The Role of Histamine Degradation Gene Polymorphisms in Moderating the Effects of Food Additives on Children's ADHD Symptoms
Abstract
Objective:
Food additives can exacerbate ADHD symptoms and cause non-immunoglobulin E-dependent histamine release from circulating basophils. However, children vary in the extent to which their ADHD symptoms are exacerbated by the ingestion of food additives. The authors hypothesized that genetic polymorphisms affecting histamine degradation would explain the diversity of responses to additives.
Method:
In a double-blind, placebo-controlled crossover trial, challenges involving two food color additive and sodium benzoate (preservative) mixtures in a fruit drink were administered to a general community sample of 3-year-old children (N = 153) and 8/9-year-old children (N = 144). An aggregate ADHD symptom measure (based on teacher and parent blind ratings of behavior, blind direct observation of behavior in the classroom, and—for 8/9-year-old children only—a computerized measure of attention) was the main outcome variable.
Results:
The adverse effect of food additives on ADHD symptoms was moderated by histamine degradation gene polymorphisms HNMT T939C and HNMT Thr105Ile in 3- and 8/9-year-old children and by a DAT1 polymorphism (short versus long) in 8/9-year-old children only. There was no evidence that polymorphisms in catecholamine genes COMT Val108Met, ADRA2A C1291G, and DRD4-rs7403703 moderated the effect on ADHD symptoms.
Conclusions:
Histamine may mediate the effects of food additives on ADHD symptoms, and variations in genes influencing the action of histamine may explain the inconsistency between previous studies. Genes influencing a range of neurotransmitter systems and their interplay with environmental factors, such as diet, need to be examined to understand genetic influences on ADHD symptoms.
Attention deficit hyperactivity disorder (ADHD) in children is characterized by symptoms of inattention, impulsivity, and overactivity. There are marked individual differences in these behaviors in the general population. A diagnosis of ADHD is usually reserved for those children with severe symptoms and a pervasive pattern of behavior from a young age that impairs other aspects of functioning, such as educational attainment (1).
Genetic factors are a major contributor to these individual differences in ADHD symptoms (2). A number of genes contributing to this effect have been identified, especially those influencing the dopamine system (e.g., dopamine D4 receptor [DRD4] and dopamine transporter [DAT1] genes). Other genes in the serotonin and noradrenergic neurotransmitter systems have also been implicated (2). However, the size of the effects identified are such that they account for only a small portion of the genetic risk suggested by quantitative genetic analyses. To date, five genome-wide association studies on ADHD have failed to identify other major genes of significant effect (3). The full effects of these genes may only become apparent when their association with environmental and experiential influences is taken into account.
Variations in ADHD symptoms have also been attributed to environmental effects (4–6) and a wide range of adverse experiential factors that involve CNS damage (7). Artificial food additives are among putative environmental toxins adversely affecting the CNS that are postulated to place children at risk for ADHD (8–10). A meta-analysis of double-blind, placebo-controlled studies has indicated that for children with ADHD, artificial food colors have a significantly adverse impact (11). There is less clear evidence for the adverse effect of such additives on behavior in children in the general population, and no studies have specifically attempted to identify whether there is a subgroup of children in the population vulnerable to their effects. An adverse effect of food additives in 3-year-old children in the general population has been shown in double-blind controlled challenges (12). The study on which this article is based has replicated these findings on 3-year-old children and extended them to 8/9-year-old children (13).
It is uncertain which mechanisms might mediate these effects or which factors may make children more or less susceptible. Histamine is an interesting candidate neurotransmitter system for a number of reasons. The activity of central histamine H3 receptors have been shown to affect inhibition learning, to increase hyperactivity levels in mouse models, and to promote dopamine release in the frontal cortex (14). There is evidence that histamine might mediate the effects of artificial food colors on ADHD symptoms. Azo dyes have been shown to provoke urticaria in some individuals, independent of whether or not they are aspirin sensitive, providing clinical evidence that artificial colors may result in histamine release (15–17). It has been proposed that the effect of food additives is likely to be a nonspecific pharmacological effect that would be similar in children irrespective of their atopic status or other characteristics (12, 18). For these reasons histamine release and its effects on the CNS may play a crucial role in mediating the effects of artificial food colors on ADHD symptoms.
