Reduced Amygdala Response to Fearful Expressions in Children and Adolescents With Callous-Unemotional Traits and Disruptive Behavior Disorders
Abstract
Objective: Extensive work implicates abnormal amygdala activation in emotional facial expression processing in adults with callous-unemotional traits. However, no research has examined amygdala response to emotional facial expressions in adolescents with disruptive behavior and callous-unemotional traits. Moreover, despite high comorbidity of callous-unemotional traits and attention deficit hyperactivity disorder (ADHD), no research has attempted to distinguish neural correlates of pediatric callous-unemotional traits and ADHD. Method: Participants were 36 children and adolescents (ages 10–17 years); 12 had callous-unemotional traits and either conduct disorder or oppositional defiant disorder, 12 had ADHD, and 12 were healthy comparison subjects. Functional MRI was used to assess amygdala activation patterns during processing of fearful facial expressions. Patterns in the callous-unemotional traits group were compared with those in the ADHD and comparison groups. Results: In youths with callous-unemotional traits, amygdala activation was reduced relative to healthy comparison subjects and youths with ADHD while processing fearful expressions, but not neutral or angry expressions. Functional connectivity analyses demonstrated greater correlations between the amygdala and the ventromedial prefrontal cortex in comparison subjects and youths with ADHD relative to those with callous-unemotional traits. Symptom severity in the callous-unemotional traits groups was negatively correlated with connectivity between amygdala and ventromedial prefrontal cortex. Conclusions: This is the first study to demonstrate reduced amygdala responsiveness in youths with callous-unemotional traits. These findings support the contention that callous and unemotional personality traits are associated with reduced amygdala response to distress-based social cues.
Callous and unemotional traits, including reduced empathy and emotional response, affect a subgroup of children and adolescents with severe conduct problems (1) . Callous-unemotional traits increase the risk of deleterious outcomes in youths with disruptive behavioral disorders, such as conduct disorder and oppositional defiant disorder (1 , 2) . Similar associations with poor outcomes have been observed in adults with callous-unemotional traits, highlighting the importance of understanding the developmental neurobiology of these traits. Functional MRI (fMRI) studies in adults have related callous-unemotional traits to dysfunction in the amygdala and ventromedial prefrontal cortex. These structures show a high degree of functional connectivity and play a role in neurocognitive tasks in which individuals with callous-unemotional traits show impairments (3 – 5) . However, no fMRI studies have been conducted to assess whether callous-unemotional traits in adolescents are associated with dysfunction in these structures.
Both children and adults with callous-unemotional traits exhibit low reactivity to emotional stimuli. This deficit is manifest in diverse paradigms, including stimulus-reinforcement learning, aversive conditioning, fear-potentiated startle, passive avoidance learning, and fearful expression recognition (6) . Such deficits have also been observed after the occurrence of amygdala lesions, which supports an association between callous-unemotional traits and amygdala dysfunction (6 , 7) . Also supporting this association are fMRI studies in which amygdala dysfunction was observed in adults with callous-unemotional traits (3 – 5 , 8 , 9) .
The severity of callous-unemotional traits in children and adolescents can be measured by the Youth Psychopathic Traits Inventory, which focuses on features such as remorselessness and unemotionality (2) . Scores on this instrument accurately predict various forms of deviant conduct, including interpersonal aggression, theft, and drug selling (10) . Scores on this and other measures of callous-unemotional traits also correlate with measures of attention deficit hyperactivity disorder (ADHD) (10) , which raises questions about neuropsychological distinctions between children with callous-unemotional traits and those who have ADHD but do not have callous-unemotional traits. Imaging studies have suggested that these conditions are dissociable, as children with ADHD typically show minimal evidence of amygdala dysfunction but strong evidence of dysfunction in regions such as the prefrontal and anterior cingulate cortices (11 , 12) .
