Severe Eating Disorder in a 28-Year-Old Man With William’s Syndrome
Case Presentation
“Mr. T” was a 28-year-old man with William’s syndrome who was admitted to a university-based inpatient psychiatric unit for evaluation and treatment of severe malnutrition and food refusal. At 5 feet 5 inches tall, his presentation weight of 64.6 lb rendered a body mass index of 10.7 kg/m 2 . Elfin faced, grinning, genial, with limited intellectual functioning, and in the company of his parents, he arrived on the unit carrying a book on drag racing and a magazine for drummers. Although never in his life heavier than 78 lb, as a result of increasingly restricted patterns of eating, Mr. T lost 10 lb during the 2 months before admission. His parents and primary care physician were deeply concerned and requested inpatient evaluation, medical stabilization, and treatment of Mr. T’s presumed eating disorder.
Because Mr. T’s I.Q. had been previously assessed at about 80, although his parents provided much of the history, Mr. T offered what information and perspectives he could regarding his symptoms, behaviors, and his understanding of their causes. Mr. T’s stated chief complaint was “I get backed up when I eat, so I’m losing weight.” He was not concerned about his current weight but realized that his parents were worried about his weight and health. For their part, Mr. T’s parents worried about not having had formal training in parenting and managing the dietary habits of an adult with a possible eating disorder. They wanted instruction in how to constructively monitor and encourage healthy eating without being overbearing.
History of Present Illness
According to his parents, Mr. T’s lifelong struggle with eating adequate amounts of food resulted in his always being exceptionally slim. Over a period of months before admission Mr. T became guarded and secretive about meals and ate only single food items for weeks at a time, such as pizza, tuna sandwiches, or chicken sandwiches. His parents described him as increasingly preoccupied with food thoughts, becoming distraught when forced to exceed his usual caloric intake boundaries and break his ritualistic eating patterns. For several weeks immediately preceding hospitalization, his poor appetite and caloric intake declined even further, and he progressively lost weight he could ill afford to lose. His habitually limited diet became further restricted, so that he ate only carefully quartered chicken sandwiches, and only on rare days finished an entire sandwich. Daily fluid intake consisted of no more than 16 ounces of bottled water and six ounces of chocolate boost. His parents repeatedly caught him surreptitiously disposing of food into the garbage or in the bathroom, so that they were unable to accurately assess his actual nutritional intake.
Mr. T consistently attributed his aversion to eating to feeling “backed up” after a meal, as though the food were trying to “make its way back up” his throat. He reported no binge eating or purging and was not known by his parents to have ever done so.
Mr. T’s parents believed that his decline in eating resulted from several recent adverse life events affecting the family that disrupted his previous psychological homeostasis: first, approximately a year and a half before admission, Mr. T’s maternal grandfather, a close, warm, and supportive figure, died. Although Mr. T reportedly grieved appropriately at the time of death, he clearly still yearned for his grandfather. Second, about 2 years before admission, Mr. T’s father retired from his long-time career and spent much more time with him than previously. Third, about 6 months before Mr. T’s recent decline, his sister married and left the home.
Although Mr. T’s mood had previously been characterized as generally stable and pleasant, in the wake of these events during the previous 2 years, his parents noted several periods of anxious and depressed mood, corresponding primarily to the aforementioned life events and lasting several months, during which Mr. T also complained of frustration with his low weight.
Two months before presentation, Mr. T’s parents arranged for outpatient psychiatric treatment. His outpatient psychiatrist initially prescribed paroxetine for Mr. T’s compulsive and ritualistic dietary habits, and megestrol to stimulate appetite. Mr. T took just one dose of each medication before stopping them, owing to fear of the potential adverse effects he’d learned about through online research.
Mr. T had never been manic or psychotic, had no history of other compulsive behaviors, such as picking or checking, and had never mentioned or displayed obsessional concerns about any specific aspect of his physical appearance other than weight. He never mentioned feeling suicidal and had no history of self-harm. No other formal psychiatric diagnoses had ever been given.
