Daily Left Prefrontal Repetitive Transcranial Magnetic Stimulation for Acute Treatment of Medication-Resistant Depression
Daily left prefrontal transcranial magnetic stimulation (TMS) over several weeks was first proposed as a treatment for depression in 1993, with double-blind study beginning in 1997. TMS for the treatment of depression was approved by the U.S. Food and Drug Administration (FDA) in October 2008 (1). More recently, a large trial sponsored by the National Institutes of Health using an innovative sham treatment technique found that a course of active treatment for 3–5 weeks was superior to sham treatment (remission rates were 15% in the active treatment group and 5% in the sham treatment group) and achieved a 30% remission rate in the open-label extension. These findings led to the implementation of the first new Current Procedural Terminology codes for psychiatry since the American Medical Association launched the system in 1966.
In the vignette we describe the case of a 55-year-old woman with treatment-resistant recurrent unipolar depression who was successfully treated with repetitive TMS (rTMS) in each of two episodes 3 years apart. Numerous questions remain on the use of TMS for depression, several of which are raised by the case description. They include how to most effectively deliver rTMS—for example, the appropriate scalp location, the optimal “dose” (frequency, train, number of stimuli per day, and pattern of delivery), its use in combination with medications or talking/exposure therapy, and whether one can use maintenance rTMS to prevent relapse after a patient achieves remission. Daily left prefrontal rTMS reflects a paradigm shift in psychiatry in that it uses noninvasive and nonconvulsive circuit-based physiological processes to treat depression in patients who have not responded to medications or who cannot tolerate them.
A 55-year-old woman with a long history of recurrent treatment-resistant depression participates in a randomized controlled trial of daily left prefrontal transcranial magnetic stimulation and continues in the study's open-label extension.
“Mrs. M” is a 55-year-old actress with recurrent unipolar depression. Although she was dysthymic in high school, her first suicide attempt was at age 23 during an episode of depression and bulimia, for which she received counseling and medication. She partially responded but then showed a repeated pattern of partial to complete response to antidepressant medications followed by a gradual loss of efficacy. After a second suicide attempt at age 35, she was hospitalized, and she partially responded to treatment with an antidepressant and psychotherapy. She made a third suicide attempt at age 48; she was hospitalized again, and she partially responded to venlafaxine at 300 mg/day and psychotherapy.
At age 50 Mrs. M relapsed again, and over the next few years she tried the following medications, either alone or in combination: venlafaxine, lamotrigine, olanzapine, trazodone, bupropion, ziprasidone, aripiprazole, oxcarbazepine, lithium, desipramine, imipramine, fluoxetine, sertraline, citalopram, and buspirone. She was offered but refused ECT, citing concerns that the potential cognitive side effects of the treatment could affect her ability to remember her lines as an actress.
In 2007, Mrs. M enrolled in a multisite randomized trial of repetitive transcranial magnetic stimulation (rTMS) (2). After being tapered off antidepressant medications, she had an entry score of 31 on the 24-item Hamilton Depression Rating Scale. At randomization she was assigned to receive sham rTMS and was treated daily over the left prefrontal cortex for 3 weeks, with no improvement in her depressive symptoms. She then exited the double-blind phase and was offered open-label treatment with the same TMS settings as were used in the active phase (120% MT, 10 Hz, 4 seconds on and 26 seconds off over 26.7 minutes, 3,000 stimuli per day). Her symptoms improved, and she remitted after 4 weeks (Figure 1). She was then tapered from the TMS (three treatments per week for 2 weeks, then two treatments per week for 2 weeks) and restarted on venlafaxine at 300 mg/day. Although the study protocol suggested that patients be started on venlafaxine with adjunctive lithium or lamotrigine after remitting on TMS, Mrs. M declined use of adjunctive medications.
Mrs. M remained in remission from depression on venlafaxine for 3 years. She resumed her acting career and remarried. At age 58, in January 2010, without changing or stopping medications, she gradually noted the return of her core depressive symptoms and recontacted our group to request another course of rTMS. Her score on the Inventory of Depressive Symptoms (IDS) was high (69 out of a possible 84 points). She then received 45 treatments of daily left prefrontal TMS for the second time, but this time while continuing to take venlafaxine. After 6 weeks of daily TMS, her IDS score was 24 (65% improvement). While being tapered from the TMS over the next 2 weeks, she was started on duloxetine at 30 mg/day, and the venlafaxine was tapered. She was then referred back to her treating psychiatrist with an exit IDS score of 7.
