Association of Poor Childhood Fear Conditioning and Adult Crime
Abstract
Objective
Amygdala dysfunction is theorized to give rise to poor fear conditioning, which in turn predisposes to crime, but it is not known whether poor conditioning precedes criminal offending. This study prospectively assessed whether poor fear conditioning early in life predisposes to adult crime in a large cohort.
Method
Electrodermal fear conditioning was assessed in a cohort of 1,795 children at age 3, and registration for criminal offending was ascertained at age 23. In a case-control design, 137 cohort members with a criminal record were matched on gender, ethnicity, and social adversity with 274 noncriminal comparison members. Statistical analyses compared childhood fear conditioning for the two groups.
Results
Criminal offenders showed significantly reduced electrodermal fear conditioning at age 3 compared to matched comparison subjects.
Conclusions
Poor fear conditioning at age 3 predisposes to crime at age 23. Poor fear conditioning early in life implicates amygdala and ventral prefrontal cortex dysfunction and a lack of fear of socializing punishments in children who grow up to become criminals. These findings are consistent with a neurodevelopmental contribution to crime causation.
Ever since Lombroso (1) proposed a brain basis to the controversial concept of "the born criminal" in the 19th century, the identification of the very early neurobiological and medical processes that give rise to adult criminal offending has been elusive. With some exceptions (2), the majority of studies of very young children have focused on psychosocial influences, with relatively little attention to early neurobiological influences (3–6). In this article, we suggest that poor fear conditioning is one of the early neurobiological risk factors that predispose some individuals to adult criminal offending.
Poor autonomic fear conditioning has been shown to be a well-replicated correlate of adult criminal and psychopathic adult offending (7–9). Individuals are hypothesized to learn to avoid antisocial and criminal acts by successfully associating stimuli that are associated with antisocial events with later socializing punishments (8–11). This association learning is hypothesized to result in an increase in anxiety and anticipatory fear whenever the individual contemplates the commission of an antisocial act, which in turn motivates the individual to avoid such stimuli and the commission of antisocial, rule-breaking behavior. Within this framework, poor conditionability, as measured by reduced responsivity to the reinforced conditioned stimulus (CS+) followed by the aversive stimuli (UCS), compared to a control stimulus not followed by an aversive event (CS–), may give rise to criminal acts in some individuals.
Both imaging and lesion studies have indicated that the amygdala is critically involved in fear conditioning (12), and clinical neuroscience research in adults has increasingly provided evidence for the primacy of amygdala dysfunction in some antisocial populations (10, 13–15). Similarly, the ventral prefrontal cortex (including the orbitofrontal cortex) plays a role in fear conditioning and the representation of information on aversive outcomes and has been found to be dysfunctional in antisocial populations (10, 15). Nevertheless, an unsolved issue in the absence of brain imaging data in very young children concerns whether poor fear conditioning, a proxy for amygdala dysfunction, occurs early in life in those on a pathway to a criminal career. In this study, we addressed this question by measuring classically conditioned emotional responses at age 3 in a birth cohort of 1,795 children and following them up after 20 years to assess outcome for criminal convictions at age 23. It was hypothesized that compared to the noncriminals, criminal offenders at age 23 would show reduced fear conditioning at age 3.
Method
Participants
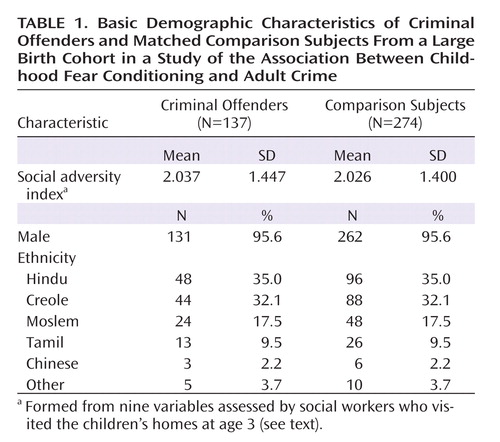
Participants consisted of a birth cohort of 1,795 children from Mauritius, a tropical island in the Indian Ocean. All children born in 1969 and 1970 in two towns (Quatre Bornes and Vacoas) were recruited at age 3 (45.9% were girls) on the basis of vaccination records. The ethnic makeup of the cohort was 68.7% Indian, 25.6% African, and 5.6% other (Chinese, English, or French descent) (16). This birth cohort has been described in our earlier reports on other early risk factors for the development of antisocial behavior (3, 17). Written informed consent and assent were obtained from parents and children.