Medically, about 6 months before admission Mr. T started to experience shortness of breath on exertion but reported no chest pain, orthopnea, or pedal edema. Other than the symptom of early satiety and “back-up” described above, he reported no significant constitutional complaints and had not recently been ill. He had no other history of abdominal pains or other subjective gastro-intestinal complaints, diarrhea or constipation, or any other complaints suggesting inflammatory bowel or other gastro-intestinal disease. His normal pattern of two to three bowel movements weekly continued unaltered.
Developmental, Medical, Personal, and Social History
Based on characteristic facies, cardiac anomalies, and failure to thrive, Mr. T was diagnosed with William’s syndrome by 6 weeks of age. As a toddler, Mr. T. showed a distinct lack of social fear, impaired visuo-spatial construction, and continued failure to thrive. He attended day school for developmentally delayed children, and since completing his special education curriculum at age 18, equivalent to an eighth grade education, he attended a day treatment program. As mentioned above, his IQ was estimated to be approximately 80. Neuropsychological testing was performed when Mr. T was school-aged, but these records were not available.
Medically, by age 19 he required surgical repair of pectus excavatum and supravalvar aortic stenosis, both of which are known to be associated with William’s syndrome (1) . Annual echocardiograms consistently demonstrated stable cardiac function until 2 years before admission, when Mr. T began to show evidence of moderate aortic regurgitation, with an ejection fraction of 50% and normal ankle-brachial indices.
Mr. T’s primary care physician, concerned with his precariously low weight and poor nutritional status, ordered bone densitometry studies when Mr. T was 25 years old. The DEXA revealed a T score consistent with both disordered eating and William’s syndrome-associated hypercalcemia. He had no history of hypertension, diabetes mellitus, or endocrine disturbance.
Socially, for his entire life, Mr. T lived with his parents. He loved listening to music, spent much of his time online, and had recently begun to express interest in weightlifting and in having a girlfriend. Since his father’s retirement, both parents were home with him full-time and focused on his care.
Although friendly and outgoing in manner, Mr. T never developed close friendships outside his family. He had some friends at the daytime rehabilitation program he had attended for years and attended dances for developmentally disabled children on Friday evenings, always accompanied by his parents.
Family Psychiatric History
No other cases of William’s syndrome occurred in Mr. T’s immediate or extended family. His mother described suffering intermittently from depressive symptoms, but she never sought treatment. There was no other acknowledged family history of any psychiatric or substance use disorder.
Physical Examination
Upon admission, Mr. T was cachectic, hypotensive, and tachycardic. He showed the characteristic symmetric microcephaly of William’s syndrome, with full lips, a large mouth, and a short upturned nose. His dentition was healthy and without erosion of enamel, although there was increased spacing between the teeth. Jugular venous pulsations were noted over his very thin neck. His scalp hair and beard were thin. Other than diminished breath sounds bilaterally at the lung bases, his pulmonary examination was normal. His heart rate and rhythm were regular with first and second heart sounds present. There was a prominent fourth heart sound and a left-sided diastolic murmur. His abdomen was retracted and scaphoid, with hyperactive bowel sounds following meals. His extremities were strikingly thin, with severely atrophied musculature, and both brachydactyly and clinodactyly were present bilaterally. There was no detectable edema. Mr. T showed generalized psychomotor slowing, but no focal neurological deficits.