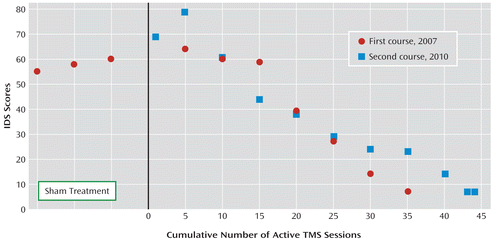
FIGURE 1. Depression Scores and Course of Improvement for a Patient Participating in a Trial of Repetitive Transcranial Magnetic Stimulation (rTMS)a
a Scores on the Inventory of Depressive Symptoms (IDS) are graphed for the first and second courses of TMS. In the first trial, in 2007, the patient initially received sham TMS, the results of which are shown to the left of the y-axis. Over 6–7 weeks, the patient responded to TMS and even remitted. The two courses were 3 years apart and had other differences, but the clinical response was similar.
Physiological Effects and Evidence Base
The patient described in the vignette is similar to many depressed patients who do not respond to or cannot tolerate antidepressant medications or who respond and then gradually experience a return of symptoms in a tolerance pattern. She failed to respond to sham rTMS during a double-blind study, responded well to open-label rTMS, and maintained her remission for 3 years on medications that had previously been inadequate to treat her acute depression. After a severe relapse, which occurred after FDA approval of TMS, she responded again to rTMS, which could now be offered in a clinical setting as an adjunct to her antidepressant medication. The case illustrates the potential use of rTMS for patients with treatment-resistant depression and the issues associated with this new therapy.
rTMS involves inducing an electrical current within the brain by a pulsating alternating magnetic field generated above the scalp (Figure 2). The essential feature of TMS is the use of electricity to generate a rapidly changing electromagnetic field, which readily crosses the scalp and skull and in turn produces electrical impulses in the brain. A typical rTMS device produces a fairly powerful magnetic field (1.5–3 Tesla), but only very briefly (a fraction of a millisecond for each pulse).
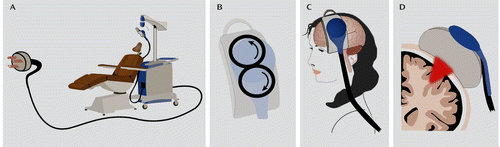
FIGURE 2. Components of a TMS Devicea
a The TMS system is about the size of a small refrigerator (panel A). The device uses a capacitor that stores an electric charge and then discharges it through an electromagnetic coil, typically in the shape of a doughnut or two round coils side by side and connected in a figure eight (panel B), resting on the scalp over the prefrontal cortex (panel C). This powerful but brief pulse induces electrical currents to flow in the cortex (panel D), depolarizing neurons locally and sending signals to distant areas, including deeper limbic regions. TMS does not involve a seizure, and patients are awake and alert during their daily sessions, which typically last an hour. Figure modified from Higgins and George (3).
TMS requires a capacitor to store and deliver a charge and an electromagnetic coil (typically in the shape of a doughnut or two round coils side by side and connected in a figure eight) to induce an electrical field in the brain. The system is about the size of a small refrigerator, weighs less than 20 lbs, and can be made portable (4, 5).
Early TMS devices emitted only a single brief pulse. Modern devices can generate a rapid succession of pulses, called repetitive TMS. The typical treatment for depression is a 20- to 40-minute session delivering 3,000 to 6,000 pulses, 5 days a week for 4 to 8 weeks. In order to keep the patient still and the device correctly placed, the patient reclines in a chair and the device is held securely against the head over the left prefrontal cortex. The patient described in the vignette received 3,000 pulses per session in her first treatment course (a total of 105,000 pulses) and 5,000 per session in the second course 3 years later (a total of 220,000 pulses).
Conventional TMS coils generate a magnetic field impulse that can only reach the portion of the cerebral cortex that lies on the brain surface (6), just 2–3 cm below the device (7, 8). A TMS device that penetrates more deeply is in early clinical trials for depression and several other indications (9–11). It has been speculated that complex assemblies of coils could be built to summate and stimulate deep within the brain while sparing the superficial cortex (12).