The present study examines whether the effect of food additives on ADHD symptoms is moderated by genetic differences between children. Genetic polymorphisms were selected from the dopamine (dopamine transporter [DAT1] and dopamine D4 receptor [DRD4]) and catechol O-methyltransferase (COMT) genes and adrenergic neurotransmitter systems (adrenergic receptor alpha 2A [ADRA2A]), since these have previously been implicated in ADHD (2, 19, 20). Since there is a suggestion that histamine may be involved in the effects of food additives, genetic polymorphisms from this system were also included (histamine N-methyl transferase [HNMT]) to address this hypothesis.
The first single nucleotide polymorphism (SNP) of HNMT, Thr105Ile (rs1801105), is the only nonsynonymous polymorphism identified in the gene in the Caucasian population. It is associated with reduced thermal stability and decreased activity of HNMT (21, 22). HNMT T939C (rs1050891) is in the 31 untranslated region of the gene and correlates with HNMT activity, although given the strong linkage disequilibrium between the Thr105Ile SNP and the T939C SNP, it is not possible to distinguish the effects of one independent of the other (21). The strong linkage disequilibrium between these two SNPs is confirmed by analysis of the HapMap phase III data for the Caucasian population, which shows the existence of a strong linkage disequilibrium block across the 3′ end of the HNMT gene. However it has been established that the C939T/ rs1050891 polymorphism (also referred to as 939A<G) does appear to have an independent effect on HNMT activity. Using in vitro mRNA stability assays, Kim et al. (23) showed that the C allele correlated with increased stability of transcripts containing the HNMT 3′ untranslated region and consequently increased enzyme activity. In addition, in vivo studies of HNMT activity in patients with aspirin-intolerant urticaria also demonstrated an increase in HNMT activity and decreased histamine release in basophils of patients carrying one or two copies of the C allele. However, the possibility that this reflected linkage disequilibrium between this SNP and the Thr105Ile SNP was not controlled for.
Given the difficulties in accurately genotyping large variable number tandem repeat polymorphisms using DNA from cheek cells (the only means of access to DNA for general population samples of children) we used a SNPs-based strategy. Consequently, results are presented here for two SNPs in HNMT (Thr105Ile [rs1801105] and T939C [rs1050891]), one SNP in COMT (Val108Met [rs4680]), one SNP in DRD4 (rs740373), and one SNP in ADRA2A -1291<G (ADRA2A C1291G [rs1800544]). It was also possible to genotype one variable number tandem repeat: the short (9 or less repeats) and long (10 repeats or more) forms of DAT1. Extensive validation was undertaken to assess the feasibility of genotyping the DRD4 repeat. However this revealed that there was bias in favor of amplification of shorter alleles. Therefore the SNP rs740373 was genotyped instead, since a significant association of the C allele with ADHD (p = 0.008) has been reported (24) and there is evidence for linkage disequilibrium between this SNP with the exon III polymorphism (p = 0.013) (25).
Method
The study was registered as a clinical trial with Current Controlled Trials (Registration number ISRCTN74481308) and was approved by the Local Research Ethics Committee (Ref. No. 04/Q1702/61). After providing full information about the study and its dietary implications, written informed consent was obtained from the parents. Each participating early years setting received £250 and each school £500 as a contribution toward school funds for the benefit of all children.
Participants
Details of recruitment and participation in the study are provided in figures S1 and S2 in the data supplement that accompanies the online version of this article. The sample of 3-year-old children was drawn from a population of preschool children aged between 3 years and 4 years 2 months registered in early years settings (nurseries, day nurseries, preschools, playgroups) within the Southampton City Council area. The older sample was drawn from children between 8 and 9 years of age attending schools within the city of Southampton.
Parents who returned an expression of interest form were contacted by phone and a convenient time for a home visit was arranged. The study dietician also obtained a report based on 24-hour recall by the parent of the child's prestudy diet, which allowed an assessment of baseline levels of the number of foods containing additives consumed by the child in the previous 24-hour period.
Of the 3-year-old children enlisted in the study (N = 153), 16 (10.5%) failed to complete. In only one case was this related to problems with the child's behavior. Of the 8/9-year-old children enlisted in the study (N = 144), 14 (9.7%) failed to complete. In no case was this related to problems with the child's behavior. For both samples, age and gender of the child and marital status of the parents had no effect on study completion, and children were no more likely to drop out during active as compared to placebo challenge weeks.
