Sibling Recurrence and the Genetic Epidemiology of Autism
Abstract
Objective:
Although the symptoms of autism exhibit quantitative distributions in nature, estimates of recurrence risk in families have never previously considered or incorporated quantitative characterization of the autistic phenotype among siblings.
Method:
The authors report the results of quantitative characterization of 2,920 children from 1,235 families participating in a national volunteer register, with at least one child clinically affected by an autism spectrum disorder and at least one full biological sibling.
Results:
A traditionally defined autism spectrum disorder in an additional child occurred in 10.9% of the families. An additional 20% of nonautism-affected siblings had a history of language delay, one-half of whom exhibited autistic qualities of speech. Quantitative characterization using the Social Responsiveness Scale supported previously reported aggregation of a wide range of subclinical (quantitative) autistic traits among otherwise unaffected children in multiple-incidence families and a relative absence of quantitative autistic traits among siblings in single-incidence families. Girls whose standardized severity ratings fell above a first percentile severity threshold (relative to the general population distribution) were significantly less likely to have elicited community diagnoses than their male counterparts.
Conclusions:
These data suggest that, depending on how it is defined, sibling recurrence in autism spectrum disorder may exceed previously published estimates and varies as a function of family type. The results support differences in mechanisms of genetic transmission between simplex and multiplex autism and advance current understanding of the genetic epidemiology of autism spectrum conditions.
Aside from its clinical importance in genetic counseling, the characterization of sibling recurrence is pivotal in the elucidation of mechanisms of inheritance for any genetically influenced condition. Categorical estimates of recurrence risk have previously indicated that the siblings of probands with autistic disorder have a 22-fold relative risk of developing the disorder (1). However, recent discoveries in the field of autism research have suggested that there exists a diversity of genetic mechanisms that give rise to the autistic syndrome (2), that each mechanism is associated with its own pattern of intergenerational transmission, and that autistic symptoms exhibit a wide, continuous distribution both in the general population and among clinically ascertained cases (3–5). An additional complexity of the quantitative variation of autistic symptoms is that when considering samples largely comprising sporadic (nonfamilial) cases of autism, there appear to exist—within the families—separable, discrete populations of affected and unaffected children whose respective severity distributions partially overlap (6, 7). Thus, a reexamination of the phenomenon of recurrence accounting for these developments is warranted.
For the 10%–20% of all autism cases whose origins are attributable to known genetic causes, there is an emerging understanding of how specific molecular mechanisms of transmission might map to a given pattern of recurrence in families. For example, large, de novo chromosomal rearrangements (mutations of typically major effect) have been observed in some 10% of children with autism (8, 9), compared with substantially lower rates in the general population. Common allelic variations of small but statistically significant effect have been associated with incremental increases in susceptibility to autism, primarily among multiple-incidence autism families (10–12). Rare mutations in a number of synapse-related genes, singly or in combination (13), have also been associated with a diverse array of full and intermediate autism phenotypes.
It is with this background that the present clinico-epidemiologic family study attempts to advance understanding of the relative proportions of autism cases in the population that might be attributable to these various mechanisms of genetic transmission, recognizing that the vast majority of cases of autism currently remain idiopathic. This study has the following two primary objectives: 1) to derive an updated estimate of recurrence risk in a large, volunteer registry of autism-affected families in which the children were both categorically and quantitatively characterized and 2) to explore the distributions of quantitative (subclinical) autistic traits in families with and without categorically defined recurrence. Additionally, the study presents the opportunity to consider (in a large sample) whether any aggregation of language delays in autism-unaffected children might constitute an additional type of recurrence among siblings in some families. Lindgren et al. (14) recently summarized the existing literature on the aggregation of language impairment in first-degree relatives of children with autism spectrum disorder and reported additional data on 52 families. Their findings generally supported estimates of 20%–25% from prior studies of comparable sample size (15, 16), with a greater predominance of pragmatic versus structural language deficits.
