Stimulant Therapy and Risk for Subsequent Substance Use Disorders in Male Adults With ADHD: A Naturalistic Controlled 10-Year Follow-Up Study
Abstract
Objective: The extant literature does not provide definite answers pertaining to whether stimulant treatment increases, decreases, or does not affect the risk for subsequent substance use disorders in youths with attention deficit hyperactivity disorder (ADHD). The authors examined the association between stimulant treatment in childhood and adolescence and subsequent substance use disorders (alcohol, drug, and nicotine) into the young adult years. Method: The authors conducted a 10-year prospective follow-up study. One hundred forty male Caucasian children with ADHD, ages 6 to 17, were examined at baseline. Of these, 112 (80%) were reassessed at the 10-year follow-up (mean age at follow-up=22 years). Assessments were made using Cox proportional hazards survival models. All models were adjusted for conduct disorder, since conduct disorder is a potent predictor of subsequent substance use disorders. Results: Of the 112 ADHD subjects who were reassessed at the 10-year follow-up, 82 (73%) had been treated previously with stimulants and 25 (22%) were undergoing stimulant treatment at the time of the follow-up assessment. There were no statistically significant associations between stimulant treatment and alcohol, drug, or nicotine use disorders. Conclusions: The findings revealed no evidence that stimulant treatment increases or decreases the risk for subsequent substance use disorders in children and adolescents with ADHD when they reach young adulthood.
Attention deficit hyperactivity disorder (ADHD) is a childhood-onset neuropsychiatric behavioral disorder (1) that affects up to 10% of children (2) and is associated with a wide range of functional impairments (3 – 6) . Although stimulants remain the mainstay of treatment for ADHD, there are questions regarding the risk for subsequent substance use disorders (7) . One group of investigators found that cocaine and nicotine abuse were associated with previous stimulant treatment in children with ADHD (8 – 10) . In contrast, a growing body of literature supports the alternative hypothesis that stimulant treatment does not increase susceptibility to the development of subsequent substance use disorders (11 – 15) , and some studies, including a meta-analysis (16 , 17) , have shown that stimulant treatment may even exert some protective effects against the development of substance use disorders (18 , 19) .
In addition to contradictory findings, the extant literature suffers from important methodological limitations. The studies that have reported only on adolescent outcomes (18 , 19) do not address whether stimulants increase the risk for substance use disorders during the young adult years, a developmental period of heightened risk. Many of these studies did not control for the presence of comorbidity with conduct disorder, a well documented risk for substance use disorders (20) . Another shortcoming in the literature pertains to inadequate attention to the different substances of abuse (alcohol, drug, and nicotine) separately (10 , 16 , 19) . Other studies did not use DSM-defined attention deficit disorder/ADHD as the ascertainment criterion to recruit subjects (11 – 13) , limiting generalizability to the ADHD population. Finally, some studies used logistic regression models (8 , 12 – 14 , 18) , which do not account for subjects who did not develop substance use disorders but were not beyond the period of risk. Considering the clinical and public health implications, further examination of this concern is warranted.
The objective of the present study was to re-examine the association between stimulant treatment and subsequent substance use disorders, addressing the shortcomings of the extant literature. We used Cox proportional hazards survival models to examine the risk for alcohol, illicit drug, and nicotine use disorders in a sample of youths with ADHD, who were followed prospectively for 10 years as a function of stimulant treatment, while accounting for conduct disorder. Our primary research objectives were as follows: 1) to examine whether prior stimulant treatment increased the risk for subsequent substance use disorders in ADHD youths grown up; 2) to examine age at stimulant treatment onset as a predictor of subsequent substance use disorders in ADHD subjects; and 3) to examine the duration of stimulant treatment as a predictor of subsequent substance use disorders in ADHD subjects.
