Personalized Medicine for Depression: Can We Match Patients With Treatments?
Abstract
Objective:
Response to specific depression treatments varies widely among individuals. Understanding and predicting that variation could have great benefits for people living with depression.
Method:
The authors describe a conceptual model for identifying and evaluating evidence relevant to personalizing treatment for depression. They review evidence related to three specific treatment decisions: choice between antidepressant medication and psychotherapy, selection of a specific antidepressant medication, and selection of a specific psychotherapy. They then discuss potential explanations for negative findings as well as implications for research and clinical practice.
Results:
Many previous studies have examined general predictors of outcome, but few have examined true moderators (predictors of differential response to alternative treatments). The limited evidence indicates that some specific clinical characteristics may inform the choice between antidepressant medication and psychotherapy and the choice of specific antidepressant medication. Research to date does not identify any biologic or genetic predictors of sufficient clinical utility to inform the choice between medication and psychotherapy, the selection of specific medication, or the selection of a specific psychotherapy.
Conclusions:
While individuals vary widely in response to specific depression treatments, the variability remains largely unpredictable. Future research should focus on identifying true moderator effects and should consider how response to treatments varies across episodes. At this time, our inability to match patients with treatments implies that systematic follow-up assessment and adjustment of treatment are more important than initial treatment selection.
On average, antidepressant medication and specific psychotherapies have similar success in the first-line treatment of moderate depression (1–3). And on average, different antidepressants show equal or similar efficacy (4, 5). But the fact that treatments have similar efficacy on average does not imply that treatment selection is unimportant (6, 7). Individuals vary widely in response to specific treatments, and poor response to one treatment does not necessarily imply poor response to others. For example, among patients who do not benefit from initial treatment with one antidepressant, up to one-half experience significant improvement after switching to an alternative medication (8), adding a second medication (9), or adding psychotherapy (10). Unfortunately, many patients treated in community practice, especially in primary care, have no chance to benefit from second-line treatments. After starting antidepressant treatment, nearly one-half make no follow-up visits, and only one-quarter return often enough to pursue additional treatment options (11, 12). Accurate selection of the best initial treatment could have tremendous benefits for people living with depression.
Personalized medicine promises to move beyond data regarding the average effectiveness of treatments to identify the best treatment for any individual. In order to provide personalized medicine for depression, we must identify characteristics of individuals that reliably predict differences in benefits and/or adverse effects of alternative depression treatments, including both biological and psychosocial treatments. These personalizing factors might include sociodemographic characteristics, clinical characteristics (such as symptom patterns or comorbidities), and biological markers (such as neuroimaging or genetic variation).
This review examines evidence that specific patient characteristics can guide selection of initial treatment for adult outpatients with unipolar depression. We begin by presenting a conceptual model for personalized treatment in order to clarify the type of evidence relevant to treatment selection. We then consider the following three specific clinical decisions: the choice between psychotherapy and antidepressant medication, selection of a specific antidepressant medication, and selection of a specific psychotherapy. For each of these three decisions, our review first clarifies the types of evidence that can and cannot inform treatment selection. We then review potentially informative evidence regarding specific factors hypothesized to inform initial treatment choice. Since fewer data exist to guide subsequent treatment choices, we do not consider second-line therapies or therapies for treatment-resistant depression.
Conceptual Model for Personalized Treatment: What Evidence Is Relevant?
We hope to identify measurable characteristics of individual patients that can guide selection of treatment. Our question concerns differential efficacy (Do patients with Characteristic X show better response to Treatment A than to Treatment B?). This is often expressed as a moderator effect (Does Characteristic X moderate the difference in response rates between Treatment A and Treatment B?). It is essential to distinguish moderators or predictors of differential efficacy from more general predictors of depression outcome (13). Previous research has often conflated these two concepts.
Two study designs could produce the evidence needed to personalize treatment selection. First, we might compare alternative treatments in an unselected group of patients and examine whether a specific patient characteristic moderates the relationship between treatment type and outcome (14–16). One example would be a randomized trial comparing cognitive therapy and interpersonal psychotherapy in which we examine whether co-occurring personality disorder moderates (or interacts with) the effect of treatment type (17). Second, we might select a group of patients with a specific characteristic and then compare outcomes in patients receiving alternative treatments. One example would be a study limited to patients with depression and co-occurring personality disorder in which we compare cognitive therapy and interpersonal therapy (18). The former strategy is more flexible, allowing study of multiple potential moderators, including moderators (such as genetic variations) that were not identified prior to treatment. The latter strategy (limiting the sample to patients with a specific characteristic of interest) may be more efficient, but it only permits study of a single predictor or potential moderator that is identified in advance.
If a study does not include a direct comparison of alternative treatments, it cannot accurately identify moderators or predictors of differential treatment response. For example, if we study a cohort of patients receiving medication A and observe that characteristic X predicts better outcomes, we cannot determine whether characteristic X is a true moderator (predicting differential response to medication A compared with some alternative medication), a general predictor of good response to any medication, or simply a general predictor of good prognosis regardless of treatment. Alternatively, if we conduct a randomized trial comparing medication A with placebo and observe that characteristic X predicts a greater drug-placebo difference, we still cannot determine whether characteristic X is a true moderator (predicting differential response to medication A compared with some alternative medication) or simply a general predictor of good response to any medication. Since neither of these hypothetical studies includes a comparison of medication A with a specific alternative, neither could possibly yield data to inform the choice between medication A and any alternative.