Method
Sample
This study is based on data obtained from the Interactive Autism Network, a national, Internet-based, voluntary autism family register (http://ianproject.org/). Parents who enroll their families complete standardized questionnaires about their autism-affected children and the biological siblings of those children. Families of any U.S. children <18 years old who are diagnosed with an autism spectrum disorder by a professional are eligible to be enrolled in the network's research database by a willing English speaking parent or legal guardian. Autism spectrum disorder includes all conditions encompassed within the current epidemiologic surveillance protocols for autism spectrum conditions maintained by the U.S. Centers for Disease Control, for which current U.S. prevalence is estimated at nine per 1,000 individuals (17).
Approximately 9 months after the registry was initially launched (2007), parents (one per family, usually the mother) were asked to provide quantitative characterizations of autistic symptoms in each of the 4- to 18-year-old children in their families using the parent-report version of the Social Responsiveness Scale. The present report encompasses those families who had an autism-affected child with at least one full biological (nonidentical) sibling, in the age range from 4 to 18 years and for whom the Social Responsiveness Scale was completed. A total of 1,235 families met these inclusion criteria. We note that although it was not possible to specifically cross-identify these subjects with those in the Autism Genetic Resource Exchange registry, who comprised the multiplex subjects of our earlier report (7), parents in the Interactive Autism Network were asked whether their children had ever been in a research study about the genetics of autism, and 14.4% of families endorsed this question affirmatively. The Autism Genetic Resource Exchange exclusively enrolled multiple-incidence families; therefore, a conservative upper limit for the proportion of families in the present report who coparticipated in our prior study is 2%.
As part of the Interactive Autism Network registration protocol for all families, each reporting parent indicated specifically whether each child in the family was or was not affected by an autism spectrum disorder diagnosed by a clinician or educational professional in the community. We refer to this as categorical designation of an autism spectrum disorder diagnosis. Among the families included in the present study, 71% of the reported autism spectrum disorder diagnoses were made by individual doctoral-level professionals, 25% by a team in a health or school system, and 4% were unspecified. In addition, it was reported that 68% of the children designated by their parents as affected had previously undergone standardized assessment using the Autism Diagnostic Observation Schedule, the Autism Diagnostic Interview-Revised, or both. Among these, 98.5% were scored as autism-affected by one or both instruments, according to parents' retrospective reports. Furthermore, in a recent study of verbal children with a history of autism diagnosis randomly ascertained from the Interactive Autism Network registry and scoring >12 on the Social Communication Questionnaire, 98% were confirmed to have a clinical autism spectrum disorder by the Autism Diagnostic Interview-Revised, by expert clinical observation, or both (18).
By parent report, 134 of the 1,235 families (10.9%) had more than one child affected by an autism spectrum disorder. In addition, however, among all of the presumed-unaffected children in the sample, 20% were reported by their parents to have a history of a diagnosis of language delay or speech problems, which is at least double the reported prevalence in the general population, especially when considering community diagnosis (19, 20). This observation prompted an attempt to more carefully characterize language impairment in the sample in order to identify subsets of language-delayed subjects who might account for the observed excess in language impairment prevalence. Selected sample characteristics are presented in Table 1. A caveat is that the Interactive Autism Network registry is overrepresentative of Caucasian families (92.7%).
| Offspring Group | N | Age (Years) | Gender | Social Responsiveness Scale T Score | Social Communication Questionnaire Score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Male | Female | Mean | SD | Mean | SD | ||
| First-affected childa | 1,235 | 9.2 | 3.4 | 1,052 | 183 | 86.3 | 15.2 | 23.2 | 7.2 |
| Subsequent-affected sibling | 138 | 8.3 | 3.2 | 100 | 38 | 85.8 | 17.2 | 22.2 | 7.9 |
| Autism spectrum disorder-unaffected sibling with a history of language delay with autistic speechb | 150 | 8.6 | 3.4 | 80 | 70 | 55.9 | 15.3 | 6.9 | 6.0 |
| Unaffected sibling | 1,397 | 9.7 | 3.8 | 651 | 746 | 45.0 | 10.4 | 2.6 | 4.2 |
TABLE 1. Selected Sample Characteristicsa
Measures
Categorical designation of affected status.
Categorical designation of affected status was provided by the parent and supported by prior clinical diagnosis. The Interactive Autism Network data set also includes parent-reported data derived from the Social Communication Questionnaire, a developmental history checklist that ascertains whether a child has ever manifested the presence of categorically defined symptoms in fulfillment of DSM-IV criteria for autistic disorder (21). A total symptom score of 15 has been used as a clinical cutoff for affected status in previous research (22).