Study Design and Challenge Protocols
Children were entered into this randomized, double-blind, placebo-controlled food challenge with a within-subject crossover. There were two active mixes of additives. Mix A contained sunset yellow, carmoisine, tartrazine, and ponceau 4R; mix B contained sunset yellow, carmoisine, quinoline yellow, and allura red AC. In addition, both additive mixes incorporated sodium benzoate, which had been included in the challenge in our earlier study (12) and in previous studies (10, 26). After a week on their normal diet, the target food colors and sodium benzoate were excluded from their diet over a 6-week period when challenge and withdrawal occurred as follows: week 0: baseline/normal diet; week 1: withdrawal period but receiving placebo; weeks 2, 4, and 6: challenge with randomization to two active and one placebo period; weeks 3 and 5: washout continuing on placebo. Full details of the challenges have been published previously (13) and are also given in supplementary data. We set a minimum of 85% of drinks consumed to constitute a per protocol population and the analyses in the present paper were restricted to these 132 3-year-old and 119 8-year-old children.
Global Hyperactivity Aggregate
For the 3-year-old children, three measures of behavior were used to calculate the global hyperactivity aggregate. The first was the Abbreviated ADHD Rating Scale—IV (Teacher version) (27, 28). A total score was obtained for 10 of the 18 items (inattentive = 5, hyperactive = 5) in this questionnaire, which was completed by the early year setting practitioner who described the frequency of the specific behaviors displayed over the past week for each week of the study. The second was a parent behavior measure: Abbreviated Weiss-Werry-Peters hyperactivity scale (29) .This scale has been used in a number of studies to assess ADHD symptoms (30, 31). Interparent agreement is good (r = 0.82) (32). Parents rated their child's behavior over the previous week for 7 items previously used (12) and a total score was obtained. The third measure was the Classroom Observation Code (33). This instrument assesses the occurrence of 12 mutually exclusive behaviors during structured didactic teaching and during periods of independent work under teacher supervision. The interrater reliability of the method, tested prior to and in mid-study, exceeded 0.87. Each child was observed for a total duration of 24 minutes each week (three observation sessions each of 8 minutes duration) and a total weekly mean score was derived from the total score over each session. The code was slightly modified for use in the present study, since preschool children in the U.K. have relatively little structured or didactic teaching sessions and tend to engage in “activities” rather than “tasks.”
For the 8/9-year-old children, four measures of behavior were used to calculate the global hyperactivity aggregate. Two were the same as for the 3-year-old children: the Abbreviated ADHD Rating Scale—IV (Teacher version) and the Classroom Observation Code. Parental ratings of behavior were obtained using a parent version of the Abbreviated ADHD Rating Scale—IV (unpublished 1994 instrument, G.J. DuPaul et al.), which has the same format as the teacher version. The final component was Connors' Continuous Performance Test II (34). This is a test using visual stimuli of 14 minutes duration and is widely used to evaluate attention and the response inhibition component of executive control. Four measures (standard error of reaction time, percentage of commission errors, d′, and β) were used to derive a weekly Continuous Performance Test II aggregate score. This subset of indicators has been shown to be highly correlated with the ADHD Rating Scale employed in this study (35).
Statistical Analysis
The weekly scores from the teacher, parent, Classroom Observation Code, and, for 8/9-year-old children only, the Continuous Performance Test II measures for each child were standardized to time 0 at baseline [T0]) for the same measure:
Weekly standardized (z) aggregate score=

The primary outcome measure, the global hyperactivity aggregate, is an unweighted aggregate of the weekly teacher, parent, Classroom Observation Code, and Continuous Performance Test II z scores. This was calculated only when at least two (for 3-year-old children) or three (for 8/9-year-old children) of these component behavior scores were present for any week, one of which had to be the Classroom Observation Code score. A high global hyperactivity aggregate indicates more ADHD symptoms. Preliminary analyses using a Kolmogorov-Smirnov one-sample test showed that these aggregate scores and the difference between the scores under placebo and active mix challenges were normally distributed.