In this study, we compared fMRI blood-oxygen-level-dependent (BOLD) responses to fearful, neutral, and angry expressions in youths with callous-unemotional traits, youths with ADHD, and healthy comparison subjects. Children with callous-unemotional traits exhibit abnormal behavioral responses to distress-related expressions, such as fearful expressions, relative to other expressions, such as anger (13) , and fearful expressions engage the amygdala more than do neutral or angry expressions (14) . We hypothesized that amygdala activity in response to fearful expressions would be reduced in youths with callous-unemotional traits relative to youths with ADHD and healthy comparison subjects, but that no group differences would be seen in responses to angry expressions. Moreover, given previous work suggesting that connectivity between the amygdala and the ventromedial prefrontal cortex plays a role in fearful expression processing and callous-unemotional traits (15) , we hypothesized that callous-unemotional traits would be associated with reduced amygdala-ventromedial prefrontal cortex connectivity.
Method
Participants
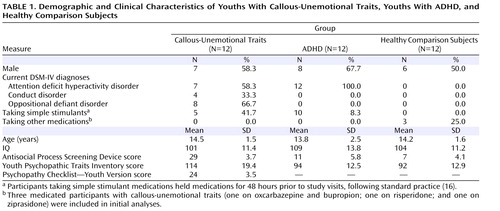
Participants were 36 children and adolescents 10–17 years of age; 12 had callous-unemotional traits and either conduct disorder or oppositional defiant disorder, 12 had ADHD, and 12 were healthy comparison subjects. Participants were matched on age, gender, and IQ ( Table 1 ). The Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL; 17) was administered to all potential participants. Exclusion criteria included psychosis, pervasive developmental disorders, Tourette’s syndrome, mood or anxiety disorders, neurologic disorders, IQ <80, or medical illness severe enough to require treatment. Participants in the callous-unemotional group had to have scores ≥20 on the Antisocial Process Screening Device (10) and the Psychopathy Checklist—Youth Version (18) . Participants in the comparison group and the ADHD group could not have scores ≥20 on the Antisocial Process Screening Device.
The study was approved by the institutional review board of the National Institute of Mental Health. After receiving a complete description of the study, participants and their parents or legal guardians provided written informed assent or consent.
Clinical Measures
The Antisocial Process Screening Device is a 20-item parent-completed scale indexing antisocial processes, including callous-unemotional traits and conduct and impulsivity problems, in children and adolescents. This instrument was completed by a parent or legal guardian for each participant. There is no established threshold score on the Antisocial Process Screening Device for classification of adolescents with callous-unemotional traits (19) . In studies of adolescents, researchers have used cutoff scores (such as a score of 25) (20) , median splits (e.g., >11 for males, 9 for females) (21) , or percentile rankings (e.g., the top 33%) (22) . For this study, we selected a cutoff score of 20, one-half the maximum possible score of 40 points.
The Psychopathy Checklist—Youth Version is a 20-item scale assessing interpersonal, affective, and behavioral features related to callous-unemotional traits in youths 12–18 years of age. Scores are based on semistructured interviews and collateral information. This instrument was completed by two trained experimenters whose scores showed good reliability (Spearman-Brown reliability, r SB =0.91); disagreements in scoring were resolved through discussion. The Psychopathy Checklist—Youth Version was completed for participants with callous-unemotional traits. A cutoff score of 20 or greater (one-half the maximum possible score) was used to classify adolescents with callous-unemotional traits, as no standard threshold scores have been established for classifying youths on this measure.
The Youth Psychopathic Traits Inventory was used to measure symptom severity on core interpersonal and affective callous-unemotional traits in all participants. This instrument is a 50-item self-report measure assessing personality domains including callousness, unemotionality, and remorselessness.
The K-SADS-PL was administered by trained clinicians. Based on an independent study, each clinician had been shown to have excellent reliability (kappa >0.75) with senior clinicians for all diagnoses.
fMRI Task
Each run of the paradigm presented participants with photographs of emotional expressions of 10 men and women from the Pictures of Facial Affect series (23) . The expressions were neutral, fearful, or angry, and the latter two types showed parametrically modulated intensity (50%, 100%, and 150% intensity). Intensity was modulated by morphing neutral and emotional expressions to create composites (50% intensity) or extrapolating from emotional expressions to create exaggerated expressions (150% intensity). Morphing introduced a diversity of expressions, as encountered in the environment. Because neutral expressions may appear threatening (24) , we followed previous studies in morphing neutral and happy expressions to create 25% happiness expressions, which are seen as affectively neutral (25) . Expressions were presented in random order across participants. In keeping with the design of previous studies (26 , 27) , participants performed an implicit processing task in which they indicated the gender of the faces using two response buttons. This method has been found to enhance BOLD responses to emotional expression stimuli (28) . Responses and latencies were recorded. Stimuli were presented on a computer display that was projected onto a mirror in the MRI scanner. Stimulus presentation occurred in four runs, each lasting 5 minutes, 27 seconds, and comprising 100 randomly ordered 3-second events (80 face trials, consisting of a 2-second face presentation and a 1-second fixation cross, and 20 interspersed “jittered” trials). Runs were preceded by four fixation trials and concluded by five fixation trials.