When the TMS device produces a pulse over the motor cortex, descending fibers are activated and volleys of electrical impulses descend through connected fibers into the spinal cord and out to a peripheral nerve, causing a muscle to twitch. The minimum amount of energy needed to produce contraction of the thumb is called the “motor threshold” (MT) (13–16). Because it is easy to generate and varies widely across individuals, the MT is used as a measure of general cortical excitability. Most TMS studies, both research and clinical, report the TMS intensity or dose as a function of individual MT, not as an absolute physical value (17). In general, a stronger, more intense TMS pulse—for example, 110%–120% of MT—results in greater activation of the CNS tissue as well as a wider and deeper area of activation (18–22).
The manipulation of the frequency of stimulation is more complex. In general, frequencies of less than 1 per second (<1 Hz) are inhibitory (23). This may be because low-frequency TMS more selectively stimulates inhibitory γ-aminobutyric acid neurons. This frequency resembles the frequencies used in animal and cell studies that produce long-term depression. One particular TMS sequence builds directly on the neurobiological studies of long-term depression and uses short bursts of TMS at theta frequencies (24, 25). Conversely, higher-frequency stimulation is excitatory (26). Interestingly, high-frequency TMS over some brain regions can, in some instances, temporarily block the function of that part of the brain (27, 28).
Putative Mechanisms of Action
TMS can produce different brain effects depending on the brain region being stimulated, the use parameters (intensity, frequency, duty train), and whether the brain region is engaged or “resting.” Thus, TMS may have several different mechanisms by which it improves mood. In general, however, a single pulse of TMS at an intensity at or above MT over a cortical region like the motor cortex causes large neurons to depolarize. That is, the powerful transient magnetic field induces current to flow in neurons in the superficial cortex. Both modeling and simple testing have shown that the fibers that are most likely to depolarize are those that are perpendicular to the coil and bend within the gyrus (16, 29–32). Some lower TMS intensities do not cause large neuron depolarization but can still affect resting membrane potentials and thus alter brain activity and behavior.
The most striking positive phenomena that TMS can produce are motor twitches (thumb, hand, arm, or leg movement) when applied over motor cortex regions and “phosphenes” when applied over the occipital cortex. TMS does not produce acute memories, thoughts, or sensations or percepts such as those typically induced by intracerebral stimulation.
rTMS over some cortical regions can produce a disruption of behavior. This is most striking when the coil is placed over Broca's area, where one can produce a transient expressive aphasia or speech arrest. Much interest is focused on whether TMS can produce short-term or even longer-term changes in plasticity (26, 33). Simple studies in motor and visual systems clearly indicate the potential for this approach (34), which is now being applied in studies of poststroke recovery and other forms of rehabilitation (35, 36).
Coupling TMS with electrophysiological measures allows one to use TMS as a measure of motor cortex excitability and then measure how behavior, medications, or other interventions change that excitability. This technique is being used to investigate new CNS-active compounds (26, 37–39).
Brain imaging techniques (positron emission tomography [PET], single photon emission computed tomography, functional MRI [fMRI], and blood-oxygen-level-dependent fMRI) allow one to directly access the changes generated by rTMS (40, 41). With respect to the neuropsychiatric uses of TMS for depression or pain, TMS is known to have many molecular effects similar to those seen with ECT, such as increased monoamine turnover, increased brain-derived neurotrophic factor, and normalization of the hypothalamic-pituitary-adrenal (HPA) axis.
The initial use of daily prefrontal TMS to treat depression was based on the theory that clinical depression involves an imbalanced relationship between prefrontal (cortical) and limbic regions (insula, cingulate gyrus, amygdala, and hippocampus) involved in mood regulation and that in many patients the prefrontal cortex was hypometabolic (42). The basic hypothesis in 1993 was that repeated subconvulsive stimulation of the prefrontal cortex would activate circuits involving regulatory pathways interacting with the limbic system (42, 43). Such circuits had been described in motor, sensory, and prefrontal systems (44).
Early work showed that single sessions of prefrontal rTMS in healthy adults had no side effects but produced evidence of HPA interaction (serum thyroid levels) and slight mood changes (45), clearing the way for case series in treating depression (46), followed by a double-blind trial (47).
There is now accumulating support, primarily from brain imaging studies (18, 38, 48), that prefrontal rTMS in depressed patients is changing cortical and limbic activity and regulatory circuits. No one has yet linked these changes directly to the antidepressant effects of the treatment, although an important recent study using a serotonin PET ligand in depressed patients undergoing rTMS (49) found that a prefrontal serotonin deficiency at baseline normalized after several weeks of treatment.