This distinction between general predictors of prognosis, general predictors of treatment response, and predictors of differential treatment response (true moderators) is illustrated in Figure 1. Characteristic X might predict better outcome regardless of treatment or might predict better outcome with any treatment or might predict better outcome with treatment A than with treatment B. Only in the third situation could we conclude that characteristic X can guide our choice between treatments A and B.
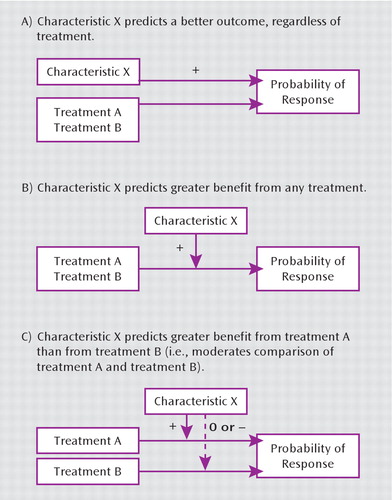
FIGURE 1. General and Differential Predictors (Moderators) of Treatment Response
We can also illustrate this distinction by examining the evidence that depression severity predicts better response to a specific antidepressant medication. Regardless of treatment, more severe depression at baseline predicts a poorer outcome (19, 20). In contrast, more severe depression at the initiation of treatment typically predicts greater benefit from medication compared with placebo (21, 22), perhaps because benefits of treatment are more apparent in those with a poorer general prognosis. Finally, more severe depression does not appear to predict better response to any specific antidepressant relative to others (4). More severe symptoms prior to starting treatment are therefore negative predictors of overall prognosis, positive predictors of benefit from medication in general (relative to placebo), and null predictors of differential response to specific medication. Severity of depression may have clinical utility as a predictor of benefit from treatment, but it has no apparent utility for predicting better response to one antidepressant over another.
Because identifying moderators or differential predictors requires a comparison of alternative treatments, it is often linked (both conceptually and practically) to comparative effectiveness research. Comparative effectiveness research examines the average effects of alternative treatments, while research to personalize treatment examines individual characteristics predicting differential response. In statistical terms, comparative effectiveness research considers main effects while personalized treatment research considers moderators or interaction effects.
Initial Choice Between Antidepressant Medication and Psychotherapy
We should first emphasize that many previous studies cited as guides to choosing between psychotherapy and pharmacotherapy do not directly address this clinical decision. Examples of these include cohort studies of patients treated with cognitive therapy or interpersonal psychotherapy showing that sleep EEG abnormalities or dexamethasone nonsuppression predict poorer outcomes (23–25). Studies of patients receiving a single treatment cannot determine whether these biomarkers are true moderators of treatment efficacy (i.e., specifically predict poorer response to psychotherapy than to pharmacotherapy) or simply general predictors of poor prognosis (i.e., predict poorer outcome with any treatment).
Three reports (26–28), including data from six randomized trials, compared the efficacy of specific antidepressants with that of specific psychotherapies among patients with more severe depression, defined as a Hamilton Depression Rating Scale (HAM-D) score ≥20 (Table 1). None of these found a significant advantage of either pharmacotherapy or psychotherapy.
| Study | Medication | Psychotherapy | Medication vs. Psychotherapyb | Analysis (test statistic for difference) |
|---|---|---|---|---|
| DeRubeis et al. (26) | Imipramine or nortriptyline (N=102) | Cognitive-behavioral therapy (N=67) | 13 vs. 12 | t=0.43, p=0.67 |
| DeRubeis et al. (27) | Paroxetine (N=120) | Cognitive therapy (N=60) | 13 vs. 14 | F=0.56, df=1, 231, p=0.46 |
| Dimidjian et al. (28) | Paroxetine (N=57) | Cognitive therapy (N=25) | 8.6 vs. 10.3 | n.s. |
| Dimidjian et al. (28) | Paroxetine (N=57) | Behavioral activation therapy (N=60) | 8.6 vs. 7.6 | n.s. |
TABLE 1. Randomized Comparisons of Antidepressant Medication and Specific Psychotherapies in Outpatients With Severe Depressiona
Six reports (29–34), including data from three randomized trials, examined clinical characteristics as moderators of response to specific medications versus specific psychotherapies (Table 2). Two such analyses (29, 31) found that personality disorder or maladaptive personality traits predicted better response to selective serotonin reuptake inhibitors (SSRIs) than to cognitive therapy or cognitive-behavioral therapy (CBT), while a third analysis limited to patients with chronic depression (32) found no such moderator effect. In a comparison of paroxetine and cognitive therapy (30), recent life stress, unemployment, and being married or living with a partner predicted more favorable outcome with psychotherapy. Among patients with chronic depression (33), a history of childhood trauma predicted better response to psychotherapy than to nefazodone, while those without trauma histories showed the opposite pattern. In this same trial (34), a stated preference for psychotherapy or pharmacotherapy strongly predicted better response to the preferred treatment, but this analysis was limited to the small minority of patients expressing such a preference.