Method
Subjects
Subjects were derived from a longitudinal case-control family study of ADHD (21 , 22) . At baseline, we ascertained male Caucasian youths with DSM-III-R ADHD (N=140) and without DSM-III-R ADHD (N=120). These subjects were 6 to 17 years old and from pediatric and psychiatric clinics. Potential subjects who were excluded from this sample were those who had been adopted or whose nuclear family was not available for study. We also excluded potential subjects if they had major sensorimotor disabilities (paralysis, deafness, blindness), psychosis, autism, inadequate command of the English language, or a Full Scale IQ score less than 80. All of the ADHD subjects met full DSM-III-R diagnostic criteria for ADHD at the time of clinical referral, and all had active symptoms of the disorder at the time of recruitment into the study. This sample (ADHD and healthy comparison subjects) was followed up at 1, 4, and 10 years after baseline. The present study reports on the 10-year follow-up of the ADHD probands only, of which 112 were successfully reascertained.
Parents and adult offspring provided written informed consent to participate in the study, and parents provided consent for offspring under the age of 18. Children and adolescents gave written assent. The Massachusetts General Hospital Human Research Committee approved the study.
We selected subjects from two independent sources, one psychiatric and one pediatric, who provided the index of children. The psychiatric referral source we used is a major academic medical center, and we selected ADHD subjects from patients who were consecutively referred to the center’s pediatric psychopharmacology clinic. We selected healthy comparison subjects from outpatients referred for routine physical examination to pediatric medical clinics of this same medical center. The pediatric referral source we used is a major health maintenance organization (HMO), and we selected ADHD subjects from consecutively ascertained pediatric clinic outpatients who were identified in the HMO’s records as having ADHD. We selected healthy comparison subjects from outpatients referred to the HMO’s pediatric medical clinics for routine physical examination who were identified in the HMO’s records as not having ADHD. We have previously demonstrated that there were no clinically or statistically significant differences among the ADHD subjects ascertained from these two referral sources on measures of psychopathology, cognitive performance, or psychosocial functioning (23) .
We used a three-stage ascertainment procedure to select subjects in order to decrease false positives and improve the accuracy of psychiatric diagnoses (24 , 25) . For ADHD subjects, the first stage was their referral, resulting in a clinical diagnosis of ADHD by a child psychiatrist or pediatrician. Since many different clinicians using different clinical standards made these diagnoses, we included a second systematic stage that confirmed the diagnosis of ADHD by administering a telephone questionnaire to the patients’ mothers. Eligible case children who met study entry criteria were recruited for the study and underwent the third stage, which was a diagnostic assessment with a structured interview. Only patients who received a positive diagnosis at all three stages were included.
Follow-Up Assessment Procedures
Psychiatric assessments at the 10-year follow-up relied on the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version (K-SADS-E) (26) for subjects younger than 18 years of age and the Structured Clinical Interview for DSM-IV (SCID) (27) (supplemented with modules from the K-SADS-E to assess childhood diagnoses) for subjects 18 years of age and older. We conducted a direct interview with each subject and an indirect interview with each subject’s mother (i.e., the mothers completed the structured interview about their offspring). We combined data from both the direct and indirect interviews by considering a diagnostic criterion positive if it was endorsed in either interview.
We considered a disorder positive if DSM-IV criteria were unequivocally met. Although standardized algorithms were used to determine each diagnosis, interviewers needed a mechanism to determine the clinical relevance of symptoms when subjects were only able to provide unclear or imprecise information. Thus, a committee of board-certified child and adult psychiatrists who were blind to the subjects’ ADHD status, referral source, and all other data resolved diagnostic uncertainties. Diagnoses presented for review were considered positive only when the committee determined that diagnostic criteria were met to a clinically meaningful degree.
The interviewers were blind to the subjects’ baseline ascertainment group, the ascertainment site, and all prior assessments. The interviewers had undergraduate degrees in psychology and were extensively trained. First, they underwent several weeks of classroom style training, in which they learned interview mechanics, diagnostic criteria, and algorithm coding. Then, they observed interviews by experienced raters and clinicians. They subsequently conducted at least six practice (nonstudy) interviews and at least three study interviews while being observed by senior interviewers. Trainees were not permitted to conduct interviews independently until they executed at least three interviews that achieved perfect diagnostic agreement with an observing senior interviewer. The principal investigator (Dr. Biederman) supervised the interviewers throughout the study. We computed kappa coefficients of agreement by having experienced, board-certified child and adult psychiatrists and licensed clinical psychologists diagnose subjects from audiotaped interviews. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98. Kappa coefficients for individual diagnoses included the following: ADHD, 0.88; conduct disorder, 1.0; major depression, 1.0; mania, 0.95; separation anxiety, 1.0; agoraphobia, 1.0; panic disorder, 0.95; substance use disorder, 1.0; and tics/Tourette’s syndrome, 0.89.