Conditioning at Age 3
The conditioning paradigm consisted of 12 tones (nine CS+ and three CS–) with a 66% partial-reinforcement schedule (see Figure 1). The CS+ was a 1000-Hz, 60-dB, 12.5-second tone with a rise and fall time of 25 msec, and CS– was a 500-Hz, 60-dB, 12.5-second tone with a rise and fall time of 25 msec. The UCS was a 90-dB, 4.5-second auditory stimulus recorded from white noise played in a tin can with jangling metal objects to add unpleasant low- and high-frequency components. The interstimulus interval was 10 seconds (onset to onset), with a mean randomized intertrial interval of 38 seconds (range=34–42 seconds). Three CS– trials and three CS+ trials that were contiguous in time with CS– were used. Electrodermal responses greater than 0.05 microsiemens within a latency window of 1–3 seconds after the onset of the CS were scored. Trials were averaged within class (CS+, CS–) to form overall measures of responding to reinforced (CS+) and unreinforced trials (CS–).
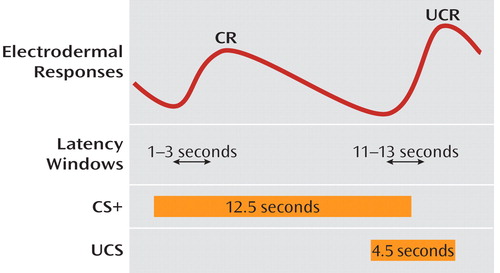
aCR=conditioned response; UCR=unconditioned response; CS+=reinforced conditioned stimulus; UCS=aversive stimulus.
Electrodermal data were collected using a Grass Model 79 polygraph with a constant voltage system, Beckman miniature silver/silver chloride electrodes, and an electrolyte consisting of 0.5% KCl in 2% agar-agar. Electrodermal activity was recorded from the medial phalanges of the index and middle fingers of the left hand (16).
Criminal Offending at Age 23
When the cohort was 23 years of age, official court records were searched for registration of offenses that included property, drug, violence, and serious driving offenses. Petty offenses, such as parking fines and lack of vehicle registration, were excluded. A total of 137 subjects (131 male and 6 female, 7.63% of the original cohort) who had at least one court conviction were categorized as criminal offenders (18).
Social Adversity Index
A social adversity index was formed from nine variables assessed by social workers who visited the children's homes at age 3 (19). One point was scored for each of the following variables: father uneducated (30%), mother uneducated (29.4%), father in semiskilled or unskilled occupation (55.5%), teenage mother (14.2%), single-parent status (2.1%), separation from parents (0.9%), large family size (30.0%), poor health of mother (3.3%), and overcrowded home (28.8%). Higher scores indicate higher adversity.
Statistical Analyses
To maximize power, we used a case-control design matching each offender to two nonoffenders on age, gender, ethnicity, and social adversity index score. The demographic characteristics of the criminal offenders and matched comparison subjects are summarized in Table 1. A repeated-measures analysis of variance with stimulus type (CS+, CS–) as within-subject factor and criminal offender group as between-subject factor was conducted on electrodermal conditioning responses. Paired-sample t tests were conducted when interaction effects were significant. Cohen's d was computed for effect sizes of group differences (20). A square root transformation was performed on the electrodermal responses before inferential statistical analyses were conducted to help attain normality (21).
 |
Results
No main effects of criminal offender group or stimulus type were found. A significant group-by-stimulus interaction indicated that the criminal offender group failed to show fear conditioning at age 3 (F=4.554, df=1, 409, p=0.033) (see Figure 2). The comparison group (N=274) showed a greater response to the CS+ than to the CS– (t=2.852, df=273, p=0.005; d=0.345), whereas the offender group (N=137) failed to show this effect (t=–0.604, df=136, p=n.s.; d=–0.104).
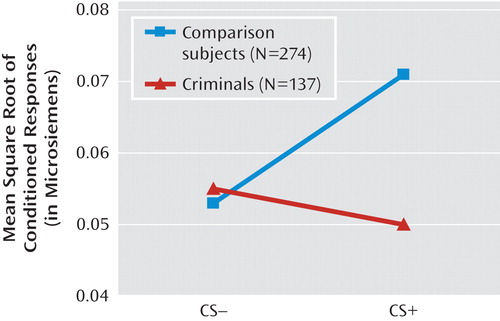
aCS+=reinforced stimulus; CS–=unreinforced stimulus. Results show conditioning (enhanced CS+) in comparison subjects but not in criminal offenders.