| Study | Proposed Moderator | Medication | Psychotherapy | Outcome | Medication vs. Psychotherapy | Analysis (Test Statistic for Interaction) | |
|---|---|---|---|---|---|---|---|
| Moderator | No Moderator | ||||||
| Fournier et al. (29) | Personality disorder | Paroxetine (N=120) | Cognitive therapy (N=60) | HAM-D response rate | 66% vs. 44% | 49% vs. 70% | χ2=6.8, p=0.009 |
| Maddux et al. (32)a | Personality disorder | Nefazodone (N=226) | Cognitive-behavioral analysis therapy (N=228) | Mean HAM-D posttreatment score | F=0.88, df=2, 473, p=0.41 | ||
| Bagby et al. (31) | High neuroticism | SSRI (N=129) | Cognitive-behavioral therapy (N=146) | Mean HAM-D posttreatment score | 8 vs. 5 | 6 vs. 6 | t=2.12, p=<0.04 |
| Fournier et al. (30) | Recent life stress | Paroxetine (N=120) | Cognitive therapy (N=60) | Mean HAM-D posttreatment score | 12. vs. 6 | 9 vs. 10 | t=2.17, p=0.03 |
| Fournier et al. (30) | Unemployment | Paroxetine (N=120) | Cognitive therapy (N=60) | Mean HAM-D posttreatment score | 15 vs. 6 | 7 vs. 8 | t=3.04, p=0.003 |
| Fournier et al. (30) | Single marital status | Paroxetine (N=120) | Cognitive therapy (N=60) | Mean HAM-D posttreatment score | 10 vs. 10 | 12 vs. 4 | t=3.13, p=0.002 |
| Nemeroff et al. (33) | Childhood trauma | Nefazodone (N=226) | Cognitive-behavioral analysis therapy (N=228) | Mean HAM-D posttreatment score | 38% vs. 48% | 40% vs. 29% | χ2=6.9, p=0.009 |
| Kocsis et al. (34) | Preference for psychotherapy | Nefazodone (N=24) | Cognitive-behavioral analysis therapy (N=32) | Mean HAM-D posttreatment score | 8% vs. 50% | 45% vs. 22% | χ2=13, p=0.04 |
TABLE 2. Randomized Comparisons of Antidepressant Medication and Specific Psychotherapies Evaluating Specific Clinical Characteristics as Moderators
Two trials examined response to combined treatment, finding that comorbid personality disorder predicted greater benefit from pharmacotherapy combined with either interpersonal psychotherapy (35) or brief psycho-dynamic psychotherapy (36) compared with pharmaco-therapy alone.
Selection of a Specific Antidepressant Medication
Clinical Predictors of Differential Benefit From Antidepressant Medications
Two lines of research have examined symptom patterns as predictors of differential response to alternative antidepressants. McGrath et al. demonstrated that the pattern of atypical depression (oversleeping, overeating, anergy, rejection sensitivity) predicted better response to phenelzine than to imipramine (37). More recent research indicates that this symptom pattern does not predict better response to SSRIs than to imipramine (38), and thus relevance to current practice is limited. Numerous trials have examined whether pretreatment anxiety or insomnia predicts better response to drugs thought to have sedating or anxiolytic effects. In a systematic review of 13 published reports including 3,114 patients, Gartlehner et al. (4) found no evidence that higher levels of either insomnia or anxiety predicted differential response to alternative antidepressant drugs. Papakostas et al. (39) reanalyzed pooled data from 10 trials including 1,275 patients with depression and high levels of anxiety, finding slightly more favorable outcomes (approximately 1 point on the HAMD) with various SSRIs compared with bupropion.
Past treatment response is often suggested as a guide to medication selection (40). Surprisingly, almost no empirical data exist regarding consistency of response to specific medications over time. Remillard et al. (41) described 59 inpatients with a history of good antidepressant response in a prior inpatient episode. Of 35 patients prescribed the same medication, 57% responded. Of 24 patients prescribed a different antidepressant, 79% responded. We are aware of no other studies examining whether history of favorable response to a specific medication predicts another favorable response in a subsequent treatment episode. Prior treatment during the current depressive episode has also been proposed as a guide to medication selection. Several studies have evaluated this question with respect to SSRIs, but none found that poor response to one SSRI drug predicted response to a subsequent drug in the same class. For example, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (8) found that poor response to citalopram did not predict differential probability of subsequent response to sertraline, bupropion, or venlafaxine.
Biomarker Predictors of Differential Benefit From Antidepressant Medications
Early research on biologic predictors of antidepressant response examined markers of neurotransmitter production or metabolism. Preliminary studies indicated that urinary 3-methoxy-4-hydroxyphenylglycol levels might predict differential response to adrenergic versus serotonergic antidepressants (42), but these findings were not replicated (43).
Subsequent research examined a range of biological predictors of treatment response, including endocrine measures (markers of corticosteroid, neuroactive steroid, and thyroid activity), neuroimaging measures, and electroencephalographic measures. While several of these measures were found to predict overall prognosis or general treatment response, none were found to predict greater response to one antidepressant (or type of antidepressant) than to another (13, 44).
Genetic Predictors of Differential Benefit From Antidepressant Medications
As we have noted, many previous studies cannot, by design, inform the selection of one antidepressant over another. This group includes numerous cohort studies examining the associations between specific genetic variations and favorable treatment outcomes in patients treated with a single antidepressant (45–52) or class of antidepressants (53, 54). Cohort studies of this type cannot distinguish between general predictors of prognosis and true moderators that predict differential response to alternative drugs. Similarly, studies identifying genetic variations associated with better response to one active antidepressant than to placebo (55) cannot distinguish general predictors of benefit with antidepressant treatment from specific predictors of differential response to alternative medications. Because none of the aforementioned reports compare alternative active treatments, none can provide evidence to personalize antidepressant selection.