Image Acquisition
Data were acquired on a 1.5-T General Electric Signa scanner (Milwaukee). Structural images were generated from a T 1 -weighted acquisition of the entire brain in the axial plane (three-dimensional spoiled gradient-recall acquisition in the steady state with inversion recovery prep pulse; 256×256 matrix; 128 1.5-mm axial slices; 24-cm field of view). This sequence was used for spatial normalization to a standard atlas (29) . Functional imaging was performed axially by using a multislice gradient-echo echo-planar sequence, 24-cm field of view, and an acquisition matrix of 64×64 (31 contiguous slices, 4-mm thickness, 3-second repetition time, 30-millisecond echo time). This sequence provided a voxel resolution of 3.75×3.75×4 mm.
Image Analysis
The fMRI data were preprocessed and analyzed with the Analysis of Functional NeuroImages (AFNI) software package (30) . The first four volumes in each scan series were discarded, leaving 608 repetition times per participant. Preprocessing included slice time correction, motion correction, and spatial normalization. The resulting motion parameters were examined to ensure that motion did not exceed 4 mm in any plane. For each participant, linear regression was used to model baseline drift and residual motion artifact. Regressors were created for each event type, including fearful expressions, angry expressions, neutral expressions, and all incorrect behavioral responses. Fearful and angry regressors were weighted according to emotion intensity. Regressors were then convolved with a gamma-variate hemodynamic response function.
Using AFNI, we conducted two group-by-emotional expression (32) analyses of variance (ANOVAs) comparing responses to fearful versus neutral expressions and angry versus neutral expressions. We focused on the resulting interaction effects (group by expression) in order to address our hypothesis that healthy youths and those with ADHD would show increased amygdala activation in response to fearful expressions relative to neutral expressions but that adolescents with callous-unemotional traits would not. We also wished to show that no group-by-expression interaction would emerge in response to angry versus neutral expressions. Clusters from resulting group maps of differential activation were selected according to our a priori hypotheses. Average signals in the clusters resulting from the analyses of functional neuroimages were extracted, and follow-up group-by-expression (32) ANOVAs and t tests (two-tailed) were performed in SPSS. Anatomical locations were labeled according to the Talairach-Tournoux Daemon. Spatial clustering using AFNI’s AlphaSim program resulted in a mapwise (entire echo planar imaging matrix) false positive probability of p<0.05.
Functional connectivity analyses were performed by examining covariation across the brain with the activation in the maximally activated voxel in the amygdala cluster created by the original analysis using AFNI. For each participant, voxelwise correlation analyses were conducted between each individual voxel’s time series and that of the identified seed. We used t tests to compare correlation coefficients in the resulting regions of interest across groups.
Results
Behavior
Behavioral data indicated that participants successfully performed the gender rating task (mean accuracy 92.2% [SD=8.3], mean reaction time=880.1 milliseconds [SD=162.0]). A main effect of emotional expression was observed for accuracy (F=4.31, df=2, 54, p<0.05); accuracy was greater for neutral expressions than for angry expressions (t=2.86, df=32, p<0.01). No effect of emotional expression was observed for reaction times. No group differences in accuracy were observed. Group differences in reaction times were observed (F=5.34, df=2, 27, p<0.01); the ADHD group responded less quickly than the other groups, but no differences were observed between the comparison group and the callous-unemotional traits group (p>0.05). No group-by-expression interactions for reaction time or accuracy were observed. (Because of a computer error, behavioral responses were not recorded for three participants in the callous-unemotional traits group. However, responses were visually monitored throughout the task, and performance rates for these participants were noted to be high and comparable to those of the rest of the group.)