Mental Status Examination
Mr. T was alarmingly cachectic. He was alert, extremely pleasant, and outgoing, and he greeted the team with a broad smile and a weak handshake, made good eye contact, but cocked his head slightly in order to focus his vision during conversation. In general, he moved slowly, but this seemed natural to him. His stated mood was “good,” and he appeared euthymic. His affect was easygoing and reactive, although he seemed almost indifferent to the severity of his physical presentation. His speech was childlike, slow yet enthusiastic, relevant, and responsive. He presented himself as goal directed: “I am determined to gain weight,” but this assertion was clearly contradicted by his inability and frank refusal to consume adequate amounts of food to maintain reasonable body weight. He reported no perceptual disturbances and was not responding to internal stimuli. The nature and origins of his somatic complaints of feeling “backed up” when he ate were unclear, and, at least manifestly, he reported no preoccupying fears of food, weight, or eating. He displayed insight regarding the fact that his weight was much too low; however, consistent with his limited intellectual abilities, despite repeated encouragement and prompting, Mr. T was unable to add any additional details to his limited description of his inner experiences regarding the eating disorder, any greater understanding of why this episode might have occurred, or any further conceptualization about his eating disorder. Based on bedside tests of cognition and higher integrative functioning, the findings were consistent with mild mental retardation.
Investigations and Consultations
Laboratory investigations revealed low serum albumin (3.6 g/dl) and pre-albumin (17.0 mg/dl), and mild anemia (hemoglobin 10.6 g/dl; hematocrit=33%). There were no electrolyte, hepatic, or thyroid function disturbances. Electrocardiogram demonstrated inverted T waves in inferior leads and pronounced P waves in lead 2, showing previous myocardial changes as a direct result of his supravalvar aortic stenosis, and possible nonspecific atrial changes.
Other examinations obtained during hospitalization were as follows: a T 1 -weighted non-contrasted magnetic resonance imaging scan of the brain demonstrated normal anatomy without mass, a midline-shift, hemorrhage, or infarction. The ventricular system and calvarium were unremarkable.
A chest X-ray demonstrated a normal cardiac profile, but hyper-inflated lung volumes with flattened diaphragms, suggesting obstructive pulmonary disease. There was a large left-sided pleural effusion, presumed to be due to hypoalbuminemia. Mild thoracic scoliosis, pectus excavatum, and evidence of prior sternotomy were noted.
An echocardiogram revealed moderate aortic regurgitation and an ejection fraction of approximately 55%.
A computerized tomography scan of the abdomen demonstrated a paucity of intra-abdominal fat and ureteral diverticulum, but no structural anatomic variant of the upper abdomen. An upper endoscopy to the third portion of the duodenum was unremarkable. Barium-contrasted upper gastrointestinal series demonstrated no stricture, hiatal hernia, or inflammatory changes. A gastric emptying study demonstrated markedly delayed and prolonged gastric emptying but no specific outlet obstruction.
DEXA bone densitometry yielded a T score of –1.5, indicating osteopenia.
Consultations
The internal medicine department reported Mr. T was cardiovascularly stable but noted concerning evidence of cor pulmonale on electrocardiogram and recommended follow-up with annual echocardiograms and prophylactic antibiotics before dental work and invasive procedures.
Gastroenterology summarized the studies noted above to indicate delayed gastric emptying but no other abnormalities.
Initial Formulation
Despite acknowledging that he was too skinny, Mr. T presented with food refusal related to a sensation of being “backed up” when he ate, a markedly low body mass index, and a neurogenetic syndrome. Laboratory investigations demonstrated no structural etiology as the basis for his somatic complaints.
In our tentative formulation, we hypothesized that psychological, behavioral, and physical processes all interacted in a highly complex manner. This atypical presentation of an eating disorder appeared to be precipitated by three significant adverse life events. Life-event-induced fears and anxieties, in turn, appeared to provoke physiological changes (e.g., delayed gastric emptying, which led to the patient feeling “backed up,” and perhaps altered appetite), psychological responses (e.g., a perceived need to exert more personal control through food refusal), and the associated behavioral response of reduced food intake. These processes were superimposed on the pre-existing medical and neurodevelopmental challenges of William’s syndrome.