Relatively few studies have examined the association between genetic variations and outcomes in patients treated with alternative antidepressants. These include both randomized comparisons of alternative antidepressants (56–59) and nonrandomized comparisons (60, 61). In some cases, the pattern of associations is consistent with presumed mechanisms of drug action. For example, Kim et al. (60) found that response to an SSRI (either fluoxetine or sertraline) was significantly associated with variation in serotonin transporter genes but not with variation in norepinephrine transporter genes. Using data from a randomized comparison of the two drugs, Szegedi et al. (56) and Tadic et al. (57) found that variations in both monoamine oxidase and catechol-O-methyltransferase genes were associated with response to mirtazapine but not with response to paroxetine. Wakeno et al. (59) found that variation in alpha 2A-adrenergic receptor genes was associated with response to milnacipran but not to paroxetine. Primary analyses in each of these studies addressed the following question: Does a specific genetic variation predict better outcome in patients treated with a specific medication? These analyses are appropriate for testing hypotheses regarding mechanisms of drug action, but they do not directly address our question regarding differential efficacy: Does a specific genetic variation identify a group of patients with significantly more favorable response to one antidepressant than to another? This question regarding differential efficacy is formally addressed by a test for interaction or moderation. Kim et al. (60) did report a post hoc subgroup analysis in which patients with a specific variation of the norepinephrine transporter gene experienced significantly higher response rates during treatment with nortriptyline than with either fluoxetine or sertraline. If replicated, this finding could inform medication choice for this subgroup of patients. The Genome-Based Therapeutic Drugs for Depression project (62) used genome-wide association analyses in patients treated with nortriptyline or escitalopram to identify moderator effects (at a suggestive level of statistical significance) for two novel genetic variations. If replicated, these findings could inform the choice between these two medications.
Genetic Predictors of Differential Adverse Effects From Antidepressant Medications
We again begin by pointing out that most previous studies cannot, by design, guide treatment selection. Included in this group are cohort studies of patients treated with a single medication or group of medications that examine genetic predictors of specific adverse effects, such as insomnia (63), sexual dysfunction (64, 65), and development of suicidal ideation (66). These cohort studies cannot distinguish general predictors of experiencing adverse effects from moderators or differential predictors of more adverse effects with one treatment than with another. For example, the serotonin receptor variation previously associated with insomnia during fluoxetine treatment may instead be associated with a general tendency to experience adverse effects with a range of medications (67, 68). In the same way, finding that a particular genetic variation is associated with suicidal ideation during treatment with citalopram (66, 69) could simply imply that this variation is associated with either suicidal ideation in general or suicidal ideation during treatment with any antidepressant. Neither of these latter interpretations would argue for or against the use of citalopram in this subgroup of patients.
Of studies examining adverse events, only one has included patients treated with alternative drugs (70), finding that variation in a gene coding for the HTR2A serotonin receptor was associated with adverse effects with paroxetine but not with mirtazapine. Because no test for interaction or moderation was reported, this finding does not definitively address our question regarding personalized treatment: Does variation in HTR2A identify a group of patients who experience significantly fewer adverse effects during mirtazapine treatment than during paroxetine treatment? The finding of a significant relationship in patients treated with one drug and not in those treated with the other can sometimes reflect low statistical power rather than a true interaction or moderator effect.
Selection of a Specific Psychotherapy
While research on differential response to medications has focused on biomarkers and genetic variation, the limited research on differential response to psychotherapies has focused more on clinical characteristics (Table 3). Four reports (17, 18, 71, 72) have examined avoidant and/ or borderline personality traits or disorders as moderators of response to interpersonal therapy versus cognitive therapy or CBT. This evidence does suggest that borderline personality disorder or avoidant attachment style predicts better response to cognitive therapy. Evidence from one study each suggests that obsessive-compulsive personality disorder may predict better response to interpersonal therapy than cognitive therapy (71) and that more severe depression may predict better outcome with behavioral activation than with cognitive therapy (28).
| Study | Proposed Moderator | Therapy A | Therapy B | Outcome | Therapy A vs. Therapy B | Analysis | |
|---|---|---|---|---|---|---|---|
| Moderator | No Moderator | ||||||
| Barber and Muenz (71) | Avoidant personality disorder | Cognitive therapy (N=37) | Interpersonal therapy (N=47) | Mean HAM-D post-treatment score | 5.7 vs. 10.7 | 8.5 vs. 5.5 | t=3.8, p<0.001 |
| Joyce et al. (17) | Personality disorder (primarily avoidant and borderline) | CBT (N=76) | Interpersonal therapy (N=83) | Decline in Montgomery-Åsberg Depression Rating Scale score (%) | 58% vs. 38%* | 58% vs. 66% | a |
| Bellino et al. (18) | Borderline personality disorder | Cognitive therapy (N=12) | Interpersonal therapy (N=14) | Mean HAM-D post-treatment score | 13.7 vs. 14.1b | a | a |
| McBride et al. (72) | Avoidant attachment style | CBT (N=28) | Interpersonal therapy (N=27) | Mean Beck Depression Inventory posttreatment scorec | 0.5 vs. 3.6 | 4.2 vs. 3.7 | z=3.1, p=0.002 |
| Barber and Muenz (71) | Obsessive-compulsive disorder | Cognitive therapy (N=37) | Interpersonal therapy (N=47) | Mean HAM-D post-treatment score | 8.1 vs. 4.2 | 7.7 vs. 7.0 | t=2.5, p=0.01 |
| Dimidjian et al. (28) | HAM-D score 20 | Behavioral action (N=43) | Cognitive therapy (N=45) | HAM-D response rate | 76% vs. 48% | 39% vs. 60% | a |
TABLE 3. Randomized Comparisons of Alternative Psychotherapies Evaluating Specific Clinical Characteristics as Moderators
Summary of Evidence Regarding Predictors of Differential Response
Regarding initial choice between medication and psychotherapy, severity of depression does not appear to predict greater likelihood of response to medication or psychotherapy. Modest evidence suggests that personality disorder predicts more favorable response to pharmacotherapy and that negative life events (either recent stresses or childhood trauma) predict better response to psychotherapy. One trial suggests that a clear preference for either medication or psychotherapy predicts greater success with the preferred treatment.