Safety and Side Effects
In general, rTMS appears to be safe and to have no enduring side effects. There have been no reported lasting neurological, cognitive, or cardiovascular sequelae. A recent international conference on TMS safety updated the guidelines for use (50, 51). Inducing a seizure remains the primary safety concern with TMS. There have been fewer than 20 reported cases of seizures induced with TMS, with a sample size of several thousand patients and healthy volunteers exposed to TMS. The risk is probably less than 0.5%.
Published safety tables concerning the proper intensity, frequency, and number of stimuli have helped minimize the numbers of seizures (50). Of the reported seizure cases, the majority were in healthy volunteers who were receiving TMS to the motor cortex—the most epileptogenic region of the cortex—and were receiving trains of stimulation outside of the limits now suggested. All of the seizures were self-limited, without need for medications or other interventions, and occurred during TMS administration when the subjects were sitting down and near an investigator. There are no reports of any recurrences among these individuals. These cases and the few that have occurred in patients suggest that TMS-induced seizures will remain a small but significant adverse event in patients without histories of seizures, even when rTMS is used within the suggested guidelines. For these reasons, TMS needs to be supervised by a physician in a facility capable of quickly responding to a potential seizure (52, 53).
Studies in rabbits as well as some human studies suggest that the loud click accompanying the TMS discharge can cause hearing loss, and therefore study subjects, patients, and operators should wear earplugs (54, 55). One patient reported a temporary hearing loss after rTMS. However, an extensive study of auditory threshold before and after 4 weeks of rTMS in more than 300 patients in the pivotal TMS depression study found no changes.
Headaches are the most common complaint after TMS, although there was no difference in headache frequency between TMS and sham treatment in the recent large trials (1, 2). Repeated analysis of neurocognitive functioning of TMS patients has not revealed any enduring negative effects from the procedure (56, 57). Immediately after an rTMS session, patients are able to drive home or return to work. The rTMS procedure itself can cause some scalp pain, which is usually worse during the first few sessions and then largely disappears, although a few patients drop out of studies because of this discomfort (58, 59).
Clinical Studies in Depression
Largely because of its noninvasiveness, rTMS has been investigated in a plethora of neuropsychiatric conditions. Until recently, there has not been a device industry to promote or perform this work, and thus much of the initial clinical work was conducted at single sites with relatively small sample sizes.
Depression has been the most widely studied condition with rTMS. Three initial studies in Europe in the early 1990s used TMS over the vertex as a potential antidepressant (60–62). In the United States in the mid-1990s, George et al. performed an open study and then a double-blind controlled trial of rTMS for 2 weeks (45–47). This work has now dramatically grown, but without much change in many of the initial treatment parameters (coil location, frequency, dosing). Several meta-analyses have been published, most of them concluding that left prefrontal TMS provided statistical superiority over sham treatment for patients with depression (2, 63–65). The clinical features that appear to be associated with greater response include younger age, lack of major refractoriness to antidepressants, and no psychotic features (57).
There have now been three large multisite trials of TMS for depression. A European trial used rTMS in 127 patients as an adjunctive treatment to recently started medications and failed to find an augmenting effect of TMS over sham treatment (66). A TMS manufacturer in the United States randomly assigned 301 medication-free patients with major depression to receive either active TMS or sham treatment for 4–6 weeks (1). The report was published as a positive trial, but the FDA initially rejected the manufacturer's application for use in treating depression and only agreed to approval after reviewing response data on subgroups (57, 63). Because a large effect was observed in those whose depression was less treatment resistant, the FDA labeling is for the treatment of unipolar major depressive disorder in adult patients who have failed to achieve satisfactory improvement from one prior antidepressant treatment at or above the minimal effective dosage and duration in the current episode.
The National Institute of Mental Health (NIMH) then funded the large multisite depression trial noted above. Using a new sham technique (64, 65), the researchers effectively kept patients, raters, and, to a substantial degree, the rTMS treaters, blind to treatment. A statistically significant difference was observed in remission rates between sham (5%) and real rTMS (15%) administered at 10 Hz over the left prefrontal cortex. In an open-label extension of the trial, 30% of patients remitted, which is comparable to or better than rates seen with medications in medication trials of similar treatment-resistant patients (66). When the nonresponders in that study were switched to treatment with 1-Hz rTMS over the right prefrontal cortex, another substantial group of patients remitted.