Linear mixed-model methods (36, 37), with a compound symmetry covariance matrix for 3-year-old children and unstructured covariance matrix for 8/9-year-old children (the best fit models for each age group), were used to analyze data. The study was powered to detect differences between the active and placebo periods so that in each case the effects of mix A and mix B can be compared to the effect of placebo. With a sample of 120 children there was 80% power at α = 0.05 to identify an effect size of 0.32, i.e., the magnitude of the difference in mean score changes (in SD units), a slightly smaller effect size to that found previously (12).
Genotyping
In the course of the study, buccal swabs were collected from the children for genotype analysis. These samples were refrigerated and packed in ice to be sent to the laboratory for DNA extraction and genotyping. The genotyping of the HNMT Thr105Ile (rs1801105), HNMT T939C (rs1050891), COMT Val108Met (rs4680), ADRA2A C1291G (rs1800544), and DRD4 (rs740373) SNPs was performed by Kbiosciences (Herts, U.K.) using a KASPar assay system (http://www.kbioscience.co.uk).
The variable number tandem repeat analysis of DAT1 was performed in our laboratory using a total volume of 10 μl, including 20 ng DNA template, 2 μl 5X Herculase II buffer containing 2 mM MgCl2, 0.5, mol/liter of each primer, 0.1 l of Herculase II DNA polymerase (Stratagene), 8% DMSO, 1 M Betaine, and 250μlmol/liter of each deoxynucleotide triphosphates. The polymerase chain reaction conditions were 3 minutes at 98°C, followed by 35 cycles each of 20 seconds at 98°C, 20 seconds at 65°C, 30 seconds at 72°C, and finally 3 minutes at 72°C in 96-well plates. DAT1 primer sequences were DAT1f 5′ - GCC ACT CAG GCA GCC TGT G-3 and DAT1r 5′-6FAM- AGG ACC CTC ATG GCC TTG-3′. Amplified fragments were sized using capillary electrophoresis on a CEQ8800 (Beckman Coulter, UK). Genotype data were assessed for concordance with the Hardy—Weinberg equilibrium law using a χ2 test with one degree of freedom. All genotypes were in were in Hardy—Weinberg equilibrium.
Results
There was evidence that the HNMTT939C and the DRD4 rs740373 polymorphisms were related to the overall level of the global hyperactivity aggregate at baseline in the 3-year-old children (Table 1). There were no significant effects of any of the polymorphisms on the overall global hyperactivity aggregate level at baseline in the 8/9-year-old children.
| Group and Genotype | SNP Present | SNP Absent | Main Effect of Genotype | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | t | df | p | |
| 3-year-old children | |||||||||
| HNMT Thr105Ile (T allele) | 30 | 0.09 | 0.66 | 74 | −0.17 | 0.64 | 1.83 | 102 | 0.07 |
| HNMT T939C (C allele) | 39 | 0.11 | 0.61 | 60 | −0.21 | 0.67 | 2.36 | 99 | 0.02 |
| COMT Val108Met (A allele) | 76 | −0.13 | 0.64 | 27 | 0.03 | 0.70 | 1.09 | 101 | 0.28 |
| ADRA2A C1291G (G allele) | 52 | −0.14 | 0.72 | 50 | −0.06 | 0.60 | 0.62 | 100 | 0.54 |
| DAT1 (short allele) | 63 | −0.11 | 0.71 | 41 | −0.08 | 0.59 | 0.25 | 102 | 0.80 |
| DRD4 rs740373 (C allele) | 26 | 0.31 | 0.62 | 74 | −0.02 | 0.62 | 2.09 | 98 | 0.04 |
| 8/9-year-old children | |||||||||
| HNMT Thr105Ile (T allele) | 23 | −0.24 | 0.77 | 83 | 0.08 | 1.09 | 1.35 | 104 | 0.18 |
| HNMT T939C (C allele) | 44 | 0.09 | 1.17 | 63 | −0.05 | 0.91 | 0.73 | 105 | 0.47 |
| COMT Val108Met (A allele) | 79 | 0.03 | 0.94 | 28 | 0.01 | 1.26 | 0.04 | 105 | 0.99 |
| ADRA2A C1291G (G allele) | 47 | −0.04 | 1.11 | 59 | 0.06 | 0.97 | 0.51 | 104 | 0.61 |
| DAT1 (short allele) | 75 | 0.03 | 1.10 | 32 | −0.04 | 0.86 | 0.31 | 105 | 0.76 |
| DRD4 rs740373 (C allele) | 39 | 0.00 | 0.76 | 0.67 | −0.02 | 1.13 | 0.10 | 104 | 0.92 |
TABLE 1. Association of Genotype and Global Hyperactivity Aggregate at Baseline for 3- and 8/9-Year-Old Children Before Consumption of Challenge Drinks Containing Food Additive Mixtures and Sodium Benzoate