Regarding selection of a specific medication, cooccurring anxiety disorder may predict greater improvement with SSRIs than with bupropion, but this difference appears small. Biological markers (such as neuroendocrine or imaging studies) do not appear to predict differential response to specific antidepressants. Most previous studies of genetic predictors have not used appropriate designs and analyses to identify true moderators or predictors of differential efficacy. Consequently, we have no strong evidence that any genetic variation can inform antidepressant selection. Surprisingly, we also have no evidence that history of prior medication response is useful in medication selection.
Regarding selection of a specific psychotherapy, moderate evidence suggests that borderline personality disorder or attachment difficulty predicts more favorable response to cognitive therapy than interpersonal therapy.
Sources of Error in Research to Personalize Depression Treatment
Inadequate statistical power to detect moderator effects is a likely explanation for many of the aforementioned “negative” findings. To illustrate, in a study comparing equal numbers of patients receiving alternative treatments with average response rates of 50%, we could examine whether relative efficacy of treatments A and B varies between patients with and without characteristic X. If patients with characteristic X have a 60% response rate with treatment A and a 40% response rate with treatment B and those without characteristic X show the opposite pattern (a relatively large moderator effect), a sample of approximately 300 patients would be necessary to reliably detect this difference (assuming a 5% type I error rate and 20% type II error rate). Of the aforementioned pharmacogenetic studies (56–61), none have included more than 250 patients, and most have included fewer than 150.
Research to personalize treatment, especially research focused on genetic moderators or predictors, must consider another source of error. Such research depends on the assumption (usually unstated) that an individual's response to a specific treatment will be stable across different episodes of treatment. This assumption also underlies the common clinical practice of basing medication selection on past treatment experience. This fundamental assumption has only been examined in a single small observational study (41), and it was not supported by these data. It is remarkable that an assumption so central to clinical practice and pharmacogenetic research has so little empirical support. Addressing this gap in knowledge is a priority for future research.
Because studies of depression treatment response typically consider only a single episode of treatment per person, they obscure the distinction between person-level and episode-level predictors of treatment response. Episode-level predictors are those that may vary within individuals across episodes of depression treatment. Examples of these potentially variable characteristics include pretreatment symptom severity, pretreatment episode duration, co-occurring substance abuse, and recent life stresses. In contrast, person-level predictors are expected to show no variability across treatment episodes. Examples of these stable characteristics include race or ethnicity, stable personality traits, genetic variation, family history, or the past experience of physical or sexual abuse. Traditional research designs (examining differences between individuals during a single episode of treatment) may be adequate to identify episode-level predictors of treatment response. Accurate identification of stable or person-level predictors of treatment response will require examining consistency of treatment response within individuals across multiple episodes of care.
Directions for Future Research
Research to date has identified few clinical characteristics and no biomarkers or genetic variations that reliably predict differential effectiveness or adverse effects of specific depression treatments. We have described three conceptual difficulties that may be responsible for our failure to identify differential predictors of response. First, previous research has often conflated predictors of response to specific treatments with more general predictors of prognosis. Second, response to specific treatments may actually vary across episodes of treatment. Third, response to treatment in any episode of illness may be influenced by a mixture of episode-level (or time-varying) and patient-level (or stable) characteristics. These conceptual issues have important implications for future research to identify differential predictors of treatment response.
First, research to personalize treatment for depression will probably require samples of patients considerably larger than those enrolled in traditional clinical trials. Detection of moderators or interaction effects generally requires larger samples than does detection of average or main effects, especially if a potential moderator (such as a genetic variation) influences response to one treatment but not the other.
Second, research to identify person-level predictors of treatment response may need to consider response across multiple episodes of depression. Research designs that consider only a single episode of treatment per person cannot examine whether any particular response pattern (e.g., gastrointestinal side effects with a specific SSRI, responding favorably to interpersonal psychotherapy) is stable within individuals across episodes of treatment.
Third, accurately predicting response to specific treatments may require combinations of several weak predictors rather than a single powerful one (73). For example, response to a specific antidepressant drug might be simultaneously moderated by a large number of variants of small effect. A recent genome-wide association study suggests that this may be the case (54). Developing rules for treatment selection based on multiple predictors will require useful theory regarding treatment mechanisms, large sample sizes, and healthy skepticism regarding predictors identified only after multiple comparisons.
Given the large sample sizes needed to detect moderators of treatment effectiveness, randomized trials to inform personalized treatment may need to adopt broader recruitment strategies and more efficient methods for outcome assessment. Large, pragmatic trials have been proposed as a more efficient strategy for comparative effectiveness research (74). These methods could be extended to address questions of differential treatment effectiveness.