Discussion
To our knowledge, this is the first longitudinal study to demonstrate an early deficit in autonomic fear conditioning as a predisposition to adult criminality. The findings are consistent with the hypothesis that poor amygdala functioning early in life, as indicated by poor fear conditioning, increases the risk for criminal offending (22), and they demonstrate that this fear conditioning risk factor for crime is in place early in life and is not explained by social adversity, ethnicity, or gender. Poor fear conditioning is hypothesized to predispose to crime because individuals who lack fear are less likely to avoid situations, contexts, and events that are associated with future punishment—resulting in a lack of conscience (8). The findings of this study are broadly consistent with a neurodevelopmental perspective on criminal offending.
Dysfunction in multiple brain structures, with a focus on frontal-temporal regions, has been implicated in criminal offending. Imaging studies have repeatedly reported antisocial-related impairments in orbitofrontal, dorsolateral, and limbic systems, including the amygdala, the insula, and the anterior cingulate cortex (15, 23, 24). The circuitry involved in fear conditioning is similarly complex, involving the orbitofrontal cortex, the insula, and the anterior cingulate in addition to the amygdala (12). Provisional brain imaging findings in adult offenders are beginning to document dysfunction in these other brain regions involved in fear conditioning (13). The amygdala, which is viewed as the primary structure subserving fear conditioning, is found to be less activated when psychopathic offenders contemplate a moral decision (25). Similarly, amygdala hyporesponsiveness has been reported in children with callous-unemotional traits (26, 27), although some neuroimaging and genetic studies (e.g., monoamine oxidase A polymorphism) suggest that there may be quite different neurobiological abnormalities in subgroups of antisocial individuals, particularly those with increased emotional reactivity (28–30). Furthermore, reduced connectivity between the amygdala and the orbitofrontal cortex may conceivably underlie the fear conditioning impairments in criminals rather than localized dysfunction to one or the other of these brain regions (27). We hypothesize that, taken together with the findings of this study, deficits in related brain regions give rise to both poor fear conditioning and behavior problems and that the circuitry involved in fear conditioning is compromised in early childhood in a subgroup of individuals who evidence criminal behavior in adulthood.
The findings of this study potentially provide some support for a neurodevelopmental theory of antisocial and criminal behavior (31). Cavum septum pellucidum, a marker of limbic neural maldevelopment, has been associated with higher levels of antisocial personality, psychopathy, and arrests and convictions in a community sample (A. Raine et al., unpublished 2009 manuscript), and minor physical anomalies (markers for fetal neural maldevelopment at the beginning of the second trimester of gestation) have been found to be more common in delinquents and criminals (32, 33). Child and adolescent imaging studies also provide some limited initial support for the neurodevelopmental perspective. Gray matter abnormalities in the frontal-temporal areas, including reduced volume in the left amygdala, have been found in children and adolescents with conduct disorder (34, 35). Adolescents with conduct disorder also show reduced amygdala responsiveness to negative emotional stimuli compared to age-matched comparison subjects (36). While atypical amygdala structure and function have been observed in children with conduct problems, to our knowledge no imaging studies on conduct problems have been conducted in children under age 11. Unlike some other brain regions (e.g., the polar prefrontal and temporal cortices), the amygdala is rarely susceptible to illness and injury (37), and hence amygdala dysfunction may be more likely to have an early neurodevelopmental basis. Only when it becomes possible to conduct brain scans in large samples of very young children and follow them longitudinally over several decades will it be possible to confirm early amygdala dysfunction as a specific source of poor fear conditioning in young children who become adult criminal offenders.
If crime is in part neurodevelopmentally determined, efforts to prevent and treat this worldwide behavior problem will increasingly rely on early health interventions. Prenatal programs aimed at health factors, including reducing cigarette, alcohol, and drug consumption and improving nutrition, have led to significant reductions in juvenile delinquency 15 years later (38). Enhancing the early health environment of young children from ages 3 to 5 years with better nutrition, more physical exercise, and cognitive stimulation has been shown both to improve brain functioning 6 years later, as indicated by a reduction in slow-wave EEG power (indicating faster developmental brain maturation), and to reduce adult criminal offending by 35% (18); conceivably it could also improve amygdala functioning. Such programs applied early in life and combining multidisciplinary health services from clinical, social, and educational domains have the potential to improve brain functioning and to make a public health contribution to the reduction of criminal offending throughout the world. At the same time, due caution should be exercised in the application of neurobiological markers early in life to predict later offending; crime is clearly a complex construct involving multiple interactions between genetic, brain, family, and societal influences (31) and cannot be predicted by single neurobiological markers such as fear conditioning.