Working Diagnoses and Initial Treatment Plan
Mr. T was given an axis I diagnosis of eating disorder not otherwise specified, manifested by food quantity restriction and inadequate oral intake, food selection restriction, food rituals, and the secreting of food. His complete admission diagnostic profile was recorded as follows: axis 1: eating disorder not otherwise specified, rule out obsessive-compulsive disorder; axis 2: mild mental retardation; axis 3: severe malnutrition, cachexia, William’s syndrome, delayed gastric emptying, supravalvar aortic disease status post repair, osteopenia; axis 4: changes in primary support systems; axis 5: Global Assessment of Functioning Scale=18.
Clinical Course
A protocol for the inpatient treatment of eating disorders was instituted based on the American Psychiatric Association Practice Guideline for the Treatment of Patients With Eating Disorders (2) and inpatient eating disorders protocols developed at the University of Iowa and Cornell University (personal communications). Nutritional rehabilitation, close observation, milieu therapy, and specific behavioral modification protocols to address Mr. T’s eating and anxiety issues were developed and implemented. Nutrition services were closely involved with treatment planning. Psychotherapy to provide education and support was initially conducted with Mr. T alone and with Mr. T together with his parents.
Mr. T struggled with his prescribed oral diet, appearing superficially enthusiastic about eating but actually eating very little. He gained no weight during his first hospital week. On several occasions, he was found to be hiding food in his napkin, in his bathrobe, and in garbage cans. On hospital day six, Mr. T was found to have poured out his boost shake in the bathroom sink after his mother had given him permission to do so.
Understandably, Mr. T’s parents were vulnerable to his expressed frustration with the eating protocols in place, and they were unaccustomed to allowing him to endure upsetting situations. As his parents’ role in encouraging these behaviors became increasingly clear, visits were restricted to 1 hour daily with supervision by the team therapist and social work staff. Focus during sessions shifted to normalizing Mr. T’s natural desire to develop autonomy, and to educating his parents on the importance of discouraging disordered eating behaviors. Time-limited daily visits allowed Mr. T to participate more autonomously in his nutritional rehabilitation. Close contact and behavioral management then successfully controlled many of these surreptitious behaviors, but his oral nutritional intake remained inadequate.
Having effectively failed a staged oral diet, and consistent with his immediate need to gain weight as evidenced by his symptomatically low serum albumin levels resulting in pulmonary effusion, dyspnea, and fatigue, on the eighth hospital day a nasogastric tube was placed to provide urgently needed supplementary nocturnal feeds, which Mr. T tolerated without difficulty. His parents actively supported the nasogastric feedings. By the second hospital week, Mr. T began to gain weight. Daily physical examinations, serum electrolytes, phosphorus, and liver function tests were monitored for the potential emergence of a refeeding syndrome. Mr. T’s father, assigned treatment guardianship, provided informed consent for pharmacotherapy with fluvoxamine (chosen in relation to obsessive-compulsive spectrum symptoms), which was slowly titrated to 100 milligrams daily. Mr. T complied with treatment, albeit reluctantly.
Based on the finding of delayed gastric emptying, the gastroenterology service recommended fluoroscopy-guided placement of a post-pyloric percutaneous feeding tube for long-term nutritional supplementation. This recommendation was not carried out initially.
Two weeks after admission, having gained 8 lb, Mr. T and his parents elected to remove the nasogastric tube used for overnight feedings and return home to attempt aftercare with a regular diet. However, within a day or two following discharge, Mr. T began to experience palpitations, light-headedness, and anxiety, began to lose weight, and within several days returned to the ward with his family for readmission. At that point, upon recommendations of the team, Mr. T and his family decided to go forward with placement of the percutaneous gastrostomy tube. With the successful placement of the tube and the resumption of nocturnal nutritional supplementation, Mr. T subsequently responded well to intensive nutritional rehabilitation and behavioral management. Fluvoxamine was titrated to 200 milligrams daily and tolerated well.