The results of the general linear mixed model analysis are summarized in Table 2 for both the 3- and 8/9-year-old children. We have previously shown that there were significant effects of challenge type on global hyperactivity aggregate (13). Here, interest centers on the interaction between challenge type and genotype. These interaction effect estimates in Table 2 are based on the difference in global hyperactivity aggregate score while challenged by an additive mix and by a placebo and specifically whether such an effect is different for the two genotypes for each gene (i.e., it estimates the magnitude of the difference of two differences and the associated 95% CI). If the latter include zero then the interaction is not significant at the 5% level. The interpretation of the interactions can be determined from the mean estimated marginal global hyperactivity aggregate values presented in Figure 1 (3-year-old children) and Figure 2 (8/9-year-old children). To test the effect of the genotype-by-challenge interactions, a number of other factors that may be related to overall the global hyperactivity aggregate needed to be taken into account in the mixed models analysis and were slightly different for the two age groups. In total, these included baseline global hyperactivity aggregate scores, age, gender, maternal education level, and prestudy diet. However, for the 3-year-old children these were gender, baseline global hyperactivity aggregate, prestudy diet, in addition to challenge and genotype. In this age group there were no significant interactions between these factors and the effects of challenge. For the 8-year-old children the factors in the analysis were week during study and baseline global hyperactivity aggregate in addition to challenge and genotype. In this age group there were no significant interactions between these factors and the effects of challenge.
| Genotype | Challenge Typeb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mix A Versus Placebo | Mix B Versus Placebo | |||||||||||
| 3-Year-Old Children | 8/9-Year-Old Children | 3-Year-Old Children | 8/9-Year-Old Children | |||||||||
| Estimate | 95% CI | p | Estimate | 95% CI | p | Estimate | 95% CI | p | Estimate | 95% CI | p | |
| HNMT Thr105Ile | −0.53 | −10.4 to −0.02 | 0.04 | −0.10 | −0.35 to 0.14 | 0.40 | −0.40 | −0.92 to 0.12 | 0.13 | −0.24 | −0.48 to 0.00 | 0.05 |
| HNMT T939C | −0.46 | −0.94 to 0.02 | 0.06 | −0.24 | −0.44 to −0.04 | 0.02 | −0.23 | −0.72 to 0.25 | 0.34 | −0.23 | −0.43 to −0.03 | 0.03 |
| COMT Val108Met | −0.23 | −0.75 to 0.29 | 0.38 | 0.02 | −0.20 to 0.24 | 0.87 | 0.12 | −0.41 to 0.64 | 0.66 | 0.02 | −0.20 to 0.24 | 0.86 |
| ADRA2A C1291G | 0.01 | −0.44 to 0.47 | 0.96 | −0.05 | −0.26 to 0.15 | 0.61 | 0.20 | −0.26 to 0.65 | 0.39 | 0.00 | −0.21 to 0.20 | 0.97 |
| DAT1 | 0.08 | −0.39 to 0.54 | 0.74 | 0.11 | −0.11 to 0.34 | 0.32 | −0.30 | −0.76 to 0.16 | 0.20 | 0.23 | 0.01 to 0.45 | 0.05 |
| DRD4 rs740373 | −0.27 | −0.82 to 0.27 | 0.33 | −0.08 | −0.29 to 0.13 | 0.44 | −0.25 | −0.80 to 0.30 | 0.37 | −0.14 | −0.34 to 0.07 | 0.19 |
TABLE 2. General Linear Mixed Model Analysis Estimates for Global Hyperactivity Aggregate for Challenge-by-Genotype Interaction in 3- and 8/9-Year-Old Children Consuming Challenge Drinks Containing Food Additive Mixtures and Sodium Benzoatea
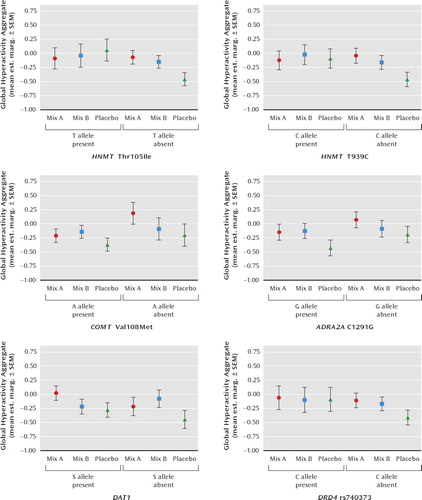
FIGURE 1. Effect of Genotype in Moderating Impact of Food Additive Challenge on Global Hyperactivity Aggregate in 3-Year-Old Childrena
a Mix A contained sunset yellow, carmoisine, tartrazine, and ponceau 4R; mix B contained sunset yellow, carmoisine, quinoline yellow, and allura red AC. Both food additive mixtures incorporated sodium benzoate. Mean est. marg.: mean estimated marginal mean when gender, baseline global hyperactivity aggregate, levels of food additives in the pre-study diet, challenge, and genotype were included in the mixed model analysis.