Imaging
Responses to fearful expressions
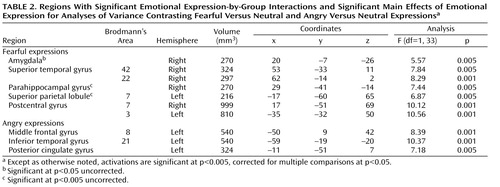
We used AFNI to test the hypothesis that the results of a 23 ANOVA (fearful and neutral expressions by group) would reveal more amygdala activity in response to fearful expressions in the comparison group relative to the callous-unemotional traits group. By contrast, no difference between the ADHD and comparison groups was expected. This hypothesis was supported by a significant group-by-expression interaction in the right amygdala (x, y, z=20, –7, –26; Figure 1 ).
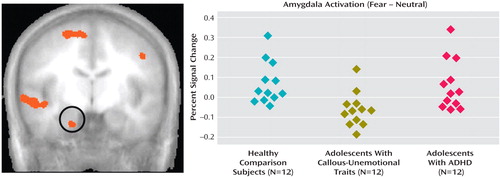
a The image on the left shows the region of the right amygdala in which an interaction effect was observed. The graph on the right summarizes amygdala activation in each group.
An ANOVA in SPSS of the BOLD responses in the identified amygdala cluster revealed a significant two-way interaction (group by expression) (F=5.56, df=1, 33, p<0.01) ( Table 2 ; Figure 1 ). Follow-up analyses decomposed this interaction. A comparison of the responses of the callous-unemotional traits group and the comparison group to fearful and neutral expressions revealed a significant group-by-expression interaction effect (F=11.06, df=1, 22, p<0.005). The comparison group showed greater amygdala activation in response to fearful expressions than did the callous-unemotional traits group (t=3.03, df=22, p<0.01), whereas no differences were seen for neutral expressions. A similar interaction emerged in comparing the ADHD and callous-unemotional traits groups (F=7.61, df=1, 22, p=0.01). The ADHD group exhibited a trend toward greater amygdala activation for fearful expressions than did the callous-unemotional traits group (t=1.85, df=22, p<0.08), whereas no similar difference emerged for neutral expressions. By contrast, a follow-up analysis comparing responses of the ADHD group and the comparison group to fearful and neutral expressions revealed only a significant main effect of expression (fearful > neutral) (F=7.43, df=1, 22, p<0.05). No group-by-expression interaction emerged ( Figure 1 ).
This pattern indicates that the callous-unemotional traits group showed significantly less left amygdala activation in response to fearful versus neutral expressions than the healthy comparison group and the ADHD group, whereas the ADHD group did not differ from the comparison group. This was confirmed by post hoc t tests using AFNI (fear minus neutral) showing increased amygdala activation to fearful expressions in the comparison group (t=3.18, p<0.005) and the ADHD group (t=4.65, p<0.005) but not in the callous-unemotional traits group (p>0.05).
Functional connectivity
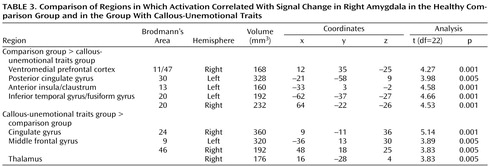
These analyses focused on differences in functional connectivity associated with the seed amygdala voxel (x, y, z=20, –7, –26) in the three groups. This voxel was chosen because of previous suggestions (15 , 31) that the amygdala communicates reward expectancies to the ventromedial prefrontal cortex. There was a stronger positive correlation between activity in the right amygdala and the right ventromedial prefrontal cortex in the comparison group relative to the callous-unemotional traits group (x, y, z=12, 35, –25; t=4.27, p<0.01 uncorrected) ( Figure 2 ), as well as in the ADHD group relative to the callous-unemotional traits group (x, y, z=–15, 14, –19, t=6.79, p<0.001).
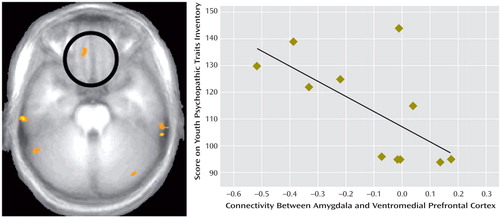
a The image on the left shows a region of the ventromedial prefrontal cortex with greater positive connectivity in healthy comparison subjects than in youths with callous-unemotional traits. The graph on the right shows the correlation between scores on the Youth Psychopathic Traits Inventory and connectivity between the amygdala and the ventromedial prefrontal cortex in youths with callous-unemotional traits (N=11; data for the Youth Psychopathic Traits Inventory missing for one participant in this group).