1 : L'uomo delinquente. Turin, Italy, Bocca, 1876 Google Scholar
2 : Research review: DSM-V conduct disorder: research needs for an evidence base. J Child Psychol Psychiatry 2008; 49:3–33 Crossref, Medline, Google Scholar
3 : Low resting heart rate at age 3 years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Project. J Am Acad Child Adolesc Psychiatry 1997; 36:1457–1464 Crossref, Medline, Google Scholar
4 : Relationships between CNS and ANS measures of arousal at age 15 and criminality at age 24. Arch Gen Psychiatry 1990; 47:1003–1007 Crossref, Medline, Google Scholar
5 : A 2-year prospective follow-up study of children and adolescents with disruptive behavior disorders. Arch Gen Psychiatry 1992; 49:429–435 Crossref, Medline, Google Scholar
6 : Early temperamental and psychophysiological precursors of adult psychopathic personality. J Abnorm Psychol 2007; 116:508–518 Crossref, Medline, Google Scholar
7 : Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull 2004; 130:531–552 Crossref, Medline, Google Scholar
8 : Getting to the heart of psychopathy, in Psychopathy: Theory, Research, and Social Implications. Edited by Herve HYuille JC. Hillsdale, NJ, Erlbaum, 2006, pp 207–252 Google Scholar
9 : The Psychopathology of Crime: Criminal Behavior as a Clinical Disorder. San Diego, Academic Press, 1993 Crossref, Google Scholar
10 : The amygdala and ventromedial prefrontal cortex in morality and psychopaths. Trends Cogn Sci 2007; 11:387–392 Crossref, Medline, Google Scholar
11 : Crime and Personality. London, Routledge & Kegan Paul, 1977 Google Scholar
12 : Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 1998; 20:947–957 Crossref, Medline, Google Scholar
13 : Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science 2000; 289:591–594 Crossref, Medline, Google Scholar
14 : Fear conditioning and affective modulation of the startle reflex in male adolescents with early-onset or adolescence-onset conduct disorder and healthy control subjects. Biol Psychiatry 2008; 63:279–285 Crossref, Medline, Google Scholar
15 : Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci 2006; 1:203–213 Crossref, Medline, Google Scholar
16 : Psychophysiology and psychometrics. Psychophysiology 1978; 15:302–315 Crossref, Medline, Google Scholar
17 : Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Dev Psychopathol 2002; 14:25–44 Crossref, Medline, Google Scholar
18 : Effects of environmental enrichment at ages 3–5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. Am J Psychiatry 2003; 160:1627–1635 Link, Google Scholar
19 : Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: evidence from the Mauritius Child Health Project. Psychophysiology 2001; 38:254–266 Crossref, Medline, Google Scholar
20 : Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988 Google Scholar
21 : The electrodermal system, in Handbook of Psychophysiology. Edited by Cacioppo JTTassinary LGBerntson G. New York, Cambridge University Press, 2007, pp 159–181 Google Scholar
22 : Deafness to fear in boys with psychopathic tendencies. J Child Psychol Psychiatry 2005; 46:327–336 Crossref, Medline, Google Scholar
23 : A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system function. Psychiatry Res 2006; 142:107–128 Crossref, Medline, Google Scholar
24 : Emotion in criminal psychopath: fear image processing. J Abnorm Psychol 1994; 103:523–534 Crossref, Medline, Google Scholar
25 : The neural correlates of moral decision-making in psychopathy. Mol Psychiatry 2009; 14:5–6 Crossref, Medline, Google Scholar
26 : Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 2009; 166:95–102 Link, Google Scholar
27 : Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008; 165:712–720 Link, Google Scholar
28 : Understanding genetic risk for aggression: clues from the brain's response to social exclusion. Biol Psychiatry 2007; 61:1100–1108 Crossref, Medline, Google Scholar
29 : Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry 2008; 49:781–791 Crossref, Medline, Google Scholar
30 : Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychiatry 2009; 80:203–211 Crossref, Google Scholar
31 : From genes to brain to antisocial behavior. Curr Dir Psychol Sci 2008; 17:323–328 Crossref, Google Scholar
32 : Minor physical anomalies and family adversity as risk factors for violent delinquency in adolescence. Am J Psychiatry 2000; 157:917–923 Link, Google Scholar
33 : Minor physical anomalies: modifiers of environmental risks for psychiatric impairment? J Am Acad Child Adolesc Psychiatry 1997; 36:395–403 Crossref, Medline, Google Scholar
34 : Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:540–547 Crossref, Medline, Google Scholar
35 : A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage 2007; 37:335–342 Crossref, Medline, Google Scholar
36 : Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 2005; 57:7–15 Crossref, Medline, Google Scholar
37 : Neuropsychological correlates of bilateral amygdala damage. Arch Neurol 1990; 47:349–355 Crossref, Medline, Google Scholar
38 : Long-term effects of nurse home visitation on children's criminal and antisocial behavior: 15-year follow-up of a randomized controlled trial. JAMA 1998; 280:1238–1244 Crossref, Medline, Google Scholar