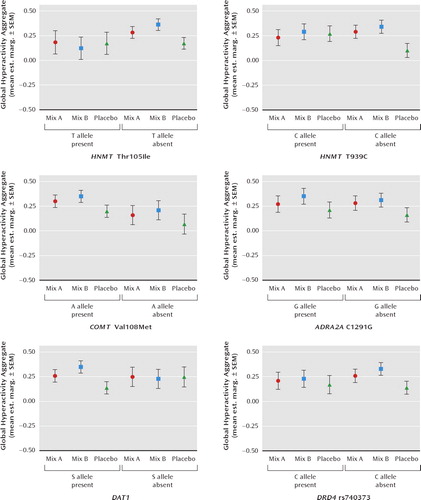
FIGURE 2. Effect of Genotype in Moderating Impact of Food Additive Challenge on Global Hyperactivity Aggregate in 8/9-Year-Old Childrena
a Mix A contained sunset yellow, carmoisine, tartrazine, and ponceau 4R; mix B contained sunset yellow, carmoisine, quinoline yellow, and allura red AC. Both food additive mixtures incorporated sodium benzoate. Mean est. marg.: mean estimated marginal mean when baseline global hyperactivity aggregate, week during study, challenge, and genotype were included in the mixed model analysis.
Considering 3-year-old children first, the estimated marginal means plotted in Figure 1 show that the effects of mix A (relative to placebo) on ADHD symptoms were limited to those children not carrying T (Ile) allele of HNMT Thr105Ile (p = 0.04) and to children not carrying the carrying the C allele of HNMT T939C, although this interaction just failed to reach significance (p = 0.06). The effects of mix B (relative to placebo) were the same regardless of the HNMT polymorphisms in this age group. The effects of mix A and mix B were not moderated in 3-year-old children by the COMT Val108Met, ADRA2A C1291G, DAT1 or DRD4 rs740373 polymorphisms.
For the 8/9-year-old children, there were no significant genotype-by-challenge interactions for COMT Val108Met, ADRA2A C1291G, or DRD4 rs740373. However, as for the 3-year-old children, interactions were present for HNMT Thr105Ile and HNMT T939C. For mix A (relative to placebo) the effects were limited to those without the C allele of HNMT T939C (p = 0.02). The effects of mix B (relative to placebo) were found only for children without the T allele of HNMT Thr105Ile (p = 0.05), the C allele for HNMT T939C (p = 0.03), and those with the short form allele of DAT1 (p = 0.05)
Discussion
These findings indicate a link between histamine and ADHD symptoms, with polymorphisms in the HNMT gene moderating behavioral responses to food additives. They suggest that the current focus on catecholamines in studies of ADHD needs to be extended to other neurotransmitters. They open up one avenue of explanation as to why the genes so far shown to be associated with ADHD explain so little of the known variance. The histamine risk alleles in this study have two actions: to influence the overall level of the global hyperactivity aggregate in the study (significantly so in the case of HNMT T939C for the younger children), and to make the child more vulnerable to the effects on behavior of food additives in the diet.
