2004; 61:765–773Crossref, Medline, Google Scholar