The goals of psychosocial interventions undertaken by his social worker were to help Mr. T understand and cooperate with nutritional and physical rehabilitation, understand, and change the behaviors and dysfunctional attitudes related to his eating disorder, and improve interpersonal and social functioning. On the fifth day of his rehospitalization, for the first time Mr. T confided to his treatment team that he felt all along that his dietary intake was one of the few aspects of life over which he exercised any control. This admission was supported by his concurrent acknowledgement that he desired more independence. He timidly expressed interest in pursuing placement in a group home, away from his parents, where he hoped he would enjoy new opportunities to assert and challenge himself. This opening provided a theme to enhance his motivation to change.
Family therapy was also included, given the centrality of Mr. T’s parents. Conducted by his psychiatrist and social worker, the focus attempted to unite the parents and Mr. T in developing a consistent approach to 1) refeeding, 2) disclaiming “blame” behavior, and 3) addressing his burgeoning wish for autonomy, which conflicted with his parents’ concerns for his safety, given his intellectual and physical limitations (3) .
His parents agreed and worked with our staff to identify suitable options. After 3 weeks, Mr. T was discharged home at our previously set goal weight of 80 lb, slightly above his previous lifetime maximum of 78 lb.
Eight-Month Follow-Up
Mr. T continued to live at home with his parents. Although they had made several inquiries into potential group homes, following discharge, Mr. T and his parents seemed less enthusiastic about the idea of placement, underscoring the challenge of reconciling his desire for autonomy with both his and his parents’ discomfort with actually attaining those goals. His g-tube was revised 5 months post-discharge without complication. At eight months post-discharge, his weight plateaued at 85 lbs, hovering within lb. He continued to see his psychiatrist and continued taking fluvoxamine 200 mg/day. According to his family, Mr. T’s moods remained good and stable, and he had no anxiety symptoms. He continued to take nocturnal g-tube feeds, and his oral appetite varied somewhat. There were no recent episodes of purging or hiding food in the trash. He had recently turned 29 and was looking forward to visiting his sister, about to have a baby, in another state. Best of all, he had acquired sufficient strength to use his brand new drum kit in the garage.
Discussion
William’s syndrome is a neurodevelopmental disorder caused by a hemizygous deletion of approximately 1.6 megabases containing approximately 28 genes on chromosome 7q11.23, resulting in a gene defect associated with elastin (ELN) that is pathognomonic of the syndrome. The diagnosis is made via a fluorescent in-site hybridization probe demonstrating the absent region. These microdeletions are due to spontaneous failure of meiosis, so that William’s syndrome is not ordinarily an inherited condition (1) .
Clinical characteristics include an “elfin” or “pixie-like” appearance of the ears and face. Microcephaly is often seen. Other physical manifestations include a stellate blue iris (thought to be due to the role of elastin in functional localization of pigment), curly hair, undeveloped dentition, brachydactyly, and clinodactyly. The elastin gene defect accounts for the hallmark cardiovascular impairment, supravalvar aortic stenosis, which ultimately results in left ventricular hypertrophy and global cardiopulmonary disease. Calcium dysregulation, hypercalcemia, gastrointestinal problems, orthopedic impairments, hypotonia, and a life expectation of approximately 40 years are common (1) .
Neurological problems include hyperreflexia, strabismus, nystagmus, hyperacusis with hypersensitivity to sound, sensori-neural hearing loss, and coordination difficulties (1 , 4) . Neuropsychiatrically, William’s syndrome is often associated with mild to moderate mental retardation or learning difficulties, with impairments of long-term visual and verbal memory, although short-term memory remains intact. Typically, patients demonstrate a severe visuo-spatial construction deficit. As with our patient, individuals with William’s syndrome are characteristically absorbed with music, and many are highly musical (1 , 4) . Interpersonally, individuals with William’s syndrome are ordinarily socially fearless, engaging eagerly in social interactions with both acquaintances and strangers (1 , 2) .