Characterization of language disorder excess among autism spectrum disorder-unaffected children.
Parent-reported data regarding each child's history of communicative development were retrievable from the Interactive Autism Network by the ratings on the Social Communication Questionnaire, in which a key item set (corresponding to symptoms in fulfillment of the communication criterion domain for a DSMIV diagnosis of autistic disorder) ascertains whether a child has historically exhibited pathognomonically autistic qualities of speech, including the use of odd or repetitive phrases, socially inappropriate questions, pronoun reversal, or invented words (language items in other sections of the questionnaire were not used for this purpose, since they can be interpreted in ways that are less specific to autistic impairment). In this sample, positive endorsement of any of these characteristics 1) was significantly more pronounced in autism-unaffected children with versus without parent-reported history of language delay (χ2=36.7, df=1, p<0.001); 2) was associated with a significantly higher level of subclinical autistic social impairment in unaffected children with versus without these characteristics (mean standardized Social Responsiveness Scale score, excluding language items: 49.7 versus 42.2; t=-12.1, df=722, p<0.0001); and 3) occurred in 54% of children with a history of language delay. For this reason, single-incidence families with one or more unaffected children with a history of diagnosed language delay, plus the aforementioned distinct autistic features of speech, were considered separately in a subset of the analyses, and the children who met this criterion were referred to as having “a history of language delay with autistic speech.” We note that neither quantitative IQ scores nor the timing of acquisition of language milestones was available for the majority of subjects in our sample.
Quantitative characterization of the autistic phenotype.
Affected and unaffected children in each family were assessed by a parent using the Social Responsiveness Scale. This scale is an extensively validated (23–27), 65-item questionnaire that capitalizes on observations of children in their naturalistic social contexts, quantitatively measures severity of autistic traits and symptoms, and distinguishes autism spectrum disorder from other psychiatric conditions (27). Norms have been published by gender and rater type (parent versus teacher) in order to standardize ratings that otherwise differ as a function of these variables. Scores are highly heritable (3), are stable over time (23), exhibit high interrater reliability (26), are continuously distributed in the general population (3), are nonsignificantly correlated with IQ among children representing the normal range of IQ in the general population (26), and exhibit a unitary factor structure (25), which supports the use of a single index score as a quantitative measure of autistic severity. T scores >75 (98.8th percentile) indicate a level of autistic social impairment that is generally highly clinically significant. In the Interactive Autism Network sample, the proportion of children at or above the cutoff score of 75 was highly similar (41.9%) to the proportion of children at or above the Social Communication Questionnaire cutoff score of 15 (43.6%). Pearson's coefficient of correlation between the total score on the Social Responsiveness Scale and total score on the Social Communication Questionnaire was 0.88 in the entire sample and 0.60 when considering affected children only.
Data Analysis
We first segregated the sample on the basis of whether or not the firstborn autism-affected child in each family was verbal versus nonverbal (or had a parent-reported full-scale IQ <56). Prior studies have suggested possible differences in recurrence risk among families whose autism-affected children exhibit intellectual disability and dysmorphism (28). In our sample, there was no significant difference in the risk of autism spectrum disorder in later-born siblings as a function of nonverbal status (verbal proband: 14.1% versus nonverbal proband 14.0%), and thus in order to optimize the statistical power of the sample, we did not retain this segregation in the analyses presented in this report. Next, we computed recurrence statistics considering the various indices derived from the measures and compared them using chi-square statistics. We subsequently tested a quantitative approach to the prospective prediction of sibling recurrence using standard regression methods, in which the index case was defined as the first (oldest) affected child in the family, and we considered the outcome of later-born siblings (one per family, chosen at random). Finally, using analysis of variance methods, we compared the distributions of quantitative scores on the Social Responsiveness Scale across the following three mutually exclusive groups of families: 1) those families in which more than one child was categorically affected (referred to as multiple-incidence families); 2) those families in which only a single child was affected by a categorical autism spectrum disorder but at least one additional child exhibited a history of language delay with autistic speech; and 3) single-incidence families in which no child other than the index case had either an autism spectrum disorder or a history of language delay with autistic speech.