There are limitations to these findings. Although there is internal replication, in that the same pattern of results were found in both 3- and in 8/9-year-old children, there is need to replicate the HNMT and food additive interactions in additional samples. The importance of such replication in gene/environment interactions has been emphasized (38). The effects we have shown are relatively short-term in that they reflect a 1-week exposure. We cannot tell if the effects of food additives on ADHD symptoms and the moderation by HNMT polymorphisms would hold for longer-term exposures. A strength of the study is that the environmental risk of food additive exposure was experimentally manipulated and the effects shown using within-person difference in a crossover design. Moreover, the gene/environment interaction was found for polymorphisms in a gene that had been postulated to be involved rather than on the basis of wider genome search. These two features of the design of the study were recently recommended as ways to promote the study of gene-environment interactions using hypothesis-based molecular genetic studies with candidate genes (39).
The role of genes in influencing behavior needs to be understood not by just their main effects of raising levels, for example, of ADHD symptoms but also by the interplay both with each other in gene-gene interactions, and also by interactions with environmental factors such as diet (40). HNMT polymorphisms impair histamine clearance (21) and a food additive challenge causes histamine release (17), and therefore the interaction found here is neurobiologically plausible. The presence of H3 receptors in the brain provides a potential mechanistic explanation for the effect (41). Moreover it has become apparent that both methylphenidate and atomoxetine, widely used treatments for ADHD, may have effects mediated via the histamine system (42, 43). Many environmental factors will increase histamine release including many food items as well as infections. This would explain the frequent claim that food allergy/intolerance is a cause of ADHD symptoms and the effects of infections in aggravating aberrant behavior (44). This clearly supports a potential target for therapeutic intervention in ADHD focused on the H3 receptor (45, 46).
1. : Annotation: principles of treatment for hyperkinetic disorder: practice approaches for the UK. J Child Psychol Psychiatry 1999; 40:1147–1157Crossref, Medline, Google Scholar
2. : Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 2009; 126: 51–90Crossref, Medline, Google Scholar
3. : Genome-wide association studies in ADHD. Hum Genet 2009; 126:13–50Crossref, Medline, Google Scholar
4. : Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002; 288:728–737Crossref, Medline, Google Scholar
5. ; Romanian Adoptees Study Team: Can inattentive/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol 2001; 29:513–528Crossref, Medline, Google Scholar
6. : Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry 2006; 45:1338–1345Crossref, Medline, Google Scholar
7. : Developmental neuropsychopathology of attention deficit and impulsiveness. Dev Psychopathol 1999; 11:607–628Crossref, Medline, Google Scholar
8. : Dietary replacement in preschool-aged hyperactive boys. Pediatrics 1989; 83: 7–17Medline, Google Scholar
9. : A randomised controlled trial into the effects of food on ADHD. Eur Child Ado-lesc Psychiatry 2009; 18:12–19Crossref, Medline, Google Scholar
10. : Effects of a few food diet in attention-deficit disorder. Arch Dis Child 1993; 69:564–568Crossref, Medline, Google Scholar
11. : Do artificial food colours promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr 2004; 25:423–434Crossref, Medline, Google Scholar
12. : The effects of a double blind, placebo controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Arch Dis Child 2004; 89:506–511Crossref, Medline, Google Scholar
13. : Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 2007; 370:1560–1567Crossref, Medline, Google Scholar
14. : Pharmacological properties of ABT-239[4-(2-{2-[(2R)-2-methylpyrrolidinyl]ethyl} benzofuran-5-yl)benzonitrile], II: Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H-3 receptor antagonist. J Pharmacol Exp Ther 2005; 313:176–190Crossref, Medline, Google Scholar
15. : Artificial food additive intolerance in patients with angio-oedema and urticaria. Lancet 1986; 2:907–909Crossref, Medline, Google Scholar
16. : Food additive induced urticaria: studies of mediator release during provocation tests. J R Coll Physicians Lond 1987; 21:262–266Medline, Google Scholar