Socioeconomic status was measured using the 5-point Hollingshead scale (28) . To measure psychopharmacological treatment, we collected the following information about each subject for each medication used: name of medication, age at onset of treatment, and age at treatment termination. Thus, we were able to determine each subject’s stimulant therapy history.
Substance Use Measures
Our diagnostic interviews collected data on the lifetime use of nicotine, alcohol, marijuana, cocaine, amphetamines, sedatives, hallucinogens, opiates, steroids, glue, ecstasy, and nonprescription sleeping or diet pills. Hereafter, all substances, with the exception of alcohol and nicotine, will be referred to as “drugs.” For every substance used by a given subject, we derived the age at first use, lifetime diagnosis of DSM-IV abuse or dependence, and age at onset.
Statistical Analysis
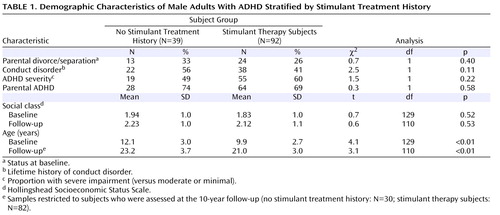
To assess the effect of attrition, we compared the baseline characteristics of subjects who were and were not assessed at the 10-year follow-up. Then, we stratified the ADHD subjects according to a lifetime history of receiving stimulant therapy. Among subjects who were followed-up at the 10-year assessment, we compared ADHD subjects with and without a lifetime history of stimulant medication on follow up demographic factors using Pearson chi square tests and t tests for binary and dimensional variables, respectively.
To estimate the lifetime risk for substance use disorders associated with stimulant therapy, we used Cox proportional hazards survival models. We evaluated the following substance use outcomes: alcohol abuse, alcohol dependence, drug abuse, drug dependence, and nicotine dependence. For each outcome, rates were defined as a positive response at any assessment (baseline, 1-year follow-up, 4-year follow-up, or 10-year follow-up) versus a negative response at all assessments. These models utilized all available data for each subject, including those who were not assessed at the 10-year follow-up. Thus, all 140 subjects were included, using as many waves of follow-up data available. We used the earliest age at onset as the survival time for case subjects and the age at most recent interview as the time of censoring for non-case subjects. Each outcome was modeled as a function of lifetime stimulant treatment, conduct disorder, and any other confounding variable.
To establish a measure of lifetime stimulant treatment, we created a binary indicator variable for each substance outcome, which was defined as positive if 1) subjects reported a lifetime history of treatment with any stimulant (amphetamine products [mixed amphetamine salts, d -amphetamine], methylphenidate products [immediate-release methylphenidate, osmotic-release oral system methylphenidate, transdermal methylphenidate, d-methylphenidate, extended-release methylphenidate], and pemoline) and 2) they did not meet criteria for the substance use outcome before the onset of treatment. Thus, the stimulant-treatment variable for a given substance outcome was coded positive only for subjects who were free from that substance use outcome at the age when their stimulant therapy began. Untreated subjects and subjects who began stimulant treatment after the onset of the substance use were defined as negative on this binary variable. Subjects whose treatment and substance outcome began at the same age were impossible to categorize and were dropped from the analysis of that outcome. Since we assessed multiple substance outcomes, each with its own age of onset, the number of subjects dropped ranged from zero for alcohol and drug dependence to two for drug abuse and smoking.