In adolescents with callous-unemotional traits, the magnitude of connectivity between the amygdala and the ventromedial prefrontal cortex correlated significantly and negatively with total score on the Youth Psychopathic Traits Inventory (Spearman r=–0.65, df=9, p<0.05). (The degrees of freedom were reduced from 10 [N–2] to nine for this analysis because data for the Youth Psychopathic Traits Inventory were not available for one participant in the callous-unemotional traits group.) Thus, functional connectivity between the amygdala and the ventromedial prefrontal cortex in callous-unemotional adolescents was inversely correlated with symptom severity ( Figure 2 ). This correlation was also negative for the comparison group but was smaller and nonsignificant, reflecting the more limited range of scores on the Youth Psychopathic Traits Inventory in this group.
These findings suggest that processing emotional expressions is associated with weaker functional connectivity between the amygdala and the ventromedial prefrontal cortex in youths with callous-unemotional traits than in those without such traits. Table 3 provides the full results comparing the healthy comparison group and the callous-unemotional traits group, using an uncorrected alpha threshold of 0.01 with an extent threshold of three voxels (168 mm 3 ).
Responses to angry expressions
We used AFNI to perform a 23 ANOVA (angry and neutral expressions by group) to assess neural responses to angry expressions. The results revealed no group-by-expression interaction in the amygdala for angry expressions comparable to that seen for fearful expressions (p>0.05). Post hoc t tests (angry minus neutral expressions) confirmed an absence of amygdala activation in response to angry expressions in the comparison group (p>0.01), the callous-unemotional traits group (p>0.01), and the ADHD group (p>0.01). Regions in which interaction effects were observed included the middle frontal gyrus, the posterior cingulate gyrus, and the inferior temporal gyrus ( Table 2 ).
ANOVAs conducted in SPSS on the BOLD responses in the identified clusters showed interactions in the middle frontal gyrus (F=8.97, df=2, 33, p<0.001) and the posterior cingulate cortex (F=7.16, df=2, 33, p<0.005). Follow-up analyses decomposed these interactions, and similar patterns of effects were seen in both clusters. No main effects or interactions were observed in either cluster when comparing the healthy comparison group and the callous-unemotional traits group. However, significant group-by-expression interactions and main effects of expression (angry > neutral) were seen in both clusters when comparing the ADHD group and the healthy comparison group or the callous-unemotional traits group (p values <0.05). Follow-up t tests on these clusters showed main effects of emotional expression (angry > neutral) in both regions in the ADHD group (posterior cingulate cortex, t=2.76, df=11, p<0.05; middle frontal gyrus, t=3.55, df=11, p<0.01) but no main effects of emotional expression in the callous-unemotional traits group or the comparison group in either region.
Discussion
We compared BOLD responses to fearful, angry, and neutral facial expressions in youths with callous-unemotional traits and disruptive behavioral disorders, youths with ADHD, and comparison subjects. We found that whereas healthy comparison subjects and youths with ADHD showed significantly greater responses to fearful expressions relative to neutral expressions, youths with callous-unemotional traits did not. No similar interaction was evident for responses to angry expressions relative to neutral expressions. Healthy comparison subjects and youths with ADHD also showed greater connectivity between the amygdala and the ventromedial prefrontal cortex than did those with callous-unemotional traits, and symptom severity in youths with callous-unemotional traits was inversely correlated with amygdala-ventromedial prefrontal cortex connectivity.
Dysfunction in the amygdala is central to several models of callous-unemotional traits (6 , 32 , 33) . The amygdala is believed to play an important role in response to distress-related emotional expressions, such as fear, and to play a role in socialization (6) . Children with callous-unemotional traits have impaired processing of the distress cues that guide healthy children away from antisocial behavior. Amygdala dysfunction may represent the locus of the impairment in distress cue processing and thereby underlie socialization problems (34) . The results of this study extend previous findings of amygdala dysfunction in adults with callous-unemotional traits (3 – 5) .