Results
Recurrence rates—conservatively operationalized as the occurrence of an autistic syndrome in one or more siblings of an index case, using as a denominator all additional children in the family (whether earlier- or later-born)—are presented in Table 2 as a function of recurrence definition. We note that use of standardized quantitative definitions of recurrence resulted in a pronounced shift in the gender ratio for recurrence events. Categorical autism spectrum disorder status in an additional child occurred in 10.9% of the families (8.2% of the individual children in the entire sibling pool). An additional 20% of presumed-unaffected siblings had a history of language delay, and of those, 54% exhibited autistic qualities of speech ascertained by the Social Communication Questionnaire, resulting in a total of 8.9% exhibiting a history of language delay with autistic speech.
| Recurrence Definition | Number of Male Siblings Affected Per Definition | Number of Female Siblings Affected Per Definition | Proportion (%) of Families With Additional Affected Siblings | Proportion (%) of All Siblings in Affected Families |
|---|---|---|---|---|
| Single criterion | ||||
| Categorical autism spectrum disorder diagnosis | 100 | 38b | 10.9c | 8.2 |
| Social Communication Questionnaire score ≥15 | 108 | 53 | 12.4 | 9.6 |
| Social Responsiveness Scale T score ≥75 | 99 | 64b | 12.5 | 9.7 |
| History of language delay with autistic speech | 80 | 70 | 10.8 | 8.9 |
| Category change when switching from categorical to quantitative threshold | ||||
| No autism spectrum disorder diagnosis but Social Communication Questionnaire score ≥15 | 27 | 22 | ||
| No autism spectrum disorder diagnosis but Social Responsiveness Scale T score ≥75 | 28 | 33 | ||
| Combined criteria | ||||
| Autism spectrum disorder or Social Communication Questionnaire score ≥15 | 127 | 60 | 14.4 | 11.1 |
| Autism spectrum disorder or Social Responsiveness Scale T score ≥75 | 128 | 71 | 15.1 | 11.8 |
| Autism spectrum disorder or history of language delay with autistic speech | 180 | 108 | 21.7c | 17.1 |
| Autistic spectrum disorder or history of language delay with autistic speech or Social Communication Questionnaire score ≥15 or Social Responsiveness Scale T score ≥75 | 211 | 142 | 26.0 | 20.9 |
TABLE 2. Rate of Recurrence of Autism as a Function of Recurrence Definition Among 831 Male Siblings and 854 Female Siblings in Autism-Affected Familiesa
The family-based recurrence rate was uniformly higher than that calculated for all individual siblings in the sample, which reflects the possible effects of stoppage (a tendency for families who have a child with a serious clinical condition to reduce subsequent childbearing). When considering families with more than two children affected by an autism spectrum disorder and the existence of at least one additional sibling, autism status in a third child occurred in 8% of these families (for the entire Interactive Autism Network registry, this figure was 18%). Linear regression revealed statistically significant effects of both proband and sibling gender on sibling standardized quantitative trait scores on the Social Responsiveness Scale (F=5.80, df=4, 622, p<0.001; R2=0.036). However, the effects were very modest in magnitude, and there was no appreciable effect of proband level of functioning (non-verbal status or IQ <56) on sibling scores.
Table 3 lists the means and standard deviations for the quantitative trait scores of specific groupings of index cases and siblings, segregated by gender and family type. The respective quantitative trait distributions are depicted in the histograms presented in Figure 1. Most striking across all subject groups and in keeping with our previous report (7), we observed an absence of quantitative autistic traits in the unaffected siblings of autism-affected children in single-incidence families. Also in keeping with our previous report, we observed a relative aggregation of quantitative autistic traits in the unaffected siblings in multiple-incidence families. This was manifested by an elevated mean and a contrasting shape of the distribution, especially for presumed-unaffected male children in those families. Unaffected siblings with a history of language delay with autistic speech contributed to an intermediate distribution, with quantitative trait scores of unaffected female children significantly overlapping with those of autism-affected girls. The differences in mean Social Responsiveness Scale scores of presumed-unaffected siblings across the three groups were highly statistically significant and remained so for boys when multiple-incidence families were directly compared with single-incidence families.