To calculate power for our Cox regression models, we used the statistical software package Power Analysis and Sample Size System (PASS) (29) , which provides power and sample size calculations for Cox regression. The power analyses we used assumed a sample size of 131 (a two-tailed test), an alpha level of 0.05, and a standard deviation of the independent variable (stimulant treatment) of 0.46. Assuming an overall substance use disorder rate of 35%, we had 81% and 93% power to detect hazard ratios of 2.5 and 3.0, respectively. As the overall substance use disorder rate increased, our power increased, and with an overall rate of 50%, our power to detect hazard ratios of 2.5 and 3.0 was 93% and 98%, respectively. Thus, we were adequately powered to detect moderate-sized effects across a range of substance use disorder rates. The statistical significance of each covariate in these regression models was determined by Wald’s test, and our alpha level was set at 0.05. All tests were two-tailed, and we reported hazard ratios and 95% confidence intervals (CIs) for each model.
Results
Attrition and Demographic Characteristics
Of the 140 ADHD subjects recruited at baseline, 112 (80%) were successfully reassessed at the 10-year follow-up. As stated in a previous report of this sample, there were no significant differences between those who were successfully followed up and those who were lost to follow-up on age, familial intactness, ascertainment source, or psychiatric outcomes (all p values ≥0.05) (6) . However, a significant difference was found in socioeconomic status, with ADHD subjects lost to follow-up having a lower mean socioeconomic status compared with subjects successfully reassessed (2.4 [SD=1.2] versus 1.8 [SD=0.9], respectively; t=3.1, df=138, p<0.01).
Of the 112 ADHD subjects assessed at the 10-year follow-up, 82 (73%) were treated with stimulant medications at some time in their lives, and 25 (22%) were being treated with stimulants during the follow-up period (i.e., in the past month at the time of the 10-year follow-up assessment). Of the entire sample of 140 ADHD probands, 92 (66%) reported a lifetime history of stimulant treatment. There was no significant difference in the rate of 10-year follow-up between ADHD probands with (89%) and without (77%) a lifetime history of stimulant therapy (χ 2 ≥3.3, df=1, p=0.07). Nine subjects did not provide information pertaining to stimulant therapy and were dropped from subsequent analyses.
The mean age at stimulant treatment onset was 8.8 years (SD=3.5). Fifty percent of subjects began their treatment between the ages of 6 and 10. The mean duration of treatment was 6 years (SD=4.7), with 50% of subjects undergoing stimulant treatment for 2 to 10 years.
As shown in Table 1 , no significant differences were detected in rates of intactness of family of origin, comorbidity with conduct disorder, baseline ADHD severity, parental history of ADHD, or social class among ADHD subjects with versus without a stimulant therapy history. However, significant differences were detected in subjects’ mean age at both baseline and follow-up, with no stimulant treatment history subjects having a younger mean age compared with stimulant therapy subjects.
Association Between Stimulant Treatment and Subsequent Substance Use Disorders
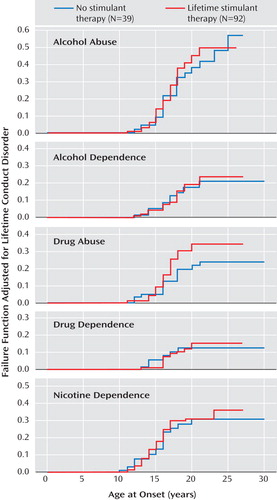
Figure 1 illustrates Kaplan-Meier failure functions adjusting for conduct disorder for each substance use outcome (i.e., alcohol abuse, alcohol dependence, drug abuse, drug dependence, and nicotine dependence) stratified by stimulant treatment status. Based on our Cox proportional hazards models controlling for conduct disorder, there was no statistical evidence for either an increased or decreased risk for any substance use disorders in subjects who received stimulant treatment (alcohol abuse: hazard ratio=1.1 [95% CI=0.6–2.1], z=0.4, p=0.66; alcohol dependence: hazard ratio=1.0 [95% CI=0.5–2.4], z=0.1, p=0.93; drug abuse: hazard ratio=1.6 [95% CI=0.8–3.2], z=1.2, p=0.23; drug dependence: hazard ratio=1.0 [95% CI=0.4–2.6], z=0.1, p=0.96; nicotine dependence: hazard ratio=1.1 [95% CI=0.6–2.1], z=0.4, p=0.72). Additional data are presented in the data supplement accompanying the online version of this article.