Our data also did not indicate impairments in the ventrolateral prefrontal cortex in youths with callous-unemotional traits. Although this region is sometimes associated with the processing of affective cues, this is primarily the case when these cues call for response modulation or modification (35) . It may be that we observed no group differences in this region because our task did not require behavior modification in response to the affective cues presented. Our data also did not indicate group differences in activation in some regions that may be impaired in individuals with callous-unemotional traits, including the ventromedial prefrontal cortex, the anterior cingulate cortex, and the insula (3 , 5 , 36) . This may be because the task we used is not typically found to enhance activity in these regions. A recent meta-analysis (37) showed that activation in the amygdala is enhanced during the presentation of fearful expressions but that the insula is instead preferentially enhanced by disgust expressions and the anterior cingulate cortex is active to a similar degree across multiple emotional expressions. The meta-analysis did not assess the medial orbitofrontal cortex, but this region is generally not found to be preferentially enhanced by implicit processing of fearful facial expressions (24 , 28) .
Nevertheless, our functional connectivity results do extend our understanding of the role of the amygdala, the insula, and the ventromedial prefrontal cortex in callous-unemotional traits. Functional connectivity is a measure of correlated activity derived from BOLD data between a reference region and a target region. This measure is widely used to characterize aspects of functional integration between neural regions, and accumulating evidence indicates that functional connectivity reflects anatomically relevant coupling within neural circuitry. The region of the ventromedial prefrontal cortex identified in our connectivity analyses is one in which activity is frequently associated with amygdala activity (35 , 38 , 39) . Animal studies indicate that this region uses input acquired from the amygdala to guide behavioral response (31) , and thus amygdala-ventromedial prefrontal cortex connectivity is important for appropriate behavioral selection (15 , 40) . Impairments in amygdala-ventromedial prefrontal cortex connectivity may be associated with the antisocial behavior seen in children and adolescents with callous-unemotional traits, which may represent instrumental behavior that is inappropriately modulated by stimuli such as others’ distress cues. In support of this interpretation, the amygdala and proximal regions of the ventromedial prefrontal cortex have been implicated in reasoning about moral and immoral behaviors (41) .
We observed no amygdala dysfunction in the ADHD group, in which amygdala response was similar to that of the healthy comparison subjects and distinct from that of the callous-unemotional traits group. This finding parallels previous behavioral work, which generally has not found deficits in the processing of fearful expressions in children with ADHD (42 , 43) . Impairments linked to ADHD appear to be associated with neural dysfunction in frontal-striatal and frontal-parietal networks (44) . Our imaging task did not assess functioning in these networks, so our analyses were not directed toward measuring activity or functional connectivity in them. However, our data, coupled with previous behavioral and neuroimaging findings, suggest that although ADHD and callous-unemotional traits are both characterized by impulsiveness and irresponsibility, the disorders are associated with dissociable neurocognitive dysfunctions.
Previous behavioral work has demonstrated that individuals with callous-unemotional traits show no impairment in the recognition of angry expressions (45 , 46) . In line with this in the current study, we identified no regions where youths with callous-unemotional traits showed significantly different BOLD responses to angry relative to neutral expressions. In contrast, youths with ADHD showed enhanced activation in regions of the frontal and posterior cingulate cortex in response to angry expressions. Previous work has also implicated hyperactivation in the frontal and posterior cingulate cortex in ADHD (12 , 47) . The underlying cause of the hyperactivation in response to angry expressions in children with ADHD is unclear, but it may reflect pathophysiology in these attention-related cortical regions.
Certain study limitations must be borne in mind when interpreting our findings. The medications of three participants in the callous-unemotional traits group could not be held prior to fMRI scanning. Mitigating this limitation, however, is that when we repeated our ANOVAs after excluding these participants, we found no differences in results (the coordinates of the maximally activated voxel remained unchanged; signal strength increased from F=5.20, to F=7.14, both p values <0.01 uncorrected). We also found no difference in the correlation between scores on the Youth Psychopathic Traits Inventory and amygdala-ventromedial prefrontal cortex connectivity after excluding these participants (the coordinates of the maximally activated amygdala voxel remained unchanged; signal strength changed minimally from F=5.20 to F=4.20, both p values <0.05 uncorrected).