| Variable | Single-Incidence Families | Single-Incidence Autism Families Who Have Unaffected Children With a History of Language Delay With Autistic Speech | Multiple-Incidence Families | Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean T Score | SD | N | Mean T Score | SD | N | Mean T Score | SD | F | df | p | |
| Boys | ||||||||||||
| Autism spectrum disorder-affected | 826 | 85.4 | 14.0 | 119 | 86.2 | 14.5 | 206 | 82.2 | 17.1 | 4.4 | 2, 1148 | 0.01 |
| Presumed unaffected | 517 | 44.3a | 9.9 | 92 | 51.5 | 13.9 | 21 | 57.5 | 15.5 | 30.4 | 2, 627 | 0.000001 |
| Girls | ||||||||||||
| Autism spectrum disorder-affected | 142 | 95.3 | 16.5 | 14 | 92.6 | 14.5 | 63 | 88.6 | 18.2 | 3.4 | 2, 216 | 0.04 |
| Presumed unaffected | 619 | 45.2 | 10.5 | 93 | 53.6 | 15.5 | 26 | 49.7 | 14.5 | 23.2 | 2, 735 | 0.000001 |
TABLE 3. Social Responsiveness Scale-Parent Report Scores Across Three Autism Family Types Segregated by Gender and Categorical Affection Statusa
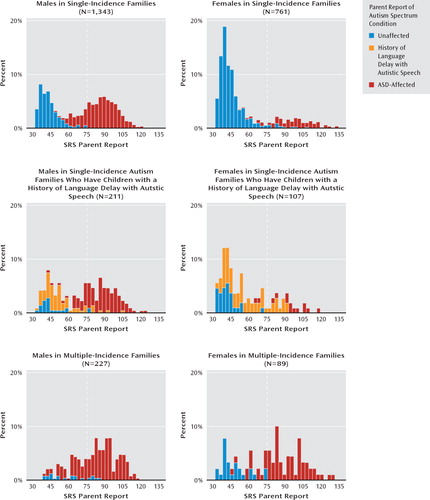
FIGURE 1. Distributions of Parent-Report Social Responsiveness Scale Scores Among Families With Autisma
a Bar graphs encompass all assessed children in the family for the respective gender and family type represented; SRS Parent Report ratings indicate T scores.
The quantitative distributions depicted in the histograms in Figure 1 reveal the manner in which parents distinguished their children with versus without an autism spectrum disorder diagnosis in this sample. The nadir in these distributions, effectively the point at which parents (on average) differentiated affected versus unaffected children in their families, fell below the Social Responsiveness Scale cutoff T score of 75. We also observed that the distributions for unaffected children in simplex families were slightly nonpathologically shifted relative to previously published general population distributions (3) suggestive of subtle rater contrast effects. In the entire sample, there were 207 families with more than one autism-unaffected child for whom parent-reported Social Responsiveness Scale data were available. For these unaffected children (predominantly from single-incidence autism families in the sample), the sibling correlation for parent-reported data was 0.38, in keeping with previously published estimates (3, 29).
Discussion
The results of this study of recurrence are notable in several respects and provide new information on the genetic epidemiology of autism spectrum conditions. First, there exists an aggregation of quantitative autistic traits among unaffected children in multiple-incidence autism spectrum disorder families—most pronounced in boys—but an absence of such traits in most single-incidence families, as initially observed in a prior study that included independent ratings by teachers, involving a smaller number of single-incidence families (7). The absence of such aggregation in single-incidence families is also consistent with our recent taxometric analysis of the entire Interactive Autism Network data set (a predominantly single-incidence sample), identifying categorical discontinuity (autism spec-t rum disorder versus nonautism spectrum disorder) rather than graded levels of symptoms within this predominantly single-incidence family sample (6). Second, we observed minimal effects of proband gender and level of functioning on the rate of sibling recurrence, but when using standardized quantitative criteria for designation of affected status, many more girls are identified, and the male:female ratio narrows to 3:2. Third, across all family types and highly consistent with prior family studies, some 20% of presumed-unaffected siblings carry a historic diagnosis of language delay, over one-half of whom exhibit distinctly autistic speech. This may constitute a form of recurrence in a substantial minority of autism-affected families. Finally, the rate of sibling recurrence of categorically defined autism spectrum disorder in this sample is in keeping with prior estimates, although distinctly lower than that reported for nonidentical twins from the same registry (31% concordance rate reported by Rosenberg et al. [30]). Whether this difference is explainable on the basis of 1) factors that might raise recurrence risk in twins versus 2) ascertainment bias favoring the enrollment of concordant over discordant twin pairs in this volunteer register will be a critical issue to resolve via future research in independent samples.