17. : The effects of food additives on leukocyte histamine release in normal and urticarial subjects. J R Coll Physicians Lond 1987; 21:251–256Medline, Google Scholar
18. : Effect of artificial food colours on childhood behaviour. Arch Dis Child 1990; 65:74–77Crossref, Medline, Google Scholar
19. : Dopamine vs noradrenaline: inverted-U effects and ADHD theories. Aust N Z J Psychiatry 2009; 43:101–108Crossref, Medline, Google Scholar
20. : COMT Val108/158Met polymor-phism and the modulation of task-oriented behaviour in children with ADHD. Neuropsychopharmacology 2008; 33:3069–3077Crossref, Medline, Google Scholar
21. : Human histamine N-methyltransferase phar-macogenetics: common genetic polymorphisms that alter activity. Mol Pharmacol 1998; 53:708–717Crossref, Medline, Google Scholar
22. : The histamine N-methyltransferase T105I polymorphism affects active site structure and dynamics. Biochemistry 2008; 47:893–901Crossref, Medline, Google Scholar
23. : Histamine N-methyltransferase 939A>G polymorphism affects mRNA stability in patients with acetylsalicylic acid-intolerant chronic urticaria. Allergy 2009; 64:213–221Crossref, Medline, Google Scholar
24. : Multiple marker analysis at the promoter region of the DRD4 gene and ADHD: evidence of linkage and association with the SNP -616. Am J Med Genet B Neuropsychiatr Genet 2004; 131B:33–37Crossref, Medline, Google Scholar
25. : 5′-untranslated region of the dopamine D4 receptor gene and atten-tion-deficit hyperactivity disorder. Am J Med Genet 2001; 105:84–90Crossref, Medline, Google Scholar
26. : Controlled trial of oligoantigenic treatment in the hyperkinetic syndrome. Lancet 1985; 1:540–545Crossref, Medline, Google Scholar
27. : AD/HD Rating Scale IV: Checklists, Norms and Clinical Interpretation. New York, Guilford, 1998Google Scholar
28. : Teacher ratings of ADHD symptoms: factor structure and normative data. Psychol As-sess 1997; 9:436–444Crossref, Google Scholar
29. : Hyperactivity, in Psychological Management of Pediatric Problems. Edited by Magrab P. Baltimore, University Park Press, 1978, pp 3–8Google Scholar
30. : The mental health of preschool children and their mothers in a mixed ur-ban/rural population: prevalence and ecological factors. Br J Psychiatry 1996; 168:16–20Crossref, Medline, Google Scholar
31. : Linking self-reported childhood behavioural inhibition to adolescent social phobia. J Am Acad Child Adolesc Psychiatry 1998; 37:1308–1316Crossref, Medline, Google Scholar
32. : Parental perceptions of child behaviour problems, parenting self-esteem and mother's reported stress in younger and older hyperactive and normal children. J Consult Clin Psychol 1983; 51:86–99Crossref, Medline, Google Scholar
33. Classroom observation code - a modification of the Stony-Brook code. Psychopharmacol Bull 1985; 21:901–909Google Scholar
34. : The Conners Continuous Performance Test. Toronto, Multi-Health Systems, 1994Google Scholar
35. : Relations between continuous performance test performance measures and ADHD behav-iors. J Abnorm Child Psychol 2003; 31:543–554Crossref, Medline, Google Scholar
36. : Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of Gen-eral Psychiatry. Arch Gen Psychiatry 2004; 61:310–317Crossref, Medline, Google Scholar
37. : Choice of the primary analysis in longitudinal clinical trials. Pharm Stat 2004; 3:161–169Crossref, Google Scholar
38. : Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 2009; 301:2462–2471Crossref, Medline, Google Scholar
39. : Gene-environment-wide interaction studies in psychiatry. Am J Psychiatry 2009; 166:964–966Link, Google Scholar
40. : Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry 2006; 47:226–261Crossref, Medline, Google Scholar
41. : Evidence for the presence of histamine uptake into the synaptosomes of rat brain. Pharmacology 2006; 78:72–80Crossref, Medline, Google Scholar
42. : Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. Eur J Pharmacol 2007; 558:96–97Crossref, Medline, Google Scholar
43. : Atomoxetine increases histamine release and improves learning deficits in an animal model of atten-tion-deficit hyperactivity disorder: the spontaneously hypertensive rat. Basic Clin Pharmacol Toxicol 2008; 102:527–532Crossref, Medline, Google Scholar
44. : ADHD as a (non) allergic hypersensitivity disorder: a hypothesis. Pediatr Allergy Immunol 2009; 20:107–112Crossref, Medline, Google Scholar
45. : The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov Today 2009; 14:509–515Crossref, Medline, Google Scholar
46. : The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol 2008; 154:1166–1181Crossref, Medline, Google Scholar