These data in part support previous observations that patients who were systematically crossed over from high-frequency (20 Hz) to low-frequency (1 Hz) rTMS responded to one of the frequencies preferentially (67, 68) and that these frequencies produced opposite effects on cerebral blood flow measured 48 hours after the last rTMS treatment. The Department of Veterans Affairs has launched a large cooperative study (No. 556) of daily left prefrontal TMS in 300 depressed veterans. This effectiveness trial allows patients to remain on stable antidepressant medication and does not exclude those with coexisting medical disorders, posttraumatic stress disorder, or past substance abuse.
Since the multisite trials augmented the larger database of more than 30 single-site rTMS depression trials, the discussion about TMS for depression has now shifted from asking whether it works to examining how large an effect it has, how durable the response is, what parameters and methods might increase its effectiveness, and who should deliver it. We discuss a few of these methodological questions here.
Methodological Questions
Where to Place the Coil?
The early NIMH studies used a rough measurement technique known as the “5 cm rule” to place the rTMS coil roughly over the prefrontal cortex (45–47). Because the location of the motor strip varies among individuals, as does skull size, this simple rule results in a large variation of actual location on the scalp across different patients. One study suggested that the 5 cm rule resulted in 30% of patients being treated over the supplementary motor area rather than the prefrontal cortex (69). Two retrospective analyses of clinical trials in which brain imaging was performed to document the coil location have independently confirmed that an anterior and lateral coil position is associated with a better clinical response to active but not sham TMS (70).
An Australian group conducted a randomized controlled trial examining different prefrontal locations and a more anterior and lateral location and indeed produced a superior antidepressant response (71). These findings suggest that the location of the coil matters, even within broad boundaries of a specific lobe. It is not clear whether individualized location will be needed or used or whether general algorithms (such as the newly suggested “7 cm” or “AF3” positioning [72]) will suffice for a probabilistic positioning for most patients.
How Intense to Make the Stimulus?
In several of the early rTMS depression studies, researchers noted that it did not work well for older patients (73). A study integrating TMS and MRI showed that this was likely a consequence of older patients having more prefrontal atrophy and thus needing a higher magnetic field in order to overcome the added distance from the coil (19, 20). An open-label study (74) and a more recent randomized trial (75) in geriatric depression showed robust responses using doses (intensities above MT) that are sufficient to bridge the distance created by the degree of atrophy seen in geriatric depression.
How Many Stimulations?
A meta-analysis (76) and a prospective clinical trial (75) suggest that use of greater numbers of rTMS stimulations is more effective. Largely because of safety concerns, researchers have used relatively low numbers of stimulations, and full safety studies have never been performed to assess the maximum tolerated daily, weekly, or lifetime number of stimulations. With continuing safety data and comfort, researchers have now delivered in 1 week of treatment doses that were formerly given in a full course (1, 47, 77–79).
To our knowledge, the largest number of stimulations given within a week (38,880 stimulations) was reported in healthy adult men participating in a sleep deprivation study (79). There were no side effects or problems, and cognition was extensively measured, with no deleterious outcome. Following this trend in the literature of the safety of higher doses of TMS and the suggestion that higher doses might have greater efficacy, we carried out a study to determine whether daily high-dose left prefrontal rTMS is effective in adult depressed patients who had concomitant medical problems and were taking antidepressant medications. Twenty patients tolerated 6,000 stimuli per day and 30,000 per week at 120% MT without side effects or problems (80).
Epstein and colleagues (78) treated 14 patients with Parkinson's disease who also had comorbid treatment-resistant depression in a 10-day open inpatient study of 10-Hz rTMS at 110% MT. Extensive psychiatric, neuropsychological, and motor testing were conducted from baseline to 6 weeks after treatment. rTMS was well tolerated in this medically fragile group, even with very high doses of 19,000 TMS pulses in a week. Highly significant improvements in depression scores were seen 3 days as well as 3–6 weeks after treatment (78). Thus, one trend in TMS for depression involves using more stimulation or using more compact and dense treatment regimens (administering treatment more frequently than merely on weekdays).
