New Possibilities in Cognition Enhancement for Schizophrenia
Cognitive remediation for schizophrenia and other mental disorders can be divided into two distinctly different approaches. Cognition-enhancing approaches train subjects with laboratory tasks designed to improve specific abilities in various cognitive domains, such as perception, learning, or memory. In contrast, compensatory approaches attempt to bypass cognitive deficits and teach strategies to compensate for them by relying on aids or other processes (1 , 2) . The article by Fisher et al. (3) in this issue of the Journal represents a new development in the cognition-enhancing approach. They applied to schizophrenia a cognitive training program that was well grounded in a neuroscientific rationale. This and similar training studies will set higher expectations for results in functional benefits for patients. To put this study in perspective, it is useful to review the key findings, establish what new terrain was covered by it, and then focus on critical missing pieces of the cognitive training puzzle.
In the study by Fisher et al., 55 patients with schizophrenia were assigned either to a cognitive training program developed by Posit Science or to a computerized game control condition to mimic the time and concentration at a computer required in the cognitive training program. Posit Science is a company founded by one of the article’s authors, Michael M. Merzenich, Ph.D., Professor Emeritus at the University of California, San Francisco. Dr. Merzenich’s work in human brain plasticity, as applied to cognitive training, particularly for memory loss in older adults, has been recognized by the National Academy of Sciences and the Institute of Medicine. The training program had a heavy emphasis on exercises to enhance basic auditory/speech perception. Participants received sessions 1 hour a day, 5 days a week, for 10 weeks (a total of 50 sessions). They could choose to take the program at the clinic or at home. The group that received cognitive training showed significantly larger improvements than the control group on global cognition, verbal working memory, and verbal learning and memory at effect sizes in the medium to large range. Improvements in a psychophysical exercise in auditory perception correlated with the degree of improvement on more general cognitive measures, suggesting that increased representational fidelity in early perceptual processes led to more efficient use of those percepts for later memory stages.
The study has several notable strengths. First, the Posit Science approach is unusual in that it rests heavily on neuroplasticity models from neuroscience that emphasize the consequences of a poor signal-to-noise ratio in initial perceptual representations in the brain, which leads to errors and poor performance as they are fed forward for cognitive and motor operations. Consequently, the program focuses exercises on early auditory perceptual processes to enhance verbal memory (4) . This emphasis on perceptual processes is a critical insight of the Posit Science approach and a clear distinction from other cognitive training programs. A model of neuroplasticity is one reason to set sights on perceptual processes, but it is not the only one. A close look at patterns of correlations among perception, cognition, and functioning in schizophrenia also highlights the importance of perceptual processes. It is increasingly clear that early auditory and visual processes contribute to cognitive impairment, including problems in social cognition (i.e., the mental operations involved with perceiving, interpreting, managing, and generating responses to socially relevant stimuli), as well as other aspects of daily functioning in schizophrenia, such as shopping, cooking, and money management (5 , 6) . On the basis of this literature, community functioning, the ultimate goal of any clinical intervention, appears to be a late stage in a cascade of processes that starts with the quality of perceptual representations.
A second strength of this study is that the program automatically and continuously adapted task parameters (i.e., making the task harder or easier) so that participants’ performance was kept at a constant level. This feature helps to keep participants engaged by maintaining a constant level of challenge. However, this strategy makes data analysis more challenging because there are always two moving parts: the subject’s performance level and the task parameters. To address this problem, Posit Science has built in “positive controls” that are repeated presentations of trials at the same parameters so that progress on the training program can be tracked.
A third strength and contribution of this article is that its assessment battery was modeled on the MATRICS Consensus Cognitive Battery (7) , which emerged from a long consensus process and provides good coverage of cognitive domains. A convergence on a set of domains and a set of measures as endpoints of trials will make it more straightforward to compare results from using this intervention with results in other cognitive training studies, including those that evaluate new cognition-enhancing drugs. The study was started before the MATRICS Consensus Cognitive Battery was available and did not have the benefits of co-norms, an automatic scoring program, and age and gender corrections. Nonetheless, the large size of the effect on the global measure (d=0.86) indicates that the treatment is effective for a well-recognized, now widely used measure.
As would be expected, many issues are left unresolved by this initial study. Among them are questions of dosing and duration of the effect. Fisher et al. speculate that the success of their methods may be partly due to the large number of sessions they used, but it has been extremely difficult to understand the relationships between hours of training and degree of cognitive improvement. The meta-analysis of studies in schizophrenia by McGurk et al. (8) found no such relationship for global measures of cognition. However, it found a significant effect of dosing on verbal learning and memory, which is also a domain targeted by Fisher et al. Highly durable effects of cognitive remediation with relatively few numbers of treatments have been seen at a 5-year follow up in healthy elderly participants who received as few as 10 training sessions (9) . Clearly, the training parameters that determine long-term benefit in schizophrenia are yet to be established.