Effect of Age at Onset of Stimulant Treatment on Subsequent Substance Use Disorders
We then estimated the effect of the age at onset of stimulant treatment on the risk for subsequent substance use disorders in Cox proportional hazards regression models adjusted for a lifetime history of conduct disorder, which was restricted to the subjects with a positive lifetime stimulant treatment history. Across the five substance use disorder outcomes, there was no significant association between the age of stimulant treatment onset and the risk for subsequent substance use disorders (alcohol abuse: hazard ratio=1.0 [95% CI=0.9–1.1]; alcohol dependence: hazard ratio=1.0 [95% CI=0.9–1.1]; drug abuse: hazard ratio=1.0 [95% CI=0.9–1.1]; drug dependence: hazard ratio=1.0 [95% CI=0.9–1.2]; nicotine dependence: hazard ratio=0.9 [95% CI=0.8–1.1]; all p values >0.05).
Effect of Stimulant Treatment Duration on Subsequent Substance Use Disorders
Next, we estimated the effect of stimulant treatment duration on the risk for subsequent substance use disorders in Cox proportional hazards regression models adjusted for a lifetime history of conduct disorder, which was restricted to the subjects with a positive lifetime stimulant treatment history. Across the five substance use disorder outcomes, there was no significant association between the duration of stimulant treatment and the risk for substance use disorders (alcohol abuse: hazard ratio=1.1 [95% CI=0.9–1.1]; alcohol dependence: hazard ratio=1.0 [95% CI=0.9–1.1]; drug abuse: hazard ratio=1.0 [95% CI=0.9–1.1]; drug dependence: hazard ratio=1.0 [95% CI=0.9–1.1]; nicotine dependence: hazard ratio=1.1 [95% CI=0.9–1.2]; all p values >0.05).
Effect of Stimulant Treatment on the Duration of Substance Use Disorders
Finally, among subjects with a substance use disorder diagnosis, we estimated the effect of stimulant treatment on the duration of specific substance use disorder outcomes using linear regression, adjusting for conduct disorder history and baseline age. The only finding was a longer duration of alcohol abuse in subjects who received stimulant treatment compared with subjects who did not receive stimulant treatment (adjusted mean increase of 1.6 years: t=2.1, df=57, p=0.04). There were no other significant associations between stimulant treatment and duration of diagnosis across the other four substance use disorder outcomes (alcohol dependence: t=0.5, df=28, p=0.60; drug abuse: t=0.4, df=40, p=0.72; drug dependence: t=0.6, df=21, p=0.53; and nicotine dependence: t=0.3, df=48, p=0.75).
Discussion
In a longitudinal sample of male subjects diagnosed with ADHD in childhood and followed up for 10 years into their young adult years, we found no evidence that prior treatment with stimulants was associated with subsequent increased or decreased risk for alcohol, drug, or nicotine use disorders. Further, we did not detect any significant association between age at stimulant treatment onset and subsequent substance use disorders or any associations between the duration of stimulant treatment and subsequent substance use disorders. These findings support the hypothesis that stimulant treatment does not increase the risk for subsequent substance use disorders. To date, this study represents the most methodologically rigorous assessment concerning the question of whether stimulant treatment increases the risk for subsequent substance use disorders, with follow-up into adult years, adjustment for conduct disorder, testing of a diverse set of substance outcomes, use of DSM criteria to define case status, and use of proportional hazards survival models.
The present results failed to replicate our previously published 4-year adolescent follow-up of this same sample, which detected a protective effect of stimulant treatment (18) . Although the reasons for these discrepant findings are not entirely clear, the most likely factor to account for this discrepancy is the additional information gained through continued follow-up. In support of this notion, the meta-analyses conducted by Wilens et al. (16 , 17) showed that stimulant-treated subjects were 5.8 times less likely to develop substance use disorders relative to untreated subjects in studies that extended their follow-up only into adolescence. In contrast, stimulant-treated subjects were only 1.4 times less likely to develop substance use disorders relative to untreated subjects in studies that followed children into adulthood. This lack of a protective effect of stimulants into adulthood was also seen in Faraone et al.’s (15) retrospective study of ADHD adults.