Another area where we have insufficient information involves management of patients who have responded to TMS. The two recent large trials found that at 6 months, only 12%–14% of patients had relapsed (81; M.S. George et al., unpublished 2010 data). These are encouraging data compared with 40% of ECT patients relapsing within the first month after ECT (82) and 71% of level III patients in the STAR*D trial relapsing (83), although such a comparison across different patient populations is tenuous. Most patients with recurrent depression do appear to need either maintenance medication or maintenance TMS. If rTMS is used, how should it be delivered? Several groups have performed maintenance TMS, but there have been no controlled clinical trials, and optimal ways of using TMS to prevent relapse remain to be defined (84, 85).
Who Should Deliver TMS?
Because it is a medical procedure with a risk of seizure, rTMS should be performed only in a medical setting under the guidance and supervision of a licensed physician (50, 51). When it is being used to treat acute depression, that physician should be a psychiatrist. As currently performed, the technique is relatively safe and not very user dependent, meaning that most psychiatrists can learn how to deliver TMS without extensive advanced training. However, as the technique evolves, it may require higher doses for greater efficacy, or psychiatrists may need to individualize coil placement with advanced imaging or other techniques that would take additional skill or training. Thus, it may be that over time TMS and other brain stimulation techniques will be offered and managed by a subset of “interventional” psychiatrists to whom treatment-resistant patients are referred. Alternatively, it may evolve into a mainstream treatment used by many, if not most, psychiatrists.
Summary and Conclusions
After much controversy over the past 15 years, the data now demonstrate that daily left prefrontal rTMS for at least several weeks treats acute depression in a subset of moderately but not extremely treatment-resistant patients with unipolar illness. The effects are about as large as those of medication in this group, but not as large as ECT. The debate and research thus now shift from determining whether rTMS works in the acute setting to trying to improve the technology and maximizing its clinical effectiveness, utility, and durability. Research is also now focusing on whether rTMS can be used as a maintenance treatment and whether it is effective in depression subgroups, such as adolescents, patients with bipolar depression, and depressed patients with anxiety disorders and other comorbidities.
It is not yet clear which subgroups of depressed patients are most likely to benefit from rTMS. The trials to date have largely been performed in mildly to moderately treatment-resistant adult unipolar patients in an acute episode. Thus, for a newly depressed patient, prescribing an antidepressant medication would be more expeditious and less expensive than delivering rTMS as it is currently performed (86–88). The place of rTMS in the treatment algorithm is likely to continue to evolve as new data become available. Currently, one might use rTMS to treat depression in patients who have tried at least one antidepressant medication and did not respond adequately (or were unable to tolerate the treatment) and some form of targeted psychotherapy. In patients who respond to rTMS, one should attempt to maintain the remission with prophylactic oral medications. If the patient relapses or does not tolerate the medication side effects, one can reapply rTMS, as in the patient described in the vignette, and perhaps even attempt maintenance TMS despite the meager supporting evidence.
1. : Efficacy and safety of transcranial magentic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62:1208–1216Crossref, Medline, Google Scholar
2. : Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67:507–516Crossref, Medline, Google Scholar
3. : Brain Stimulation Therapies for Clinicians. American Psychiatric Publishing, Inc, 2009Google Scholar
4. : Consensus: new methodologies for brain stimulation. Brain Stimul 2009; 2:2–13Crossref, Medline, Google Scholar
5. : A six-pound battery-powered portable transcranial magnetic stimulator. Brain Stimul 2008; 1:128–130Crossref, Medline, Google Scholar
6. : Modeling the effects of electrical conductivity of the head on the induced electrical field in the brain during magnetic stimulation. Clin Neurophysiol 2004; 114:2204–2209Crossref, Google Scholar
7. : Magnetic Stimulation: motor evoked potentials (the International Federation of Clinical Neurophysiology). Electroencephalogr Clin Neurophysiol Suppl 1999; 52:97–103Google Scholar
8. : Algorithm for the design of magnetic stimulation coils. Med Biol Eng Comput 1994; 32:214–216Crossref, Medline, Google Scholar
9. : A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiology 2002; 19:361–370Crossref, Medline, Google Scholar
10. : Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol 2005; 116:775–779Crossref, Medline, Google Scholar
11. : Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul 2009; 2:188–200Crossref, Medline, Google Scholar
12. : Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport 1997; 8:2535–2538Crossref, Medline, Google Scholar
13. : A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006; 117:2584–2596Crossref, Medline, Google Scholar
14. : Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Human Brain Mapp 2006; 27:478–487Crossref, Medline, Google Scholar
15. : Corticomotor responses to triple-pulse transcranial magnetic stimulation: effects of interstimulus interval and stimulus intensity. Brain Stimul 2009; 2:36–40Crossref, Medline, Google Scholar
16. : Motor threshold in transcranial magnetic stimulation: the impact of white matter fiber orientation and skull-to-cortex distance. Human Brain Mapp 2009; 30:2044–2055Crossref, Medline, Google Scholar
17. : State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul 2008; 1:345–362Crossref, Medline, Google Scholar
18. : Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry 2004; 55:882–890Crossref, Medline, Google Scholar
19. : Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. J Neuropsychiatry Clin Neurosci 2001; 13:459–470Crossref, Medline, Google Scholar
20. : The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry 2001; 49:454–459Crossref, Medline, Google Scholar
21. : How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000; 12:376–384Crossref, Medline, Google Scholar
22. : Motor cortex brain activity induced by 1-Hz transcranial magnetic stimulation is similar in location and level to that for volitional movement. Invest Radiol 2000; 35:676–683Crossref, Medline, Google Scholar
23. : Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry 2002; 159:1093–1102Link, Google Scholar
24. : Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 2005; 565:945–950Crossref, Medline, Google Scholar
25. : Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol 2009; 101:2872–2877Crossref, Medline, Google Scholar
26. : Consensus: motor cortex plasticity protocols. Brain Stimul 2008; 1:164–182Crossref, Medline, Google Scholar
27. : Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology 1991; 41:697–702Crossref, Medline, Google Scholar
28. : Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology 1996; 47:1590–1593Crossref, Medline, Google Scholar
29. : A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol 1990; 77:390–401Crossref, Medline, Google Scholar
30. : Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol 1992; 85:291–301Crossref, Medline, Google Scholar
31. : rTMS in primates: intracerebral measurement of rTMS and ECS induced voltage in vivo. Electroencephalogr Clin Neurophysiol 1998; 107:79PGoogle Scholar
32. : Intracerebral measurement of rTMS and ECS induced voltage in vivo. Biol Psychiatry 1998; 43 (suppl 1): S100.Crossref, Google Scholar
33. : A pilot feasibility study of daily rTMS to modify corticospinal excitability during lower limb immobilization. Ther Clin Risk Manag 2008; 4:1127–1134Crossref, Medline, Google Scholar
34. : Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul 2008; 1:326–336Crossref, Medline, Google Scholar
35. : Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul 2008; 1:370–382Crossref, Medline, Google Scholar
36. : Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul 2009; 2:22–35Crossref, Medline, Google Scholar
37. : Lamotrigine and valproic acid have different effects on motorcortical neuronal excitability. J Neural Transm 2009; 116:423–429Crossref, Medline, Google Scholar
38. : Interleaved transcranial magnetic stimulation/functional MRI confirms that lamotrigine inhibits cortical excitability in healthy young men. Neuropsychopharmacology 2004; 29:1395–1407Crossref, Medline, Google Scholar
39. : State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul 2008; 1:151–163Crossref, Medline, Google Scholar
40. : Neuroimaging of repetitive transcranial magnetic stimulation effects on the brain, in Transcranial Brain Stimulation in Mental Disorders. Edited by Marcolin MPadberg F. Berlin, Karger, 2007, pp 35–52Crossref, Google Scholar
41. : Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul 2009; 2:58–80Crossref, Medline, Google Scholar
42. : Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59–72Crossref, Google Scholar
43. : Why would you ever want to? toward understanding the antidepressant effect of prefrontal rTMS. Hum Psychopharmacol 1998; 15:307–313Crossref, Google Scholar
44. : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
45. : Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci 1996; 8:172–180Crossref, Medline, Google Scholar
46. : Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853–1856Crossref, Medline, Google Scholar
47. : Mood improvement following daily left prefrontal repetitive trans-cranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752–1756Link, Google Scholar
48. : Neural network dysfunction in bipolar depression: clues from the efficacy of lamotri-gine. Biochem Soc Trans 2009; 37:1080–1084Crossref, Medline, Google Scholar
49. : The influence of HF-rTMS treatment on 5-HT2A receptors in medication-resistant unipolar depression. J Affect Disord 2010; 122 (suppl 1):S40Crossref, Google Scholar