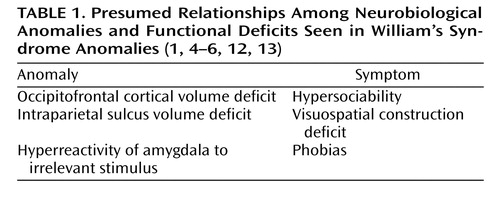
Psychiatrically, a variety of anxiety disturbances are often seen related to nonsocial objects. The most prevalent psychiatric disorders are attention deficit hyperactivity disorder and simple phobias (1 , 5 , 6) . Although patients with William’s syndrome are notably constitutionally thin when they are young, their weights typically approach normal ranges for peers in adulthood (1 , 2) . To our knowledge, eating disorders per se have not previously been described in adolescent or adult individuals with William’s syndrome. Table 1 summarizes the presumed relationships among brain structural anomalies and neurocognitive functional deficits seen in William’s syndrome.
Research into the molecular genetics and the pathophysiology of William’s syndrome is proceeding rapidly, through clinical studies and through the use of animal knockout models. Although the ELN haploinsufficiency that characterizes William’s syndrome leads to its identification as a result of cardiovascular abnormalities, ELN mutations do not affect cognitive function and elastin is not strongly expressed in the brain. The three candidate genes currently believed to contribute most strongly to the neurobehavioral abnormalities are LIM domain kinase 1 (LIMK1), CLIP-115 (Cyln2), and general transcription factor IIi (GTF2I). LIMK1, a regulator of cofilin phosphorylation and actin dynamics, has been robustly connected to the visuospatial deficit by linkage studies. Cyln2 encodes a cytoplasmic linker protein implicated in the local regulation of microtubule dynamics, especially in response to positional cues. Knockout mice lacking this gene develop coordination impairment and decreased contextual, but not cue-conditioned, fear responses. GTP2I is a ubiquitously expressed transcription factor linking signal transduction to transcription. Based on kindred analyses, a hemideletion of the GTF2I gene appears necessary for the mental retardation seen in individuals with William’s syndrome (1) .
Clinically, the life-course of patients with William’s syndrome is known to be foreshortened, with patients ordinarily living to their 40s. As with any developmentally, intellectually, and neuropsychologically challenged individuals, the difficulties to be faced by these patients, their families, and clinicians are considerable. For any given individual, the challenges include establishing appropriate social, educational, and vocational expectations, determining what can be done to maximize the individual’s capacity for autonomy and quality of life, and fostering successful and enduring relationships with caregivers (5 , 6) .
Unanswered questions abound regarding the nature and quality of attachments of individuals with William’s syndrome and their abilities to emotionally self-nourish and self-regulate. Parents of these patients are frequently preoccupied with how to best care for and protect their children, who seem open, friendly, and lacking in social fear. National support groups have been established through which families offer each other the benefits of their experiences (see http://www.wsf.org/family/support/orgs.htm). Undoubtedly, such mutual aid provides one of the most helpful resources available to patients and families. Many questions remain regarding the psychological and pharmacological management of patients with William’s syndrome who happen to develop co-morbid psychiatric disorders, including, most commonly, anxiety spectrum pathology. In the absence of a substantial database, clinicians are left to judiciously approach the problems they encounter with compassionate combinations of education, individual and family psychotherapies, and the medications known to be effective for treating such disorders in non-William’s syndrome individuals (1 , 2 , 5) .
Weight and Eating Behavior Considerations
William’s syndrome patients tend to be very thin when young, but growth chart data on these patients show that their body mass indices on average tend to approach the 50th percentile in adulthood, and some even become obese (1) .
The patient had a very slight-built body habitus to begin with. Given the fact that he never previously weighed more than 78 lb (body mass index=13.0 kg/m 3 ) in his life, how could we assess the presence of an eating disorder, and what diagnosis did this merit? Eating disorders manifest disturbed eating-associated psychological and behavioral processes often accompanied by physical impairments due to poor nutritional practices and related maladaptive routines. If we can presume that patients with William’s syndrome ordinarily eat appropriately in accord with their genetically determined nutritional needs and approximate set-point ranges, the fact that many are slim does not by itself indicate the presence of an eating disorder (7) .