We do not know why the protective effect of stimulants is not evident in adulthood. It is possible that because of parental monitoring, treatment compliance and hence efficacy is greater for youths than adults. Another possibility is that because adolescents have not fully passed through the age of risk to develop substance use disorders, stimulants may delay rather than stop subsequent substance use disorders. More research is needed to understand this developmental effect and clarify protective mechanisms. Nevertheless, these data stress the importance of continued follow-up through the period of risk in naturalistic studies examining this question.
Our results are consistent with several other studies that failed to detect meaningful associations between stimulant treatment and subsequent substance use disorders (11 – 14) . The consistency of results across different studies is particularly impressive given the diversity of case definitions used by different studies to ascertain subjects (developmental reading disorders [13] , hyperkinetic reaction of childhood/minimal brain dysfunction [11] , and hyperactivity [12] ).
Our results are not consistent with a study that documented an association between stimulant therapy and subsequent tobacco dependence in ADHD participants (30) . However, the stimulant-treated group in this study had an overrepresentation of conduct disorder, a powerful predictor of substance use, including tobacco (16) . The groups in our study were more balanced on rates of conduct disorder, and our analyses statistically adjusted for its effects. In fact, in our analyses conduct disorder was a powerful predictor of smoking dependence (hazard ratio=2.7, p=0.001), independent of stimulant treatment.
Across the 20 hypothesis tests we conducted in the assessment of our hypotheses, we only detected one statistically significant association: among subjects with alcohol abuse, stimulant treatment predicted a longer duration of alcohol abuse. Given that this finding would not survive even the most liberal correction for multiple testing and its incongruence with the rest of the results, we recommend interpreting this result with caution.
These findings must be considered in light of several methodological issues. We do not know whether our results will generalize to ADHD children in the general population, to other racial or ethnic backgrounds, or to female subjects. Our sample was originally ascertained according to DSM-III-R criteria, and it is possible that our results may not generalize to samples ascertained by DSM-IV criteria. However, considering the very high overlap between the two definitions (93% of DSM-III-R case subjects received a DSM-IV diagnosis [31] ), any effect should be minimal. Although our study was prospective, we still relied on retrospectively (i.e., within the intervals between assessments) reported ages at stimulant treatment and substance use disorder onset to establish the temporal sequence. Our results suffer from misclassification (and thus a reduction in precision) to the degree that these ages were incorrectly recalled. However, while the exact ages may not have been recalled accurately, the relative ordering of the ages at stimulant treatment and substance use disorders is likely to be correct, and thus any misclassification of our exposure and outcome variables should be minimal. Finally, our naturalistic study design cannot provide the more informative evidence that would be produced by a randomized controlled study of stimulant treatment. For example, we did not have the advantage of detailed data on dose and treatment adherence over time.