50. : Report on risk and safety of repetitive trans-cranial magnetic stimulation (rTMS): suggested guidelines from the International Workshop on Risk and Safety of rTMS (June 1996). Electroencephalogr Clin Neuro 1997; 108:1–16Crossref, Medline, Google Scholar
51. ;
52. ;
53. : Managing the risks of repetitive transcranial stimulation. CNS Spectr 2003; 8:489Crossref, Medline, Google Scholar
54. : Hearing loss from the acoustic artifact of the coil used in extracranial magnetic stimulation. Neurology 1990; 40:1159–1162Crossref, Medline, Google Scholar
55. : Effects of a 2- to 4-week course of repetitive transcranial magnetic stimulation on neuropsychological functioning, electroencephalogram, and auditory threshold in depressed patients. Biol Psychiatry 2001; 49:615–623Crossref, Medline, Google Scholar
56. : Cognitive effects of 1- and 20-hertz repetitive transcranial magnetic stimulation in depression: preliminary report. Neuropsychiatry Neuropsychol Behav Neurol 2000; 13:119–124Medline, Google Scholar
57. : Trans-cranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry 2008; 69:441–451Crossref, Medline, Google Scholar
58. : Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. J ECT 2006; 22:259–264Crossref, Medline, Google Scholar
59. : Decreasing procedural pain over time of left prefrontal rTMS for depression: initial results from the open-label phase of a multisite trial (OPT-TMS). Brain Stimul 2009; 2:88–92Crossref, Medline, Google Scholar
60. : Application of transcranial magnetic stimulation in the treatment of drug-resistant major depression. Hum Psychopharmacol 1993; 8:361–365Crossref, Google Scholar
61. : Transcranial magnetic stimulation (TMS) in the treatment of major depression: a pilot study. Hum Psychopharmacol 1995; 10:305–310Crossref, Google Scholar
62. : Transcranial magnetic stimulation in depression and schizophrenia. Eur Neuropsychopharmacol 1994; 4:287–288Crossref, Medline, Google Scholar
63. : Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology 2009; 34:522–534Crossref, Medline, Google Scholar
64. : Focal electrically administered therapy: device parameter effects on stimulus perception in humans. J ECT 2009; 25:91–98Crossref, Medline, Google Scholar
65. : Focal electrical stimulation as a sham control for rTMS: does it truly mimic the cutaneous sensation and pain of active prefrontal rTMS? Brain Stimul 2008; 1:44–51Crossref, Medline, Google Scholar
66. : Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br J Psychiatry 2007; 191:441–448Crossref, Medline, Google Scholar
67. : Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000; 48:1133–1141Crossref, Medline, Google Scholar
68. : Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry 1999; 46:1603–1613Crossref, Medline, Google Scholar
69. : Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry 2001; 50:58–61Crossref, Medline, Google Scholar
70. : More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry 2009; 66:509–515Crossref, Medline, Google Scholar
71. : A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 2009; 34:1255–1262Crossref, Medline, Google Scholar
72. : An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2009; 2:50–54Crossref, Medline, Google Scholar
73. : The use of rapid rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci 1998; 10:20–25Crossref, Medline, Google Scholar
74. : Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depress Anxiety 2004; 19:249–256Crossref, Medline, Google Scholar
75. : Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry 2008; 65:268–276Crossref, Medline, Google Scholar
76. : Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 2003; 160:835–845Link, Google Scholar
77. : A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000; 48:962–970Crossref, Medline, Google Scholar
78. : An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson's disease. Clin Neurophysiol 2007; 118:2189–2194Crossref, Medline, Google Scholar
79. : Tolerability and safety of high daily doses of repetitive transcranial magnetic stimulation in healthy young men. J ECT 2006; 22:49–53Crossref, Medline, Google Scholar
80. : Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. J ECT (Epub ahead of print, Feb 8, 2010)Google Scholar
81. : Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul 2010; 3:187–199Crossref, Medline, Google Scholar
82. : Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 2001; 285:1299–1307Crossref, Medline, Google Scholar
83. : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Link, Google Scholar
84. : Long-term maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry 2005; 66:1524–1528Crossref, Medline, Google Scholar
85. : Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depress Anxiety 2004; 20:98–100Crossref, Medline, Google Scholar
86. : Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther 2009; 26:346–368Crossref, Medline, Google Scholar
87. : Decision analysis of the cost-effectiveness of repetitive transcranial magnetic stimulation versus electroconvulsive therapy for treatment of nonpsychotic severe depression. CNS Spectr 2004; 9:476–482Medline, Google Scholar
88. : Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002; 8:270–275Crossref, Medline, Google Scholar