We are also limited in our ability to draw conclusions about the relationship between ADHD and callous-unemotional traits, given that seven of the 12 youths in our callous-unemotional sample had ADHD diagnoses. This proportion is representative of comorbidity estimates of callous-unemotional traits and ADHD (1 , 48) . Limiting our sample to adolescents who had both callous-unemotional traits and ADHD or to those who had callous-unemotional traits without ADHD would have reduced the representativeness of our sample without affecting our main conclusion, which is that callous-unemotional traits and ADHD are associated with disparate patterns of neuropsychological functioning.
The imaging task we used is limited in that it does not permit measurements of explicit cognitive or behavior responses to the emotional facial expressions. We selected this task design to reduce biases related to expectancy or participant demands (28) . Future studies might assess the extent to which BOLD responses correspond to behavioral responses. They might also include a greater variety of emotional expression stimuli, such as expressions of happiness, surprise, and disgust. This would help strengthen the conclusion that facial emotion processing impairments in youths with callous-unemotional traits are limited to distress-related emotions (namely, fear and sadness), the processing of which requires intact amygdala functioning. Previous data support the validity of this study’s task design for assessing neural responses to fearful versus neutral emotional expressions (49) . Previous studies using similar paradigms have distinguished adults with callous-unemotional traits from those without (8 , 9) . The data in this study provide the first evidence of amygdala dysfunction in youths with callous-unemotional traits and disruptive behavior disorders relative to healthy youths and youths with ADHD. The data also indicate that the severity of callous-unemotional traits is associated with the strength of the functional association between the amygdala and the ventromedial prefrontal cortex.

1. Frick P, Stickle T, Dandreaux D, Farrell J, Kimonis E: Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. J Abnorm Child Psychol 2005; 33:471–487Google Scholar
2. Andershed H, Gustafson SB, Kerr M, Stattin H: The usefulness of self-reported psychopathy-like traits in the study of antisocial behaviour among non-referred adolescents. Eur J Personality 2002; 16:383–402Google Scholar
3. Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H: Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2005; 62:799–805Google Scholar
4. Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF: Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry 2001; 50:677–684Google Scholar
5. Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO: Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry 2006; 61:1260–1271Google Scholar
6. Blair R, Peschardt K, Budhani S, Mitchell D, Pine D: The development of psychopathy. J Child Psychol Psychiatry 2006; 47:262–276Google Scholar
7. Patrick C: Emotion and psychopathy: startling new insights. Psychophysiology 1994; 31:319–330Google Scholar
8. Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, Ambikapathy A, Robertson D, Giampietro V, Brammer MJ, Clarke A, Dowsett J, Fahy T, Phillips ML, Murphy DG: Facial emotion processing in criminal psychopathy: preliminary functional magnetic resonance imaging study. Br J Psychiatry 2006; 189:533–539Google Scholar
9. Gordon HL, Baird AA, End A: Functional differences among those high and low on a trait measure of psychopathy. Biol Psychiatry 2004; 56:516–521Google Scholar
10. Poythress NJ, Dembo R, Wareham J, Greenbaum P: Construct validity of the Youth Psychopathic Traits Inventory (YPI) and the Antisocial Process Screening Device (APSD) with justice-involved adolescents. Crim Justice Behav 2006; 33:26–55Google Scholar
11. Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL: Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:374–383Google Scholar
12. Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R III, Xiong J, Liotti M: Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry 2006; 163:1052–1060Google Scholar
13. Blair RJ, Colledge E, Murray L, Mitchell DG: A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol 2001; 29:491–498Google Scholar
14. Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL: A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 2001; 1:70–83Google Scholar
15. Blair R: The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn 2004; 55:198–208Google Scholar
16. Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, Denckla MB: Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry 2006; 59:48–56Google Scholar
17. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Google Scholar