In summary, we observed a range of manifestations of sibling recurrence in autism to include 1) categorically defined autism spectrum disorder, 2) history of language delay with autistic speech qualities, and 3) aggregation of quantitative (subclinical) autistic traits. The third manifestation appears to be absent in most single-incidence autism families. These disparate manifestations of recurrence may reflect differential mechanisms of genetic transmission of autism in the population, which include (respectively) 1) rare recessive or de novo mutations (including chromosomal rearrangements) of substantial effect, which in some cases have accounted for sporadic incidence of autism; 2) inherited mutations that may be variably expressed and result in varying degrees of social and language impairment (i.e., categorically defined autism spectrum disorder, history of language delay with autistic speech) and/or sub-clinical autistic impairment; and 3) common susceptibility alleles or rare variants of minor effect, which may operate in additive or epistatic fashion. We note that even among single-incidence families in which affectation status appears categorical, the distribution of quantitative trait scores for affected children extends well into the range of the distribution for the general population. Thus, the continuum observed for autistic symptoms in nature may be composed of highly overlapping segments, each with its own mechanism (or mechanisms) of genetic transmission. Finally, the observation of a narrowing of the gender ratio when standardized quantitative criteria for affectation status are applied suggests the possibility that affected female children may be underascertained when using traditional categorical methods for diagnostic assignment.
Limitations of the study are that the sample was not fully epidemiologic (rather, a large volunteer register), not fully representative of the ethnicity of the population of U.S. children affected by autism, and the data were provided exclusively by parents, which potentially introduces a variety of biases, including rater contrast effects. Higher levels of rater contrast are expected in parent-reported data in clinically ascertained families (a reason for the use of teacher-reported data in our previous study [7]) and may have actually resulted in underestimation of the magnitude of familial aggregation among multiple-incidence families in this report. We also note that we were unable to directly compare the proportion of children with language delay in this sample with a population-based sample in which the same ascertainment methods were employed.
However, several aspects of the data validate the reports of parents, including the very high rate of reported diagnostic confirmation (98%) in families whose children underwent standardized testing for autism spectrum disorder (18), the fact that parents' reports of a diagnosis corresponded closely to quantitative characterizations of social deficiency in their children (with nadirs closely corresponding to established cutoff scores for clinical-level symptoms), and that these results replicate what was observed by both teacher-reported data (minimizing the likelihood of rater contrast) and parent-reported data in a smaller independent sample (7). It is important to note that elevations in quantitative autistic traits ascertained by the Social Responsiveness Scale and Social Communication Questionnaire have been observed in samples of children seriously affected by other primary psychiatric conditions not ascertained in the Interactive Autism Network data collection (31, 32). Future research will need to explore the extent to which the quantitative distribution of autistic traits in these populations represent distinct or overlapping continua with those traits that characterize autism spectrum disorder.
On the basis of these findings, we propose careful reconsideration of what constitutes recurrence (and therefore diagnosis), informed by an understanding of the range of symptoms that aggregate in the siblings of autism-affected probands (including girls or twins) (30), and that may more closely correspond to the manner in which autistic syndromes are intergenerationally transmitted. Among families of autism subjects in this sample, fully 21.7% exhibited a recurrence of either an autism spectrum disorder or a history of language delay with autistic speech, with a broad distribution of subclinical autistic traits among unaffected male subjects in multiple-incidence families.
Studies examining the association between autistic phenotypes and their underlying genetic (33) or neurobiologic (34, 35) determinants may be optimized by including information about recurrence of the autistic syndrome and the aggregation of relevant subclinical phenotypes among first-degree relatives. The data from the current study provide new perspectives on the relative proportions of autism cases in the general population that manifest distinct patterns of familial aggregation and should alert clinicians to the presence of both clinical and sub-clinical autism spectrum disorder-related-syndromes that occur in the siblings of children affected by autism.
1. : Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry 2005; 46:963–971Crossref, Medline, Google Scholar
2. : Autism genetics: emerging data from genome-wide copy-number and single nucleotide polymorphism scans. Expert Rev Mol Diagn 2009; 9:795–803Crossref, Medline, Google Scholar
3. : Autistic traits in the general population: a twin study. Arch Gen Psychiatry 2003; 60:524–530Crossref, Medline, Google Scholar
4. : Phenotypic and genetic overlap between autistic traits at the extremes of the general population. J Am Acad Child Adolesc Psychiatry 2006; 45:1206–1214Crossref, Medline, Google Scholar
5. : Social communication competence and functional adaptation in a general population of children: preliminary evidence for sex-by-verbal IQ differential risk. J Am Acad Child Adolesc Psychiatry 2009; 48:128–137Crossref, Medline, Google Scholar
6. : Autism spectrum disorders as a qualitatively distinct category from typical behavior in a large, clinically ascertained sample. Assessment [Epub ahead of print, Dec 29, 2009]Google Scholar
7. : Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet 2009; 150B:328–334Crossref, Medline, Google Scholar
8. : Strong association of de novo copy number mutations with autism. Science 2007; 316:445–449Crossref, Medline, Google Scholar
9. : Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 2008; 358:667–675Crossref, Medline, Google Scholar
10. : A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet 2008; 82:160–164Crossref, Medline, Google Scholar
11. : Association of MET with social and communication phenotypes in individuals with autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet 2010; 153:438–446Crossref, Google Scholar
12. : Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009; 459:528–533Crossref, Medline, Google Scholar
13. : A functional genetic link between distinct developmental language disorders. N Engl J Med 2008; 359:2337–2345Crossref, Medline, Google Scholar
14. : Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Res 2009; 2:22–38Crossref, Medline, Google Scholar
15. : A comparative study of infantile autism and specific developmental receptive language disorders, III: discriminant function analysis. J Autism Child Schizophr 1977; 7:383–396Crossref, Medline, Google Scholar
16. : Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry 1997; 154:185–190Link, Google Scholar
17.
18. : Accuracy of phenotyping of autistic children based on Internet implemented parent report. Am J Med Genet B Neuropsychiatr Genet [Epub ahead of print, June 15, 2010]Google Scholar
19. : Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J Speech Lang Hear Res 1999; 42:1461–1481Crossref, Medline, Google Scholar
20. : Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res 1997; 40:1245–1260Crossref, Medline, Google Scholar
21. : Social Communication Questionnaire (SCQ). Los Angeles, Western Psychological Services, 2003Google Scholar
22. : Efficacy of three screening instruments in the identification of autistic-spectrum disorders. Br J Psychiatry 2007; 191:554–559Crossref, Medline, Google Scholar
23. : Developmental course of autistic social impairment in males. Dev Psycho-pathol 2009; 21:127–138Crossref, Medline, Google Scholar
24. : The Social Responsiveness Scale Manual. Los Angeles, Western Psychological Services, 2005Google Scholar
25. : The factor structure of autistic traits. J Child Psychol Psychiatry 2004; 45:719–726Crossref, Medline, Google Scholar
26. : Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry 2007; 46:1668–1676Crossref, Medline, Google Scholar
27. : Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr 2000; 21:2–11Crossref, Medline, Google Scholar
28. : Essential versus complex autism: definition of fundamental prognostic subtypes. Am J Med Genet A 2005; 135:171–180Crossref, Medline, Google Scholar
29. : Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry 2006; 163:294–296Link, Google Scholar
30. : Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 2009; 163:907–914Crossref, Medline, Google Scholar
31. : Autism Spectrum Disorder Scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adolesc Psychiatry 2008; 47:652–661Crossref, Medline, Google Scholar
32. : Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:662–672Crossref, Medline, Google Scholar
33. : A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry 2007; 164:656–662Link, Google Scholar
34. : Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry 2007; 61:512–520Crossref, Medline, Google Scholar
35. : Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry 2009; 166:891–899Link, Google Scholar