But, clearly, it is more complicated than that. Why Mr. T would settle specifically on undereating as a primary interpersonal strategy around which to play out a need to exert control in relation to his parents is unclear. Certainly, his tilt in that direction may have simply resulted from an iterative snowballing of physiological vulnerabilities, the anxiety-inducing impact of his curtailed eating on his parents, and, in turn, the impact of their reflected efforts to take control of his eating on his diminished sense of autonomy. These interpersonal iterations, in which Mr. T’s parents showed increasingly anxious concerns, may have offered him some unfortunate combination of attention and gratification. However, if Mr. T truly felt painfully hungry as a result of his reduced caloric intake, he would probably not have maintained a battle around eating. Of note, neither his parents nor the staff perceived that he was experiencing or fighting off hunger pangs, consistent with the observation that after prolonged food restriction patients with eating disorders often experience little hunger.
Mr. T demonstrated the considerable challenges facing clinicians who attempt to unearth the psychological underpinnings of disordered eating in individuals who function in this intellectual range. Patients with such limited intellects are unable to adequately describe their inner experiences or conjecture about their origins or meanings. Furthermore, the patient never communicated that he was personally suffering much subjective distress as a result of his eating disorder, although we suspect that this lack of distress owed more to his eating disorder than to his intellectual limitations. Did the patient harbor a distorted self-image with regard to his shape and weight? There’s little evidence to support this idea since he repeatedly emphasized that he thought he was “too skinny” at his low weight, suggesting that he retained at least an intellectual appreciation for the unhealthiness of his status. Was he “fat phobic”? Again, at least according to his declarations, there’s no evidence to support this hypothesis.
Mr. T’s feeling of being “backed up” suggests a somatic sensation of bodily tension, perhaps a visceral somatization, in association with ingestion, something seen when one attempts to overfeed or force feed individuals experiencing true anorexia (the symptom). A gastrointestinal workup showed that he did have functionally delayed gastric emptying. Gastroparesis is often associated with satiety and nausea (1) . While malnutrition itself has been associated with delayed gastric emptying, a finding frequently found in conjunction with bloating and gastrointestinal distress during refeeding, the mechanisms by which this occurs are not well understood. Substantial deficiencies exist in current knowledge relating to the pathophysiology of gastroparesis (1 , 8 , 9) , although in the restrictive type of anorexia nervosa, delayed gastric transit has been associated with atrophy of the smooth muscles of the gastrointestinal tract (2) . Numerous other hormone and neurotransmitter agents, including CRF, CCK, NPY, agouti, glucagons, and gastric-related peptides, nitrous oxide, and a variety of cytokines, among others, affect appetite, satiety, and transit in the gut (1 , 2) . Furthermore, we know that appetite loss occurs in patients with major depression and anxiety syndromes, again resulting from poorly understood mechanisms. Cytokines such as tumor necrosis factor have been implicated in the pathophysiology of the “psychogenic” anorexia occurring in depression (1 , 2) . How the patient’s other neuropsychological limitations might create vulnerabilities for the eating disorder-related thoughts and behaviors is unclear, but we might speculate that unstudied processes involving early satiety might be present, so that food restriction might not have been as subjectively painful for Mr. T as for others. On balance, it is conceivable that as yet poorly understood physiological processes, perhaps provoked by Mr. T’s increased distress about intercurrent life events and family processes ultimately evoked the sensation of “backing up” and turned off his appetite, making it easier for him to subsequently construe his weight loss in terms of interpersonal conflict. Although some children, adolescents, elderly demented individuals, and otherwise powerless political prisoners may initiate food refusal or “hunger strikes” as a manifestation of willfulness and passive-aggressive attempts to achieve some degree of control over overpowering situations, most individuals find other means to deal with their conflicts (10 , 11) .