18. Forth AE, Kosson DS, Hare RD: The Psychopathy Checklist: Youth Version. Toronto, Ontario, Canada, Multi-Health Systems, 2003Google Scholar
19. Frick PJ, Lilienfeld S, Ellis M, Loney B, Silverthorn P: The association between anxiety and psychopathy dimensions in children. J Abnorm Child Psychol 1999; 27:383–392Google Scholar
20. Budhani S, Blair R: Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry 2005; 46:972–981Google Scholar
21. Vitale J, Newman J, Bates J, Goodnight J, Dodge K, Pettit G: Deficient behavioral inhibition and anomalous selective attention in a community sample of adolescents with psychopathic traits and low-anxiety traits. J Abnorm Child Psychol 2005; 33:461–470Google Scholar
22. Murrie D, Cornell D: Psychopathy screening of incarcerated juveniles: a comparison of measures. Psychol Assess 2002; 14:390–396Google Scholar
23. Ekman P, Friesen W: Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists, 1976Google Scholar
24. Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA: Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage 2004; 21:1484–1496Google Scholar
25. Young AW, Rowland D, Calder AJ, Etcoff NL, Seth A, Perrett DI: Facial expression megamix: tests of dimensional and category accounts of emotion recognition. Cognition 1997; 63:271–313Google Scholar
26. Blair RJ, Morris J, Frith C, Perrett D, Dolan R: Dissociable neural responses to facial expressions of sadness and anger. Brain 1999; 122:883–893Google Scholar
27. Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA: Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci 1998; 265:1809–1817Google Scholar
28. Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, Gray JA, Phillips ML: Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry 2003; 53:226–232Google Scholar
29. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme, 1988Google Scholar
30. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Google Scholar
31. Schoenbaum G, Setlow B, Saddoris M, Gallagher M: Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron 2003; 39:855–867Google Scholar
32. Kiehl K: A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res 2006; 142:107–128Google Scholar
33. Patrick C, Cuthbert B, Lang P: Emotion in the criminal psychopath: fear image processing. J Abnorm Psychol 1994; 103:523–534Google Scholar
34. Wootton J, Frick PJ, Shelton K, Silverthorn P: Ineffective parenting and childhood conduct problems: the moderating role of callous-unemotional traits. J Consult Clin Psychol 1997; 65:301–308Google Scholar
35. Budhani S, Marsh A, Pine DS, Blair R: Neural correlates of response reversal: considering acquisition. Neuroimage 2007; 34:1754–1765Google Scholar
36. Kiehl KA, Smith AM, Mendrek A, Forster B, Hare R, Liddle P: Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Res 2004; 130:297–312Google Scholar
37. Murphy F, Nimmo-Smith I, Lawrence A: Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci 2003; 3:207–233Google Scholar
38. McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS: Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007; 64:97–106Google Scholar
39. Siegle GJ, Carter CS, Thase ME: Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 2006; 163:735–738Google Scholar
40. De Martino B, Kumaran D, Seymour B, Dolan R: Frames, biases, and rational decision-making in the human brain. Science 2006; 313:684–687Google Scholar
41. Moll J, De Oliveira-Souza R, Eslinger PJ: Morals and the human brain: a working model. Neuroreport 2003; 14:299–305Google Scholar
42. Cadesky E, Mota VL, Schachar R: Beyond words: how do children with ADHD and/or conduct problems process nonverbal information about affect? J Am Acad Child Adolesc Psychiatry 2000; 39:1160–1167Google Scholar
43. Pelc K, Kornreich C, Foisy ML, Dan B: Recognition of emotional facial expressions in attention-deficit hyperactivity disorder. Pediatr Neurol 2006; 35:93–97Google Scholar
44. Dickstein S, Bannon K, Xavier Castellanos F, Milham M: The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 2006; 47:1051–1062Google Scholar
45. Blair RJR, Mitchell DGV, Peschardt KS, Colledge E, Leonard RA, Shine JH, Murray LK, Perrett DL: Reduced sensitivity to others’ fearful expressions in psychopathic individuals. Pers Individ Dif 2004; 37:1111–1122Google Scholar
46. Kosson D, Suchy Y, Mayer AR, Libby J: Facial affect recognition in criminal psychopaths. Emotion 2002; 2:398–411Google Scholar
47. Silk T, Vance A, Rinehart N, Egan G, O’Boyle M, Bradshaw JL, Cunnington R: Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry 2005; 187:282–283Google Scholar
48. Blair RJ, Colledge E, Mitchell DG: Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol 2001; 29:499–511Google Scholar
49. Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ: Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage 2005; 25:1112–1123Google Scholar