Another unresolved issue is the functional significance of the gain in cognitive function for the patient. Fisher et al. state that the key question from these efforts is whether cognitive training helps the patients in their daily functioning. This is a commonly asked question for both cognitive training and cognition-enhancing drugs, but one that is rarely framed in a way that permits a clear answer. The pivotal point that tends to get lost is that improving cognition in schizophrenia is not an end in itself. Although we talk about cognition-enhancing drugs or cognitive remediation, the missing factor being sought is something that helps individuals learn and acquire skills to enable them to navigate their world and cope with life’s daily challenges. In the meta-analysis by McGurk et al. (8) , the size of the effect of cognitive remediation on a psychosocial outcome (broadly defined) was 0.36, which is respectable and significant, but not huge. However, there is an intriguing moderator effect: for studies that did not have a rehabilitation component, the effect size was essentially zero (0.05). For studies that did include rehabilitation, the effect size was 0.47. Hence, one sees clinically meaningful effects when the cognitive remediation is used to facilitate a learning process that enables patients to engage in a broader rehabilitation effort, but not otherwise. In essence, a basic cognition-enhancing strategy has to be combined with the teaching of psychosocial skills and strategies to improve patients’ lives. Whether or not the new strategy of Fisher et al. will work better for this ultimate purpose than other cognition-enhancing strategies has yet to be tested.
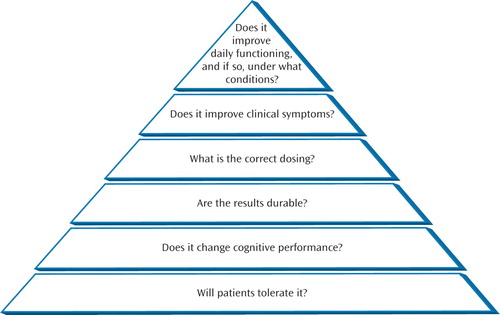
Reactions to studies of cognitive remediation for schizophrenia tend to display a bimodal distribution. One response is enthusiasm for the latest study that far exceeds the data. The other is a sympathetic nod: an appreciation of the earnestness of the endeavor with little expectation of payoff. After all, if cognitive training worked, would we not all know it by now? In fact, this field is characterized by steady progress over the past decades. In a 1993 article on cognitive remediation in schizophrenia (10) , I reviewed a relatively small number of articles, many of them feasibility studies. At that time, this field lacked basic information on every major issue ( Figure 1 ). There were fundamental concerns about whether the cognitive interventions that were primarily developed for brain-injured patients could be productively applied to patients with schizophrenia. Not any more. We now have a much stronger database, including several reviews and a meta-analysis (1 , 2 , 8) . As a result, we are working our way up the hierarchy of questions in Figure 1 . Training programs are well tolerated, and they effectively improve cognitive performance. At the top of the hierarchy, we know that cognitive training can improve the daily functioning of patients but does not always do so. The meta-analysis by McGurk et al. has given us a reason that is both clear and intuitive: studies that realize the benefits are those in which cognitive training is adjunctive to rehabilitation.
The significance of the article by Fisher et al. is that it addresses cognitive training at a more basic neurobiological level than any previous strategy. We can hope that the dramatic effects they have reported will prove to be replicable and durable and that they will extend to meaningful effects for patients’ lives.
1. Kern RS, Glynn SM, Horan WP, Marder SR: Psychosocial treatments to promote functional recovery in schizophrenia. Schizophr Bull 2009; 35:347–361Google Scholar
2. Velligan DI, Kern RS, Gold JM: Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull 2006; 32:474–485Google Scholar
3. Fisher M, Holland C, Merzenich MM, Vinogradov S: Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry 2009; 166:805–811Google Scholar
4. Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM: Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA 2006; 103:12523–12528Google Scholar
5. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF: Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry 2006; 163:448–454Google Scholar
6. Light GA, Braff DL: Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry 2005; 62:127–136Google Scholar
7. Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ III, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR: The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Google Scholar
8. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT: A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 2007; 164:1791–1802Google Scholar
9. Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E, ACTIVE Study Group: Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA 2006; 296:2805–2814Google Scholar
10. Green MF: Cognitive remediation in schizophrenia: is it time yet? Am J Psychiatry 1993; 150:178–187Google Scholar