Recruitment for randomized trials to identify moderators of treatment response might incorporate information regarding response to past treatments. Traditional clinical trials typically consider past treatment only for safety reasons, excluding patients with histories of adverse reactions to a study treatment. Future trials might consider past treatment response in order to select particularly informative samples of participants. For example, a study to identify genetic predictors of favorable response to drug A over drug B might preferentially include patients with past exposures to drug A and/or drug B. Information regarding past response could be combined with new data to more accurately distinguish true treatment effects from other sources of variation in outcome.
Another alternative approach would use observational data from large, population-based samples of patients treated under naturalistic conditions. This approach has led to significant advances in personalizing treatment for other chronic health conditions (75, 76). Observational studies permit examination of thousands of patients rather than the hundreds typically enrolled in clinical trials. Most important, longitudinal data would permit study of multiple treatment episodes per patient, including exposures to similar and dissimilar treatments. These data could serve in two ways. First, only such longitudinal data could evaluate the stability of potential treatment response phenotypes prior to expending resources searching for a corresponding genotype. Second, observational studies could identify potential moderators or differential predictors for definitive evaluation in subsequent randomized trials. Given potential biases due to nonrandom assignment of treatments, observational studies would only be appropriate for generating hypotheses regarding differential treatment response rather than confirming them.
Translating Research Prediction Into Clinical Utility
When research does identify statistically significant predictors of differential treatment response, caution will be necessary when translating those research findings into clinical practice. The rise and fall of the dexamethasone suppression test as a predictor of need for depression treatment is a useful cautionary tale regarding clinical utility of predictive tests derived from research populations (77). Data from research settings suggested that this test might accurately predict poorer prognosis and greater need for specific depression treatment. But predictive power was much weaker in representative clinical populations, reflecting the different spectrum of illness in everyday practice (78).
Replication is the first step in translating research findings to clinically useful tests or predictors. Given the number of putative predictors available to examine in any study, reliance on the 5% standard for statistical significance is likely too liberal. Many reported predictors are likely to be false positives (79).
Even after replication, calibration studies in representative populations are necessary to assess clinical utility. The expected clinical utility of testing for genetic or other predictors of treatment response could vary widely according to the prevalence of the predictor, the accuracy of the test in actual practice, and both the prevalence and clinical importance of the outcome (80–82). Predictive power often declines significantly during the translation from research to practice. Given this slippage between research accuracy and clinical utility, the number of patients whom we would need to test in order to gain an additional good clinical outcome is often surprisingly large. For example, tests for cytochrome P450 variation might accurately predict antidepressant serum concentrations. But the numerous sources of variation between serum concentrations and clinical response mean that such tests would likely have limited utility for guiding antidepressant prescribing in clinical practice (83).
Implications for Practice
Given our limited ability to predict differential response to specific depression treatments, clinical recommendations in this area derive more from what we do not know than from what we do. When selecting between psychotherapy and medication, modest evidence suggests that personality disorder predicts better initial response to medication and a history of stressful or traumatic life events predicts better response to psychotherapy. When selecting between medications, neither biomarkers nor specific symptom patterns (e.g., anxiety symptoms, insomnia) predict clinically important differences in response. While conventional wisdom would base treatment selection on past response of an individual patient or the patient's family members, we lack evidence that response to a specific treatment is consistent within families or even within individuals over time. Treatment selection should also consider patients' preferences and (in the case of psychotherapy) the availability of adequately trained providers.
Our limited ability to predict response to specific treatments does have important implications for the organization of practice, especially in the selection and prescribing of antidepressant medications. Of patients starting antidepressant treatment, only one-half will experience a good outcome with the first treatment selected. Unfortunately, we have scant evidence that attempts to match specific treatments to specific patients will improve the rate of success. We should contrast this disappointing conclusion with the very strong evidence that organized follow-up programs significantly increase the success of antidepressant treatment (84, 85). Monitoring outcomes and personalizing treatment over time will have a much greater effect on outcomes than will attempts to personalize initial treatment selection. But opportunities for monitoring and tailoring of treatment are frequently missed. Of patients initiating anti-depressant treatment, as many as one-half will not return for follow-up visits (11, 12). Of those starting psychotherapy in community practice, one-half make fewer than four visits (86). Because low motivation, discouragement, and self-blame are core features of depression, aggressive outreach may be necessary to reach those who fail to return (87).
Our limited ability to match patients with specific treatments also raises questions about how we share uncertainty with our patients. Communicating hope is an essential element of any healing relationship. But it would be less than honest to imply that we can accurately select the best treatment for any individual. Prudence and respect for patients' autonomy argue for following an honestly optimistic approach, such as: “We have several good treatment options to choose from. On the average, they have about the same chance of success. But you are not an average; you are an individual. At this time, there is no scientific way to predict which treatment will work best for you. Together we will look at your options and decide what treatment to start with. But it is important to remember that there are other options. If the first treatment we pick does not work out for you, some other treatment might work well. Regular follow-up over the next several weeks will tell us whether to stay with our first choice or try something else.”
1.
2. ;
3.
4. : Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med 2008; 149:734–750Crossref, Medline, Google Scholar
5. : Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009; 373:746–758Crossref, Medline, Google Scholar
6. : Choosing a first-line antidepressant: equal on average does not mean equal for everyone. JAMA 2001; 286:3003–3004Crossref, Medline, Google Scholar
7. : Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q 2004; 82:661–687Crossref, Medline, Google Scholar
8. ;
9. ;
10. : Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry 2007; 164:739–752Link, Google Scholar
11. : Treatment process and outcomes for managed care patients receiving new anti-depressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry 2001; 58:395–401Crossref, Medline, Google Scholar
12.