General management strategies for treating patients with eating disorders apply to the patient’s case as well (2) . These strategies, described in the American Psychiatric Association’s Practice Guideline for the Treatment of Patients With Eating Disorders, emphasize the importance of attending to physical, behavioral, psychological, and interpersonal, particularly family, issues, none to the exclusion of the others. Clinicians are obliged to assess the relative urgency and priorities of the patient’s problems in these domains for the immediate and long term. In turn, clinicians must decide on the urgency and on priorities for initiating suitable interventions addressing each of these problem areas. The first priority is the patient’s physical safety, specifically, assuring sufficient nutritional intake to prevent malnutrition and physiological collapse. When it became clear that usual behavioral and supportive interventions were insufficient and that even adding overnight supplemental feedings shown elsewhere to assist in the treatment of pediatric anorexia nervosa patients (1) was not adequate, the more definitive option of a percutaneous gastrostomy tube seemed necessary. How accessible Mr. T might be over the long run to various psychotherapeutic strategies, including educational, cognitive, behavioral, and interpersonal approaches, remains to be seen. We continue to support the idea of the patient moving to a group home environment, where he would have the necessary support system to manage his individual needs while exploring a more independent and autonomous existence. We also acknowledge the difficulty of this process for both Mr. T and his parents. Following the patient over the ensuing months and years will be informative regarding the resolution of his eating attitudes and behaviors, his interactions with his parents, and his ability to achieve a reasonable quality of life.
1. Meyer-Lindenberg A, Mervis CB, Berman KF: Neural mechanisms in William’s syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci 2006; 7:380–393Google Scholar
2. American Psychiatric Association Practice Guideline for the Treatment of Patients With Eating Disorders, 3rd Revision, Part A. Am J Psychiatry 2006; 163, pp 1–54Google Scholar
3. O’Reilly MF, Lancioni GE: Treatment food refusal in a child with Willam’s syndrome using the parent as therapist in the home setting. J Intellect Disabili Res 2001; 45(Part I); 41–46Google Scholar
4. Sacks O: A hypermusical species: William’s syndrome, in Musicophilia. New York, Knopf, 2008, pp 317–334Google Scholar
5. Stewart E: Behavioral and emotional disturbance in individuals with William’s syndrome. Am J Ment Retard 1997; 102:45–53Google Scholar
6. Dykens E: Anxiety, fears and phobias in persons with William’s syndrome. Dev Neuropsychol 2003; 23:291–316Google Scholar
7. Sarimski K: Specific eating and sleeping problems in Prader-Willi and William’s-Beuren Syndrome. Child Care Health Dev 1996; 22:143–150Google Scholar
8. Hutson WR, Wald A: Gastric emptying in patients with bulimia nervosa and anorexia nervosa. Am J Gastroenterology 1990; 85:41–46Google Scholar
9. De Caprio C, Pasansi F, Contaldo F: Gastrointestinal complications in a patient with eating disorders. Eat Weight Disorder 2000, 5:228–230Google Scholar
10. Morley JE: Anorexia and weight loss in older persons. J Gerontol 2003; 58:M131–M137Google Scholar
11. Lee JS, Kritchevsky SB, Tylavsky F, Harris TB, Ayonayon HN, Newman AB: Factors associated with impaired appetite in well-functioning community-dwelling older adults. J Nutr Elder 2006; 26:27–43Google Scholar
12. Farran E: Evidence for unusual spatial location coding in William’s syndrome: and explanation for the local bias in visuo-spatial construction tasks? Brain Cogn 2005; 59:159–172Google Scholar
13. Einfeld S: Longitudinal course of behavioral and emotional problems in Williams syndrome. Am J Ment Retard 2001: 1006:73–81Google Scholar