Despite these methodological issues, the results in the present study converge with previous studies toward helping to alleviate concerns among clinicians about future substance use disorder problems when prescribing stimulants to children with ADHD. Future research should focus on more salient predictors and moderators of substance use disorder risk in male patients with ADHD and extend these findings to minority and female samples of ADHD patients.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 1994Google Scholar
2. Faraone SV, Sergeant J, Gillberg C, Biederman J: The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2003; 2:104–113Google Scholar
3. Mannuzza S, Klein RG, Bessler A, Malloy P, Hynes ME: Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry 1997; 36:1222–1227Google Scholar
4. Mannuzza S, Klein R, Bessler A, Malloy P, LaPadula M: Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry 1998; 155:493–498Google Scholar
5. Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, Wilens TE, Frazier E, Johnson MA: Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002; 159:36–42Google Scholar
6. Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Silva J, Snyder L, Faraone SV: Young adult outcome of attention deficit hyperactivity disorder: a controlled 10 year prospective follow-up study. Psychol Med 2006; 36:167–179Google Scholar
7. Robinson TE, Berridge KC: The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993; 18:247–291Google Scholar
8. Lambert NM, McLeod M, Schenk S: Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction 2006; 101:713–725Google Scholar
9. Lambert NM: Stimulant treatment as a risk factor for nicotine and substance abuse, in Attention Deficit Hyperactivity Disorder: State of the Science, Best Practices. Edited by Jensen PS, Cooper J. Kingston, NJ, Civic Research Institute, 2002, pp 1–24Google Scholar
10. Lambert NM: Stimulant treatment as a risk factor for nicotine use and substance abuse, in NIH Consensus Development Conference Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder. Bethesda, Md, NIH, 1998, pp 191–198Google Scholar
11. Paternite CE, Loney J, Salisbury H, Whaley MA: Childhood inattention-overactivity, aggression, and stimulant medication history as predictors of young adult outcomes. J Child Adolesc Psychopharmacol 1999; 9:169–184Google Scholar
12. Barkley RA, Fischer M, Smallish L, Fletcher K: Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse?: A 13-year prospective study. Pediatrics 2003; 111:97–109Google Scholar
13. Mannuzza S, Klein RG, Moulton JL: Does stimulant treatment place children at risk for adult substance abuse?: A controlled, prospective follow-up study. J Child Adolesc Psychopharmacology 2003; 13:273–282Google Scholar
14. Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ: Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: a population-based, birth cohort study. J Child Adolesc Psychopharmacol 2005; 15:764–776Google Scholar
15. Faraone SV, Biederman J, Wilens TE, Adamson JJ: A naturalistic study of the effects of pharmacotherapy on substance use disorders among ADHD adults. Psychological Med 2007; 37:1743–1752Google Scholar
16. Wilens T, Faraone SV, Biederman J, Gunawardene S: Does stimulant therapy of attention deficit hyperactivity disorder beget later substance abuse?: A meta-analytic review of the literature. Pediatrics 2003; 111:179–185Google Scholar
17. Faraone SV, Wilens T: Does stimulant treatment lead to substance use disorders? J Clin Psychiatry 2003; 64(suppl 11):9–13Google Scholar
18. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV: Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999; 104:e20Google Scholar
19. Whalen CK, Jamner LD, Henker B, Gehricke JG, King PS: Is there a link between adolescent cigarette smoking and pharmacotherapy for ADHD? Psychol Addict Behav 2003; 17:332–335Google Scholar
20. Fischer M, Barkley RA: Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry 2003; 64(suppl)11:19–23Google Scholar
21. Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT: Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 1992; 49:728–738Google Scholar
22. Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, Mennin D, Marrs A, Ouellette C, Moore P, Spencer T, Norman D, Wilens T, Kraus I, Perrin J: A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry 1996; 53:437–446Google Scholar
23. Busch B, Biederman J, Cohen L, Faraone S, Sayer J, Monuteaux M, Mick E, Zallen B: Similar correlates of ADHD in children from pediatric and psychiatric clinics. Psychiatr Serv 2002; 53:1103–1111Google Scholar
24. Faraone SV, Tsuang MT, Tsuang D: Genetics and Mental Disorders: A Guide for Students, Clinicians, and Researchers. New York, Guilford, 1999Google Scholar
25. Faraone S, Tsuang M: Measuring diagnostic accuracy in the absence of a “gold standard.” Am J Psychiatry 1994; 151:650–657Google Scholar
26. Orvaschel H: Schedule for Affective Disorder and Schizophrenia for School-Age Children, Epidemiologic Version. Ft. Lauderdale, Fla, Nova Southeastern University, Center for Psychological Studies, 1994Google Scholar
27. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC, American Psychiatric Publishing, 1997Google Scholar
28. Hollingshead AB: Four Factor Index of Social Status. New Haven, Conn, Yale University Press, 1975Google Scholar
29. Hintze JL: PASS (Power Analysis and Sample Size System). Kaysville, Utah, NCSS, 2005Google Scholar
30. Lambert NM, Hartsough CS: Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil 1998; 31:533–544Google Scholar
31. Biederman J, Faraone SV, Weber W, Russell RL, Rater M, Park K: Correspondence between DSM-III-R and DSM-IV attention deficit hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry 1997; 36:1682–1687Google Scholar