13. : Predictors, moderators, and mediators (correlates) of treatment outcome in major depressive disorder. Dialogues Clin Neurosci 2008; 10:439–451Medline, Google Scholar
14. : The moderator-mediator distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol 1986; 51:1173–1182Crossref, Medline, Google Scholar
15. : Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002; 59:877–883Crossref, Medline, Google Scholar
16. : Moderators of treatment outcomes: clinical, research, and policy importance. JAMA 2006; 296:1286–1289Crossref, Medline, Google Scholar
17. : Temperament, character, and personality disorders as predictors of response to interpersonal psychotherapy and cognitive-behavioural therapy for depression. Br J Psychiatry 2007; 190:503–508Crossref, Medline, Google Scholar
18. : Combined therapy of major depression with concomitant borderline personality disorder: comparison of interpersonal and cognitive psychotherapy. Can J Psychiatry 2007; 52:718–725Crossref, Medline, Google Scholar
19. : Selecting among second-step antidepressant medication monotherapies: Are clinical, demographic, or first-step treatment results informative? Arch Gen Psychiatry 2008; 65:870–880Crossref, Medline, Google Scholar
20. ;
21. : Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psycho-pharmacol 2002; 22:40–45Crossref, Medline, Google Scholar
22. : Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010; 303:47–53Crossref, Medline, Google Scholar
23. : Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am J Psychiatry 1997; 154:502–509Link, Google Scholar
24. : Hypothalamic-pituitary-adrenocortical activity and response to cognitive behavior therapy in unmedicated, hospitalized depressed patients. Am J Psychiatry 1996; 153:886–891Link, Google Scholar
25. : Psychobiological correlates of poor response to cognitive behavior therapy: potential indications for antidepressant pharmacotherapy. Psychopharmacol Bull 1993; 29:293–301Medline, Google Scholar
26. : Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry 1999; 156:1007–1013Abstract, Google Scholar
27. : Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005; 62:409–416Crossref, Medline, Google Scholar
28. : Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74:658–670Crossref, Medline, Google Scholar
29. : Antidepressant medications v cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry 2008; 192:124–129Crossref, Medline, Google Scholar
30. : Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol 2009; 77:775–787Crossref, Medline, Google Scholar
31. : Personality and differential treatment response in major depression: a randomized controlled trial comparing cognitive-behavioural therapy and pharmacotherapy. Can J Psychiatry 2008; 53:361–370Crossref, Medline, Google Scholar
32. : Select comorbid personality disorders and the treatment of chronic depression with nefazodone, targeted psychotherapy, or their combination. J Affect Disord 2009; 117:174–179Crossref, Medline, Google Scholar
33. : Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 2003; 100:14293–14296Crossref, Medline, Google Scholar
34. : Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, cognitive behavioral analysis system of psychotherapy, or their combination. J Clin Psychiatry 2009; 70:354–361Crossref, Medline, Google Scholar
35. : Combined treatment of major depression in patients with borderline personality disorder: a comparison with pharmacotherapy. Can J Psychiatry 2006; 51:453–460Crossref, Medline, Google Scholar
36. : Efficacy of combined therapy and pharmacotherapy for depressed patients with or without personality disorders. Harv Rev Psychiatry 2003; 11:133–141Crossref, Medline, Google Scholar
37. : Predictive value of symptoms of atypical depression for differential drug treatment outcome. J Clin Psychopharmacol 1992; 12:197–202Crossref, Medline, Google Scholar
38. : A placebo-controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. Am J Psychiatry 2000; 157:344–350Link, Google Scholar
39. : Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies. J Clin Psychiatry 2008; 69:1287–1292Crossref, Medline, Google Scholar
40. : Rational antidepressant selection: applying evidence-based medicine to complex real-world patients. Psychopharmacol Bull 2006; 39:38–104Medline, Google Scholar
41. : Differential responses to a single antidepressant in recurrent episodes of major depression. Hosp Community Psychiatry 1994; 45:359–361Abstract, Google Scholar
42. : Urinary MHPG levels and tricyclic antidepressant drug selection: a preliminary communication on improved drug selection in clinical practice. Arch Gen Psychiatry 1979; 36:1111–1115Crossref, Medline, Google Scholar
43. : Failure of urinary MHPG levels to predict depression treatment response in patients with unipolar depression. Am J Psychiatry 1986; 143:1398–1402Link, Google Scholar
44. : Biological predictors of treatment response in affective illness. Psychiatr Clin North Am 2003; 26:323–344Crossref, Medline, Google Scholar
45. : Variation in catechol-O-methyltransferase is associated with duloxetine response in a clinical trial for major depressive disorder. Biol Psychiatry 2009; 65:785–791Crossref, Medline, Google Scholar
46. : Prediction of antidepressant response to milnacipran by norepinephrine transporter gene polymorphisms. Am J Psychiatry 2004; 161:1575–1580Link, Google Scholar
47. : Response to fluoxetine and serotonin 1A (C-1019G) polymorphism in Taiwan Chinese major depressive disorder. Pharmacogenomics J 2006; 6:27–33Crossref, Medline, Google Scholar
48. : Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry 2007; 164:1181–1188Link, Google Scholar
49. : The FKBP5-gene in depression and treatment response: an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) cohort. Biol Psychiatry 2008; 63:1103–1110Crossref, Medline, Google Scholar
50. : Influence of the tyrosine hydroxylase Val81MET polymorphism and catechol-O-methyltransferase Val158MET polymorphism on the antidepressant effect of milnacipran. Hum Psychopharmacol 2008; 23:121–128Crossref, Medline, Google Scholar
51. : Association study of two serotonin 1A receptor gene polymorphisms and fluoxetine treatment response in Chinese major depressive disorders. Eur Neuropsychopharmacol 2006; 16:498–503Crossref, Medline, Google Scholar
52. : Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol 2003; 6:339–346Crossref, Medline, Google Scholar
53. : Sequence analysis of the serotonin transporter and associations with antidepressant response. Biol Psychiatry 2005; 58:374–381Crossref, Medline, Google Scholar
54. : A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry 2009; 66:966–975Crossref, Medline, Google Scholar
55. : The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology (Berl) 2004; 174:525–529Crossref, Medline, Google Scholar
56. : The catechol-O-methyltransferase Val108/158Met polymorphism affects short-term treatment response to mirtazapine, but not to paroxetine in major depression. Pharmacogenomics J 2005; 5:49–53Crossref, Medline, Google Scholar
57. : The MAOA T941G polymorphism and short-term treatment response to mirtazapine and paroxetine in major depression. Am J Med Genet B Neuropsychiatr Genet 2007; 144:325–331Crossref, Google Scholar
58. : A monoamine oxidase B gene variant and short-term antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:1370–1377Crossref, Medline, Google Scholar
59. : The alpha 2A-adrenergic receptor gene polymorphism modifies antidepressant responses to milnacipran. J Clin Psychopharmacol 2008; 28:518–524Crossref, Medline, Google Scholar
60. : Monoamine transporter gene polymorphisms and antidepressant response in Koreans with late-life depression. JAMA 2006; 296:1609–1618Crossref, Medline, Google Scholar
61. : The G196A polymorphism of the brain-derived neurotrophic factor gene and the antidepressant effect of milnacipran and fluvoxamine. J Psychopharmacol 2007; 21:650–656Crossref, Medline, Google Scholar
62. : Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010; 167:555–564Link, Google Scholar
63. : Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry 2003; 54:879–883Crossref, Medline, Google Scholar
64. : Serotonin 2A-1438 G/A and G-protein beta3 subunit C825T polymorphisms in patients with depression and SSRI-associated sexual side-effects. Neuropsychopharmacology 2006; 31:2281–2288Crossref, Medline, Google Scholar
65. : Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology 2009; 34:1819–1828Crossref, Medline, Google Scholar
66. : Association between treatment-emergent suicidal ideation with citalopram and polymorphisms near cyclic adenosine monophosphate response element binding protein in the STAR*D study. Arch Gen Psychiatry 2007; 64:689–697Crossref, Medline, Google Scholar
67. : Serotonin transporter polymorphisms and the occurrence of adverse events during treatment with selective serotonin reuptake inhibitors. Int Clin Psychopharmacol 2007; 22:137–143Crossref, Medline, Google Scholar
68. : Association between a functional serotonin transporter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry 2007; 64:783–792Crossref, Medline, Google Scholar
69. : Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164:1460–1461Link, Google Scholar
70. : Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 2003; 160:1830–1835Link, Google Scholar
71. : The role of avoidance and obsessiveness in matching patients to cognitive and interpersonal psychotherapy: empirical findings from the treatment for depression collaborative research program. J Consult Clin Psychol 1996; 64:951–958Crossref, Medline, Google Scholar
72. : Attachment as moderator of treatment outcome in major depression: a randomized control trial of interpersonal psychotherapy versus cognitive behavior therapy. J Consult Clin Psychol 2006; 74:1041–1054Crossref, Medline, Google Scholar
73. : Common genetic variation and human traits. N Engl J Med 2009; 360:1696–1698Crossref, Medline, Google Scholar
74. : Pragmatic randomised controlled trials in psychiatry. Br J Psychiatry 1999; 175:217–223Crossref, Medline, Google Scholar
75. : Personalized medicine in the era of genomics. JAMA 2007; 298:1682–1684Crossref, Medline, Google Scholar
76. : Drug-gene interactions between genetic polymorphisms and antihypertensive therapy. Drugs 2004; 64:1801–1816Crossref, Medline, Google Scholar
77. : How to evaluate a diagnostic marker test: lessons from the rise and fall of dexamethasone suppression test. JAMA 1988; 259:1699–1702Crossref, Medline, Google Scholar
78. : Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 1978; 299:926–930Crossref, Medline, Google Scholar
79. : Replication validity of genetic association studies. Nat Genet 2001; 29:306–309Crossref, Medline, Google Scholar
80. : When is pharmaco-genetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology 2009; 34:2227–2236Crossref, Medline, Google Scholar
81. : Pharmacogenetic testing in the clinical management of schizophrenia: a decision-analytic model. J Clin Psychopharmacol 2005; 25:427–434Crossref, Medline, Google Scholar
82. : Clinically responsible genetic testing in neuropsychiatric patients: a bridge too far and too soon. Am J Psychiatry 2008; 165:952–955Link, Google Scholar
83. : Cytochrome P450 genotyping and antidepressants. BMJ 2007; 334:759Crossref, Medline, Google Scholar
84. : Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006; 166:2314–2321Crossref, Medline, Google Scholar
85. : Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA 2003; 289:3145–3151Crossref, Medline, Google Scholar
86. : The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry 2001; 58:55–61Crossref, Medline, Google Scholar
87. : Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized trial. JAMA 2004; 292:935–942Crossref, Medline, Google Scholar



